Obstetric Ultrasonography to Detect Fetal Abnormalities in a Mouse Model for Zika Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Mice
2.3. Cells and Viruses
2.4. Ultrasound Imaging
2.5. Determination of ZIKV Loads in Mouse Organs
2.6. Histopathology and Immunohistochemistry
2.7. Statistical Analysis
3. Results
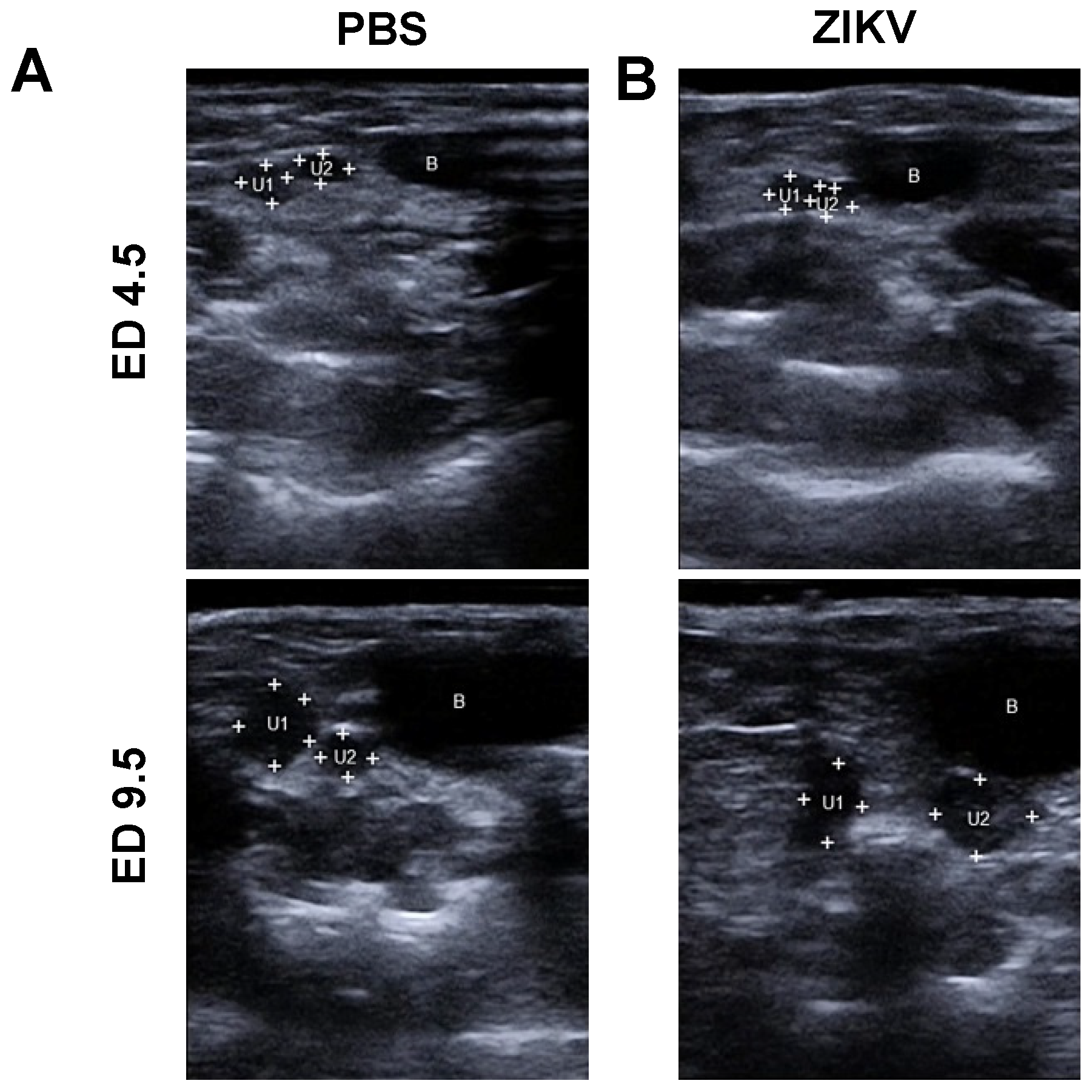
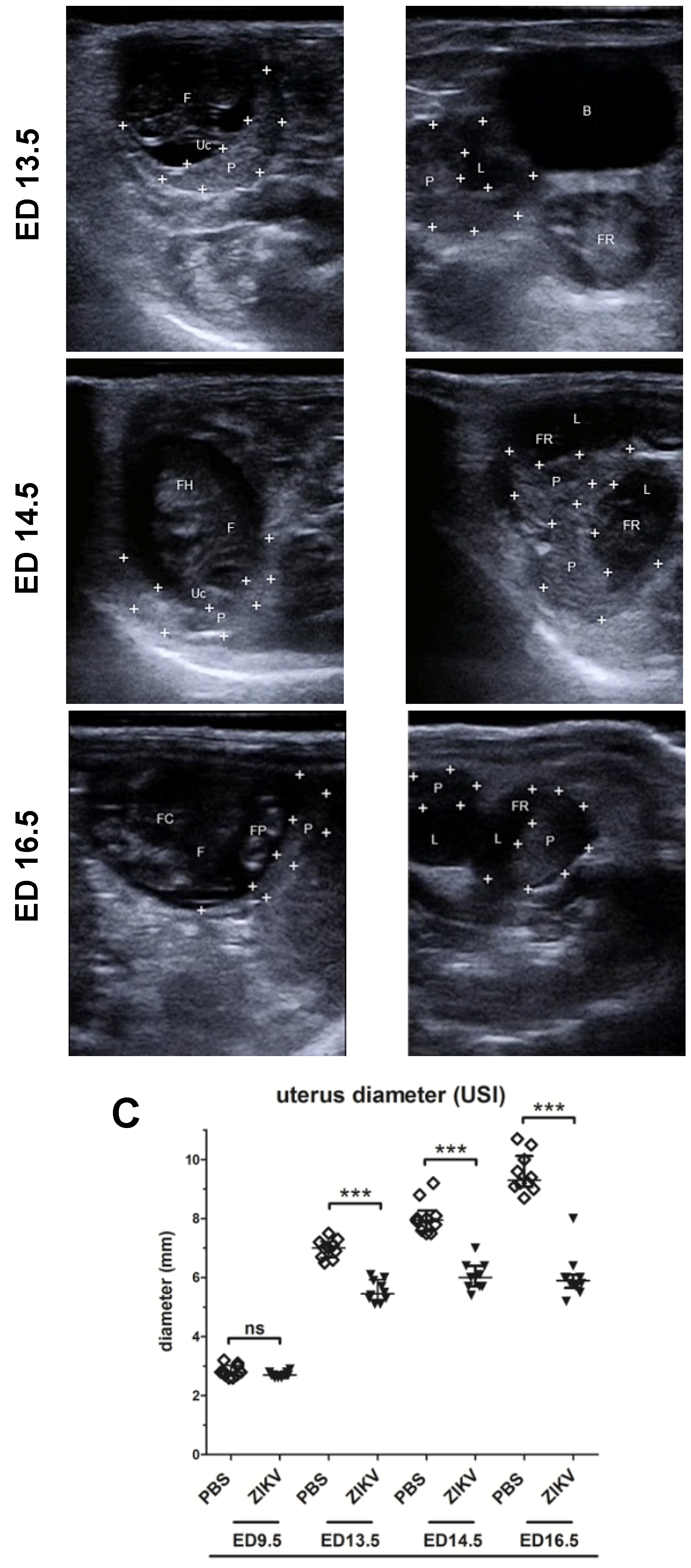
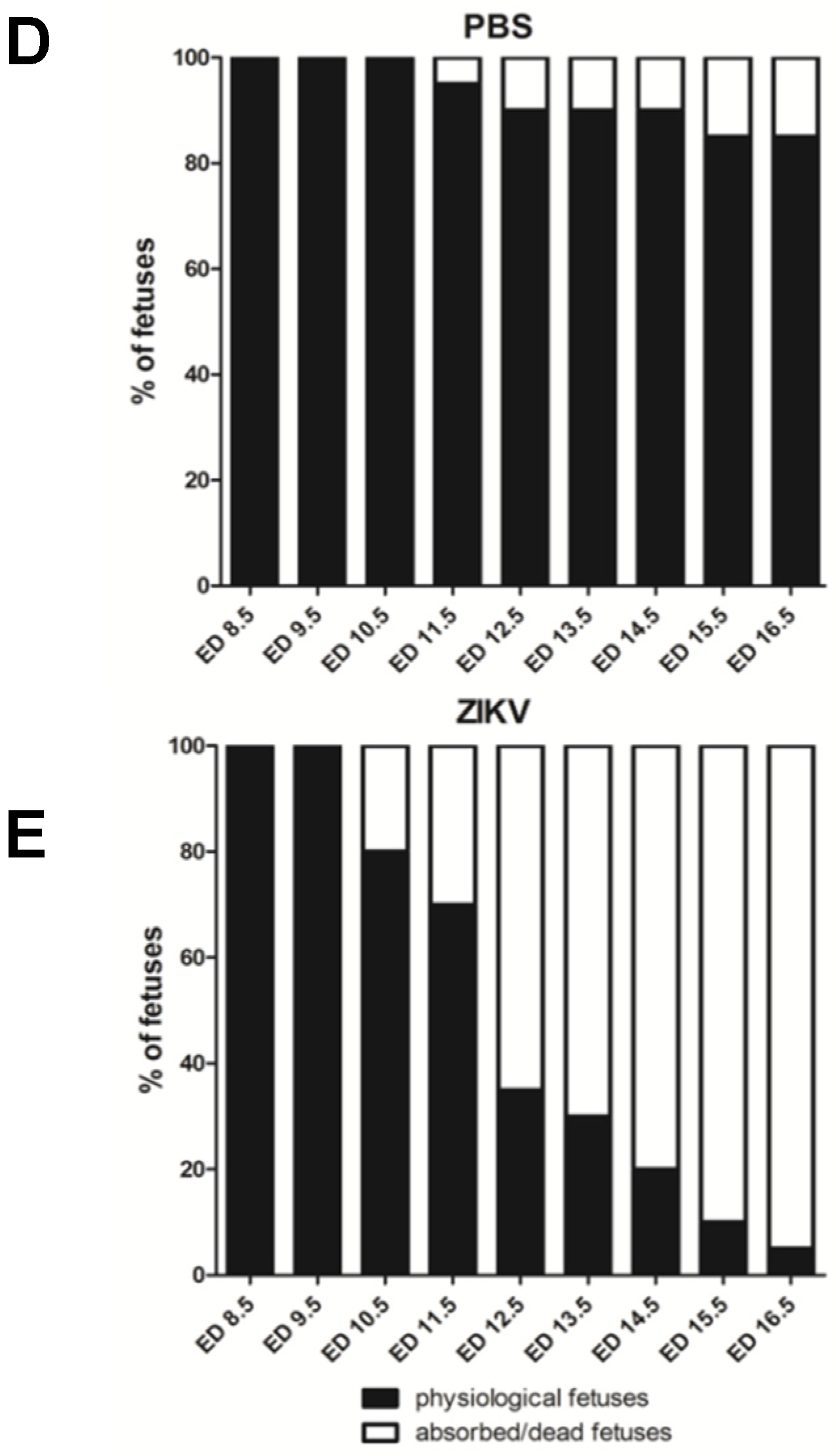
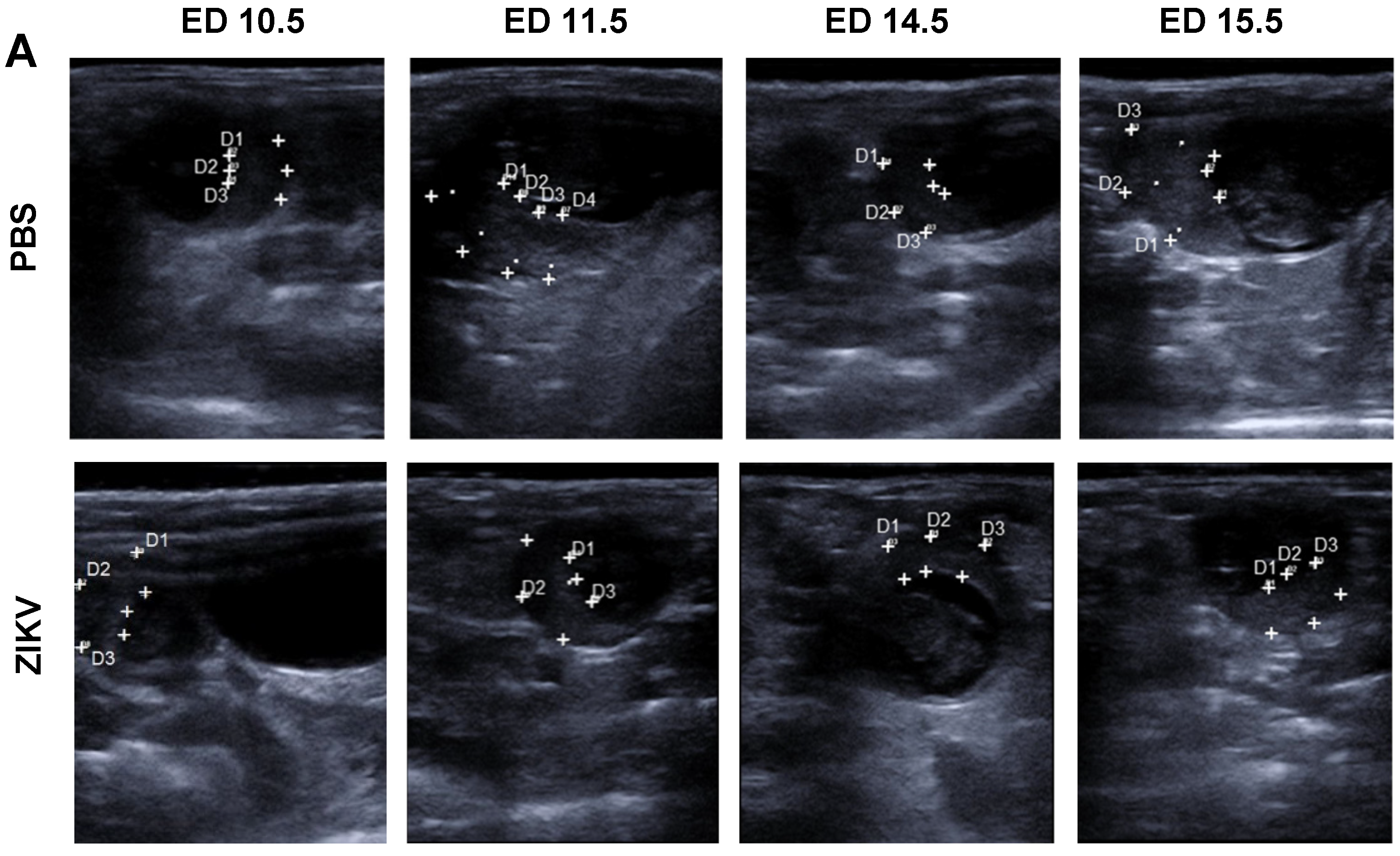
3.1. Outcome of ZIKV Infection in Pregnant IFNAR-/- Mice as Analyzed by Sonography
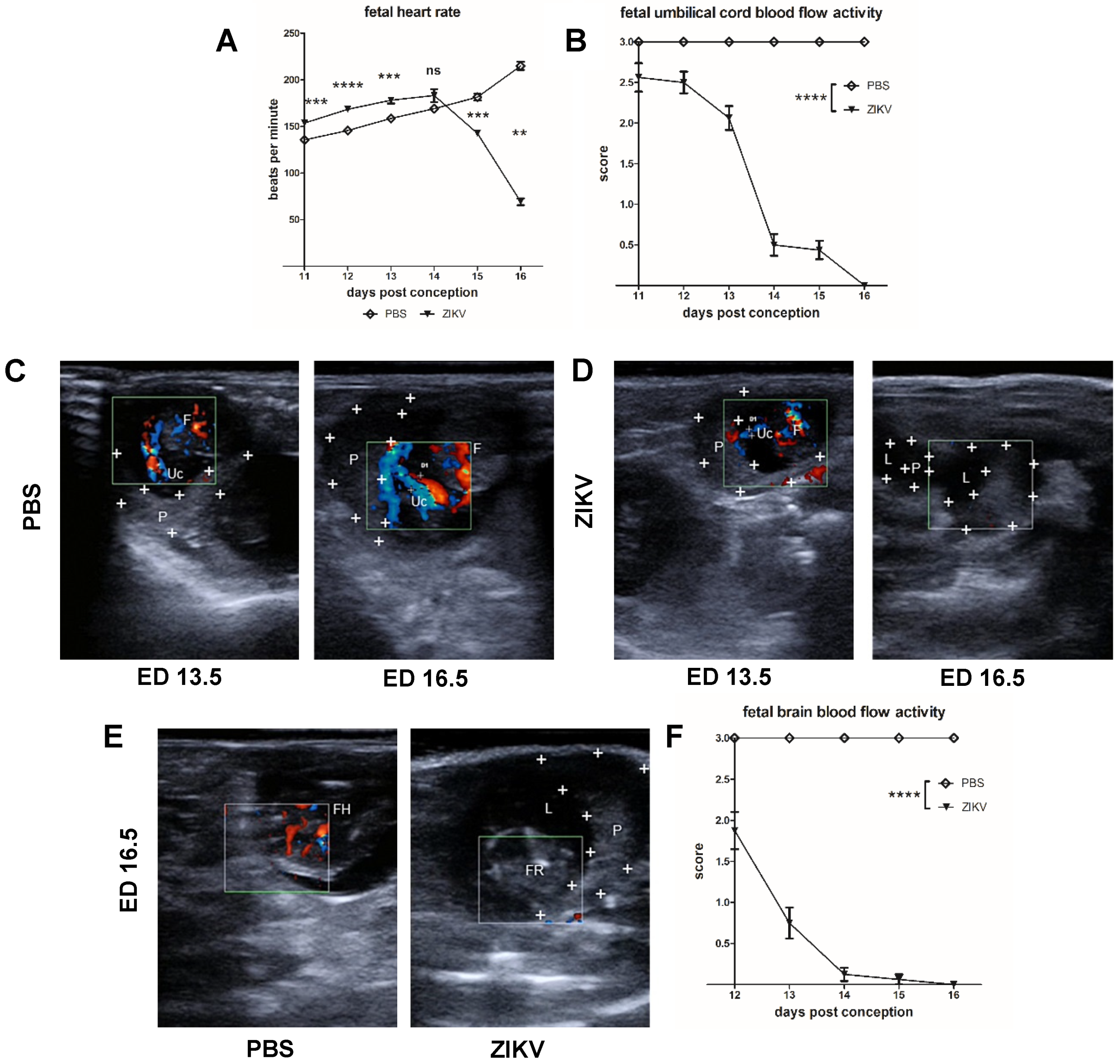
3.2. Evaluation of Uteroplacental Blood Flow Activity by Doppler Ultrasound
Direct Comparison between ED 13.5 and ED 16.5
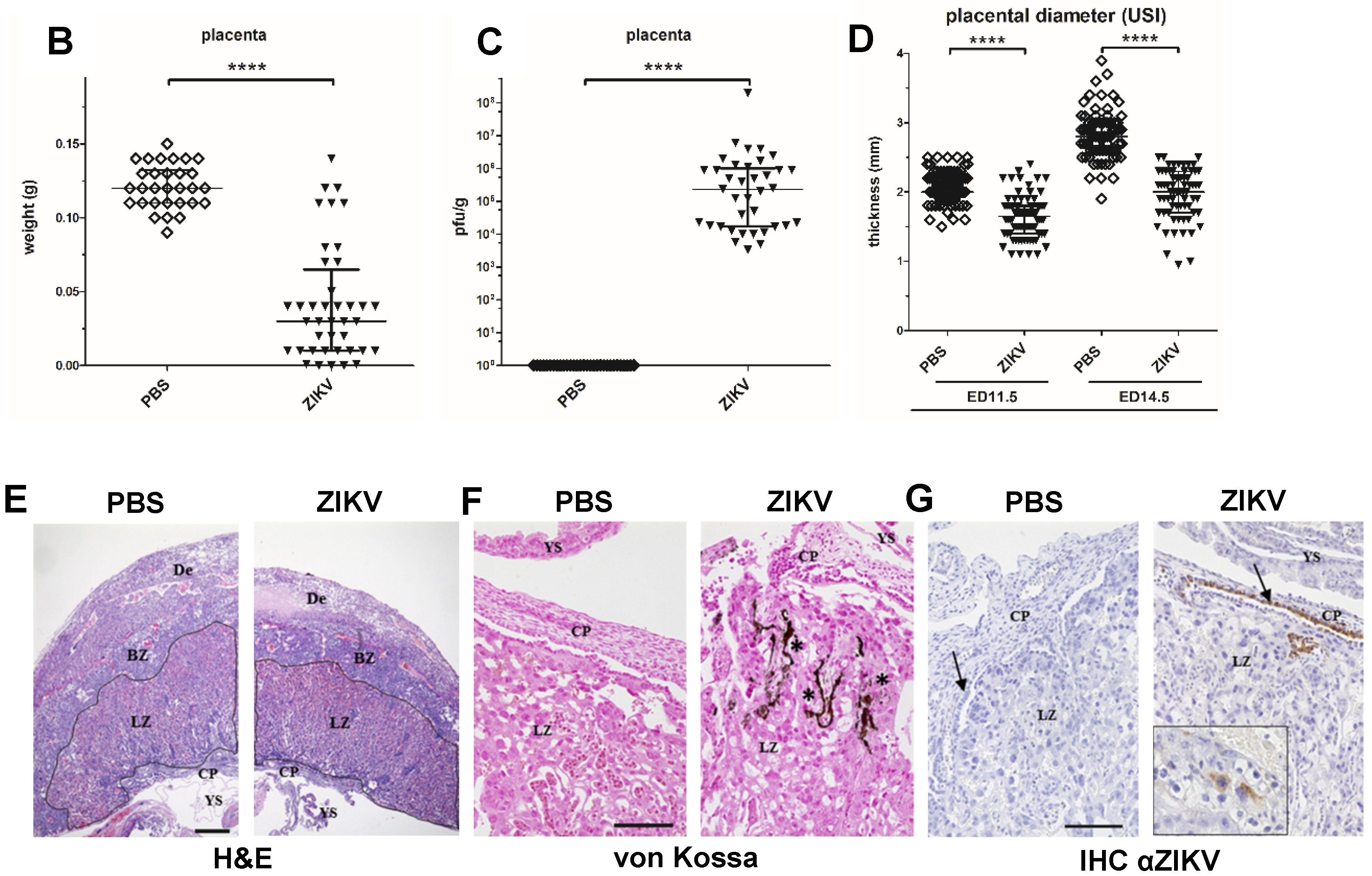
3.3. Characterization of ZIKV Infection of the Placenta
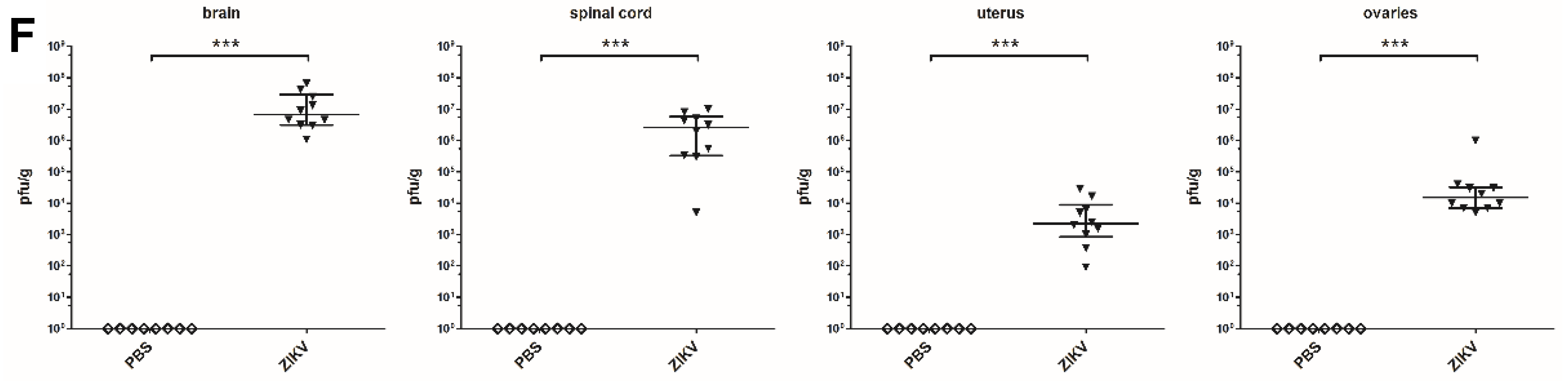
3.4. Macroscopic Outcome and Viral Burden
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Faye, O.; Freire, C.C.; Iamarino, A.; Faye, O.; de Oliveira, J.V.; Diallo, M.; Zanotto, P.M.; Sall, A.A. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl. Trop. Dis. 2014, 8, e2636. [Google Scholar] [CrossRef]
- Ioos, S.; Mallet, H.P.; Leparc Goffart, I.; Gauthier, V.; Cardoso, T.; Herida, M. Current Zika virus epidemiology and recent epidemics. Med. Mal. Infect. 2014, 44, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.; Horovitz, D.D.; Cavalcanti, D.P.; Pessoa, A.; Doriqui, M.J.; Neri, J.I.; Neto, J.M.; Wanderley, H.Y.; et al. Possible Association Between Zika Virus Infection and Microcephaly—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastere, S.; Valour, F.; Baudouin, L.; Mallet, H.; Musso, D.; Ghawche, F. Zika virus infection complicated by Guillain-Barre syndrome—Case report, French Polynesia, December 2013. Eurosurveillance 2014, 19, 20720. [Google Scholar] [CrossRef] [PubMed]
