The Conserved Tyr176/Leu177 Motif in the α-Helix 9 of the Feline Immunodeficiency Virus Capsid Protein Is Critical for Gag Particle Assembly
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
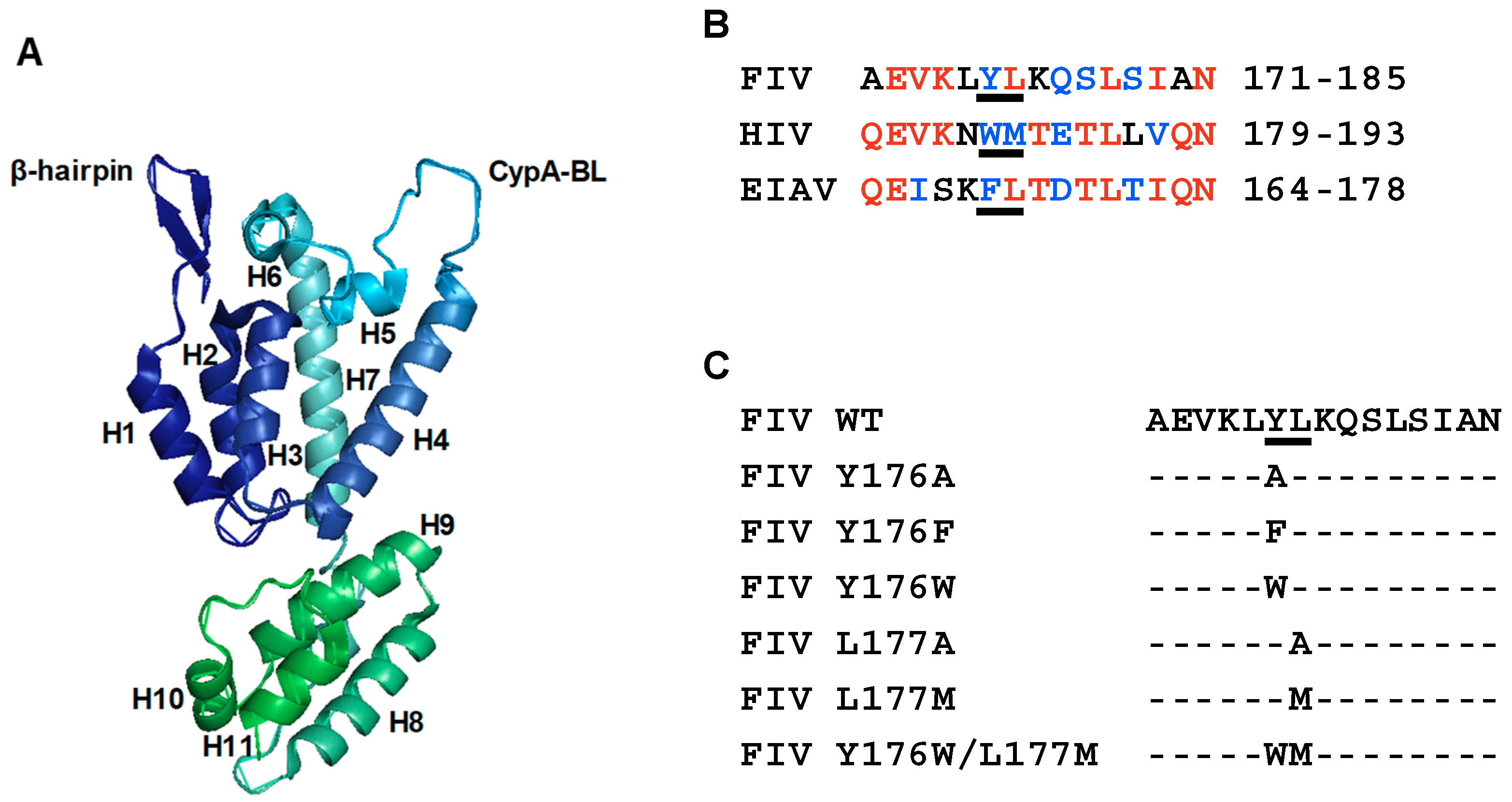
2.2. DNA Constructs and Site-Directed Mutagenesis
2.3. Sequence Similarity Searching
2.4. Transfections and Viral Protein Analysis