- do Rosario, M.S.; de Jesus, P.A.; Vasilakis, N.; Farias, D.S.; Novaes, M.A.; Rodrigues, S.G.; Martins, L.C.; Vasconcelos, P.F.; Ko, A.I.; Alcantara, L.C.; et al. Guillain-Barre Syndrome After Zika Virus Infection in Brazil. Am. J. Trop. Med. Hyg. 2016, 95, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Imperato, P.J. The Convergence of a Virus, Mosquitoes, and Human Travel in Globalizing the Zika Epidemic. J. Community Health 2016, 41, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Cao-Lormeau, V.M.; Gubler, D.J. Zika virus: Following the path of dengue and chikungunya? Lancet 2015, 386, 243–244. [Google Scholar] [CrossRef]
- Marchette, N.J.; Garcia, R.; Rudnick, A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969, 18, 411–415. [Google Scholar] [CrossRef]
- D’Ortenzio, E.; Matheron, S.; Yazdanpanah, Y.; de Lamballerie, X.; Hubert, B.; Piorkowski, G.; Maquart, M.; Descamps, D.; Damond, F.; Leparc-Goffart, I. Evidence of Sexual Transmission of Zika Virus. N. Engl. J. Med. 2016, 374, 2195–2198. [Google Scholar] [CrossRef]
- Coyne, C.B.; Lazear, H.M. Zika virus—Reigniting the TORCH. Nat. Rev. Microbiol. 2016, 14, 707–715. [Google Scholar] [CrossRef]
- Lazear, H.M.; Diamond, M.S. Zika Virus: New Clinical Syndromes and Its Emergence in the Western Hemisphere. J. Virol. 2016, 90, 4864–4875. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, J.M.; Dutertre, M.; Mengelle, C.; Fourcade, C.; Marchou, B.; Delobel, P.; Izopet, J.; Martin-Blondel, G. Zika virus: High infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016, 16, 405. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Cauchemez, S.; Besnard, M.; Bompard, P.; Dub, T.; Guillemette-Artur, P.; Eyrolle-Guignot, D.; Salje, H.; Van Kerkhove, M.D.; Abadie, V.; Garel, C.; et al. Association between Zika virus and microcephaly in French Polynesia, 2013–2015: A retrospective study. Lancet 2016, 387, 2125–2132. [Google Scholar] [CrossRef]
- Joob, B.; Wiwanitkit, V. Fetal Magnetic Resonance Imaging of Fetus with Zika Virus Infection. Pediatr. Neurol. 2017, 68, e1. [Google Scholar] [CrossRef] [PubMed]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine Publications Committee. Ultrasound screening for fetal microcephaly following Zika virus exposure. Am. J. Obstet. Gynecol. 2016, 214, B2–B4. [Google Scholar] [CrossRef]
- Adams Waldorf, K.M.; Nelson, B.R.; Stencel-Baerenwald, J.E.; Studholme, C.; Kapur, R.P.; Armistead, B.; Walker, C.L.; Merillat, S.; Vornhagen, J.; Tisoncik-Go, J.; et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat. Med. 2018, 24, 368–374. [Google Scholar] [CrossRef]
- Cao, B.; Diamond, M.S.; Mysorekar, I.U. Maternal-Fetal Transmission of Zika Virus: Routes and Signals for Infection. J. Interferon Cytokine Res. 2017, 37, 287–294. [Google Scholar] [CrossRef]
- de Carvalho Leal, M.; Ramos, D.S.; Caldas Neto, S.S. Hearing Loss From Congenital Zika Virus Infection. Top. Magn. Reson. Imaging 2019, 28, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Melo, A.S.; Malinger, G.; Ximenes, R.; Szejnfeld, P.O.; Alves Sampaio, S.; Bispo de Filippis, A.M. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet. Gynecol. 2016, 47, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.V.; Maia, M.; Bravo-Filho, V.; Gois, A.L.; Belfort, R., Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016, 387, 228. [Google Scholar] [CrossRef]
- Miner, J.J.; Diamond, M.S. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 2017, 21, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.B.; Jungmann, P.; Beltrao-Braga, P.C.B. Zika infection and the development of neurological defects. Cell. Microbiol. 2017, 19, e12744. [Google Scholar] [CrossRef]
- Li, H.; Saucedo-Cuevas, L.; Shresta, S.; Gleeson, J.G. The Neurobiology of Zika Virus. Neuron 2016, 92, 949–958. [Google Scholar] [CrossRef]
- Meertens, L.; Labeau, A.; Dejarnac, O.; Cipriani, S.; Sinigaglia, L.; Bonnet-Madin, L.; Le Charpentier, T.; Hafirassou, M.L.; Zamborlini, A.; Cao-Lormeau, V.-M. Axl mediates ZIKA virus entry in human glial cells and modulates innate immune responses. Cell Rep. 2017, 18, 324–333. [Google Scholar] [CrossRef]
- Lum, F.-M.; Low, D.K.S.; Fan, Y.; Tan, J.J.L.; Lee, B.; Chan, J.K.Y.; Rénia, L.; Ginhoux, F.; Ng, L.F.P. Zika Virus Infects Human Fetal Brain Microglia and Induces Inflammation. Clin. Infect. Dis. 2017, 64, 914–920. [Google Scholar] [CrossRef]
- Rosenfeld, A.B.; Doobin, D.J.; Warren, A.L.; Racaniello, V.R.; Vallee, R.B. Replication of early and recent Zika virus isolates throughout mouse brain development. Proc. Natl. Acad. Sci. USA 2017, 114, 12273–12278. [Google Scholar] [CrossRef]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef]
- Hermanns, K.; Göhner, C.; Kopp, A.; Schmidt, A.; Merz, W.M.; Markert, U.R.; Junglen, S.; Drosten, C. Zika virus infection in human placental tissue explants is enhanced in the presence of dengue virus antibodies in-vitro. Emerg. Microbes Infect. 2018, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.A.; Yunusov, D.; Balaraman, V.; Alexenko, A.P.; Yabe, S.; Verjovski-Almeida, S.; Schust, D.J.; Franz, A.W.; Sadovsky, Y.; Ezashi, T. Vulnerability of primitive human placental trophoblast to Zika virus. Proc. Natl. Acad. Sci. USA 2017, 114, E1587–E1596. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Lennemann, N.J.; Ouyang, Y.; Bramley, J.C.; Morosky, S.; Marques, E.T.D.A., Jr.; Cherry, S.; Sadovsky, Y.; Coyne, C.B. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 2016, 19, 705–712. [Google Scholar] [CrossRef] [PubMed]
- El Costa, H.; Gouilly, J.; Mansuy, J.M.; Chen, Q.; Levy, C.; Cartron, G.; Veas, F.; Al-Daccak, R.; Izopet, J.; Jabrane-Ferrat, N. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci. Rep. 2016, 6, 35296. [Google Scholar] [CrossRef]
- Grazel, R.; Harris-Haman, P. Zika Virus Infection: A Vector-Borne Threat to Pregnant Women and Infants. Adv. Neonatal Care 2018, 18, 350–359. [Google Scholar] [CrossRef]
- Petitt, M.; Tabata, T.; Puerta-Guardo, H.; Harris, E.; Pereira, L. Zika virus infection of first-trimester human placentas: Utility of an explant model of replication to evaluate correlates of immune protection ex vivo. Curr. Opin Virol. 2017, 27, 48–56. [Google Scholar] [CrossRef]
- Hirsch, A.J.; Roberts, V.H.J.; Grigsby, P.L.; Haese, N.; Schabel, M.C.; Wang, X.; Lo, J.O.; Liu, Z.; Kroenke, C.D.; Smith, J.L.; et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat. Commun. 2018, 9, 263. [Google Scholar] [CrossRef]
- Muller, U.; Steinhoff, U.; Reis, L.F.; Hemmi, S.; Pavlovic, J.; Zinkernagel, R.M.; Aguet, M. Functional role of type I and type II interferons in antiviral defense. Science 1994, 264, 1918–1921. [Google Scholar] [CrossRef]
- Agbulos, D.S.; Barelli, L.; Giordano, B.V.; Hunter, F.F. Zika virus: Quantification, propagation, detection, and storage. Curr. Protoc. Microbiol. 2016, 43, 15D.4.1–15D.4.16. [Google Scholar]
- Sepulveda, W.; Rojas, I.; Robert, J.; Schnapp, C.; Alcalde, J. Prenatal detection of velamentous insertion of the umbilical cord: A prospective color Doppler ultrasound study. Ultrasound Obstet. Gynecol. 2003, 21, 564–569. [Google Scholar] [CrossRef]
- Kim, W.; Seidah, N.G.; Prat, A. In utero measurement of heart rate in mouse by noninvasive M-mode echocardiography. JoVE (J. Vis. Exp.) 2013, 81, e50994. [Google Scholar] [CrossRef]
- Ang, E.S., Jr.; Gluncic, V.; Duque, A.; Schafer, M.E.; Rakic, P. Prenatal exposure to ultrasound waves impacts neuronal migration in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 12903–12910. [Google Scholar] [CrossRef] [PubMed]
- McClintic, A.M.; King, B.H.; Webb, S.J.; Mourad, P.D. Mice exposed to diagnostic ultrasound in utero are less social and more active in social situations relative to controls. Autism Res. 2014, 7, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Maltepe, E.; Bakardjiev, A.I.; Fisher, S.J. The placenta: Transcriptional, epigenetic, and physiological integration during development. J. Clin. Investig. 2010, 120, 1016–1025. [Google Scholar] [CrossRef]
- Georgiades, P.; Ferguson-Smith, A.C.; Burton, G.J. Comparative developmental anatomy of the murine and human definitive placentae. Placenta 2002, 23, 3–19. [Google Scholar] [CrossRef]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef]
- Akirav, C.; Lu, Y.; Mu, J.; Qu, D.W.; Zhou, Y.Q.; Slevin, J.; Holmyard, D.; Foster, F.S.; Adamson, S.L. Ultrasonic detection and developmental changes in calcification of the placenta during normal pregnancy in mice. Placenta 2005, 26, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Wan Masliza, W.D.; Bajuri, M.Y.; Hassan, M.R.; Naim, N.M.; Shuhaila, A.; Das, S. Sonographically abnormal placenta: An association with an increased risk poor pregnancy outcomes. Clin. Ter. 2017, 168, e283–e289. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Chen, L.R. PP096. The effect of preterm placental calcification on uteroplacental blood flow, fetal growth and perinatal outcome in hypertension complicating pregnancy. Pregnancy Hypertens. 2012, 2, 292. [Google Scholar] [CrossRef] [PubMed]
- Hastings, A.K.; Yockey, L.J.; Jagger, B.W.; Hwang, J.; Uraki, R.; Gaitsch, H.F.; Parnell, L.A.; Cao, B.; Mysorekar, I.U.; Rothlin, C.V.; et al. TAM Receptors Are Not Required for Zika Virus Infection in Mice. Cell Rep. 2017, 19, 558–568. [Google Scholar] [CrossRef]
- Browne, V.A.; Julian, C.G.; Toledo-Jaldin, L.; Cioffi-Ragan, D.; Vargas, E.; Moore, L.G. Uterine artery blood flow, fetal hypoxia and fetal growth. Philos. Trans R. Soc. B Biol. Sci. 2015, 370, 20140068. [Google Scholar] [CrossRef] [PubMed]
- Lang, U.; Baker, R.S.; Khoury, J.; Clark, K.E. Effects of chronic reduction in uterine blood flow on fetal and placental growth in the sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R53–R59. [Google Scholar] [CrossRef] [PubMed]
- PrabhuDas, M.; Bonney, E.; Caron, K.; Dey, S.; Erlebacher, A.; Fazleabas, A.; Fisher, S.; Golos, T.; Matzuk, M.; McCune, J.M.; et al. Immune mechanisms at the maternal-fetal interface: Perspectives and challenges. Nat. Immunol. 2015, 16, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.J.; Smith, J.L.; Haese, N.N.; Broeckel, R.M.; Parkins, C.J.; Kreklywich, C.; DeFilippis, V.R.; Denton, M.; Smith, P.P.; Messer, W.B.; et al. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017, 13, e1006219. [Google Scholar] [CrossRef] [PubMed]
- Platt, D.J.; Smith, A.M.; Arora, N.; Diamond, M.S.; Coyne, C.B.; Miner, J.J. Zika virus-related neurotropic flaviviruses infect human placental explants and cause fetal demise in mice. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Nishimura, M.; Umeda, K.; Suwa, M.; Furuoka, H.; Nishikawa, Y. CCR5 Is Involved in Interruption of Pregnancy in Mice Infected with Toxoplasma gondii during Early Pregnancy. Infect. Immun. 2017, 85, e00257-17. [Google Scholar] [CrossRef]
- Adams Waldorf, K.M.; Stencel-Baerenwald, J.E.; Kapur, R.P.; Studholme, C.; Boldenow, E.; Vornhagen, J.; Baldessari, A.; Dighe, M.K.; Thiel, J.; Merillat, S.; et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat. Med. 2016, 22, 1256–1259. [Google Scholar] [CrossRef]

| Pulsating Umbilical Cord Diameter | ||||||
| Score “a” | ED 11.5 | ED 12.5 | ED 13.5 | ED 14.5 | ED 15.5 | ED 16.5 |
| 3 | >0.5 mm | >0.5 mm | >0.6 mm | >0.6 mm | >0.7 mm | >0.8 mm |
| 2 | <0.5 mm | <0.5 mm | <0.6 mm | <0.6 mm | <0.7 mm | <0.8 mm |
| 1 | <0.3 mm | <0.3 mm | <0.4 mm | <0.4 mm | <0.5 mm | <0.5 mm |
| 0 | Neg. | Neg. | Neg. | Neg. | Neg. | Neg. |
| brain vessel diameter | |||||
| Score “b” | ED 12.5 | ED 13.5 | ED 14.5 | ED 15.5 | ED 16.5 |
| 3 | >0.05 mm | >0.05 mm | >0.05 mm | >0.08 mm | >0.08 mm |
| 2 | <0.05 mm | <0.05 mm | <0.05 mm | <0.08 mm | <0.08 mm |
| 1 | <0.03 mm | <0.03 mm | <0.03 mm | <0.05 mm | <0.05 mm |
| 0 | Neg. | Neg. | Neg. | Neg. | Neg. |
| Score “c” | % living fetuses |
| 3 | >80% |
| 2 | <80% |
| 1 | <50% |
| 0 | <25% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forster, D.; Schwarz, J.H.; Brosinski, K.; Kalinke, U.; Sutter, G.; Volz, A. Obstetric Ultrasonography to Detect Fetal Abnormalities in a Mouse Model for Zika Virus Infection. Viruses 2020, 12, 72. https://doi.org/10.3390/v12010072
Forster D, Schwarz JH, Brosinski K, Kalinke U, Sutter G, Volz A. Obstetric Ultrasonography to Detect Fetal Abnormalities in a Mouse Model for Zika Virus Infection. Viruses. 2020; 12(1):72. https://doi.org/10.3390/v12010072
Chicago/Turabian StyleForster, Dominik, Jan Hendrik Schwarz, Katrin Brosinski, Ulrich Kalinke, Gerd Sutter, and Asisa Volz. 2020. "Obstetric Ultrasonography to Detect Fetal Abnormalities in a Mouse Model for Zika Virus Infection" Viruses 12, no. 1: 72. https://doi.org/10.3390/v12010072
APA StyleForster, D., Schwarz, J. H., Brosinski, K., Kalinke, U., Sutter, G., & Volz, A. (2020). Obstetric Ultrasonography to Detect Fetal Abnormalities in a Mouse Model for Zika Virus Infection. Viruses, 12(1), 72. https://doi.org/10.3390/v12010072