2.5. Expression in Escherichia coli and Purification of Recombinant Proteins
2.6. In Vitro Assembly of FIV Gag Proteins
2.7. In Vitro Assembly of FIV CA Proteins
2.8. Reverse Transcriptase Assays
2.9. FIV Infectivity Assays in CrFK Cells
3. Results
3.1. Mutagenesis of Residues Y176 and L177 in the FIV CA-CTD
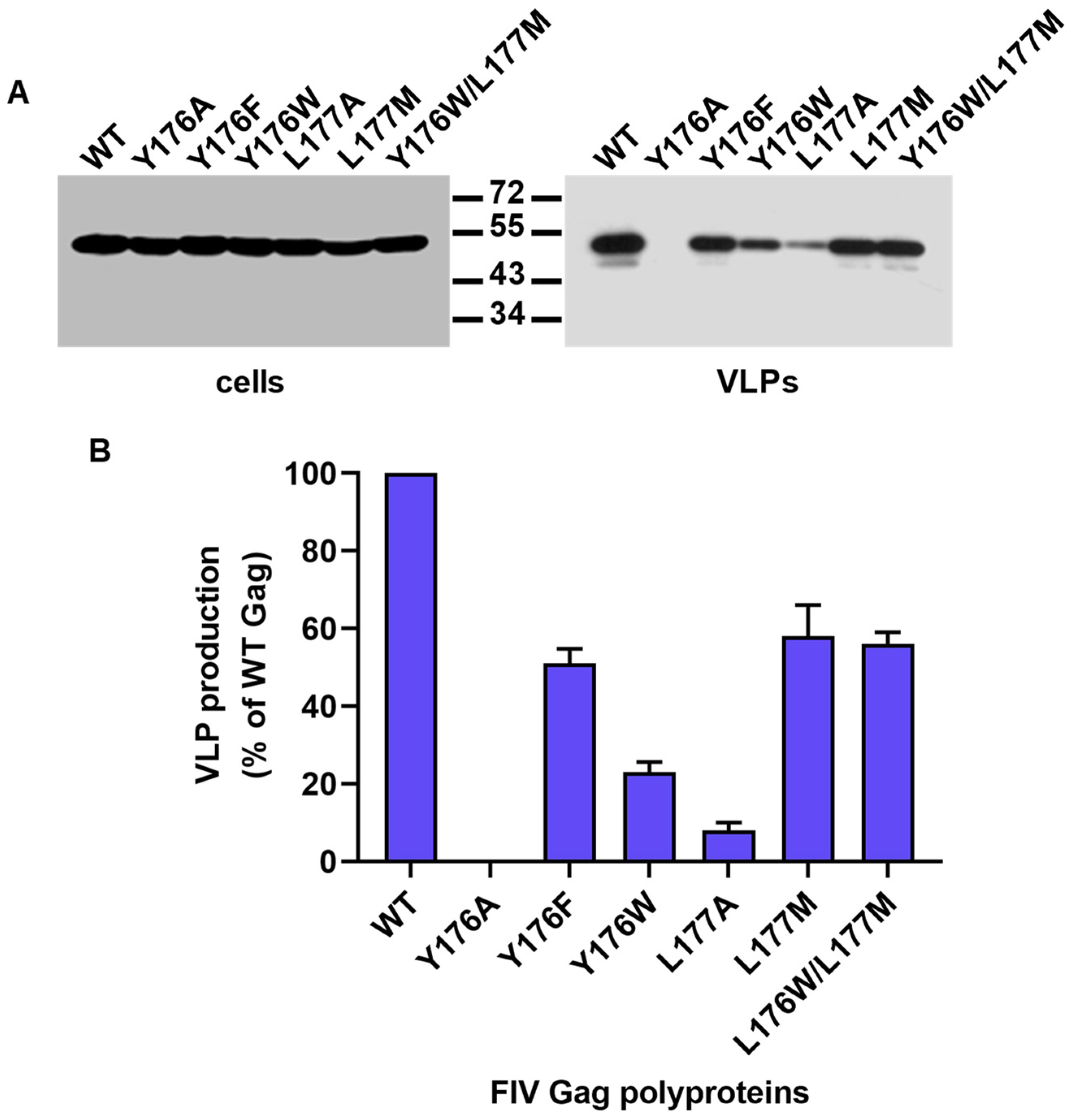
3.2. Effect of Mutations Affecting the CA-CTD Motif Y176/L177 on Gag Particle Assembly
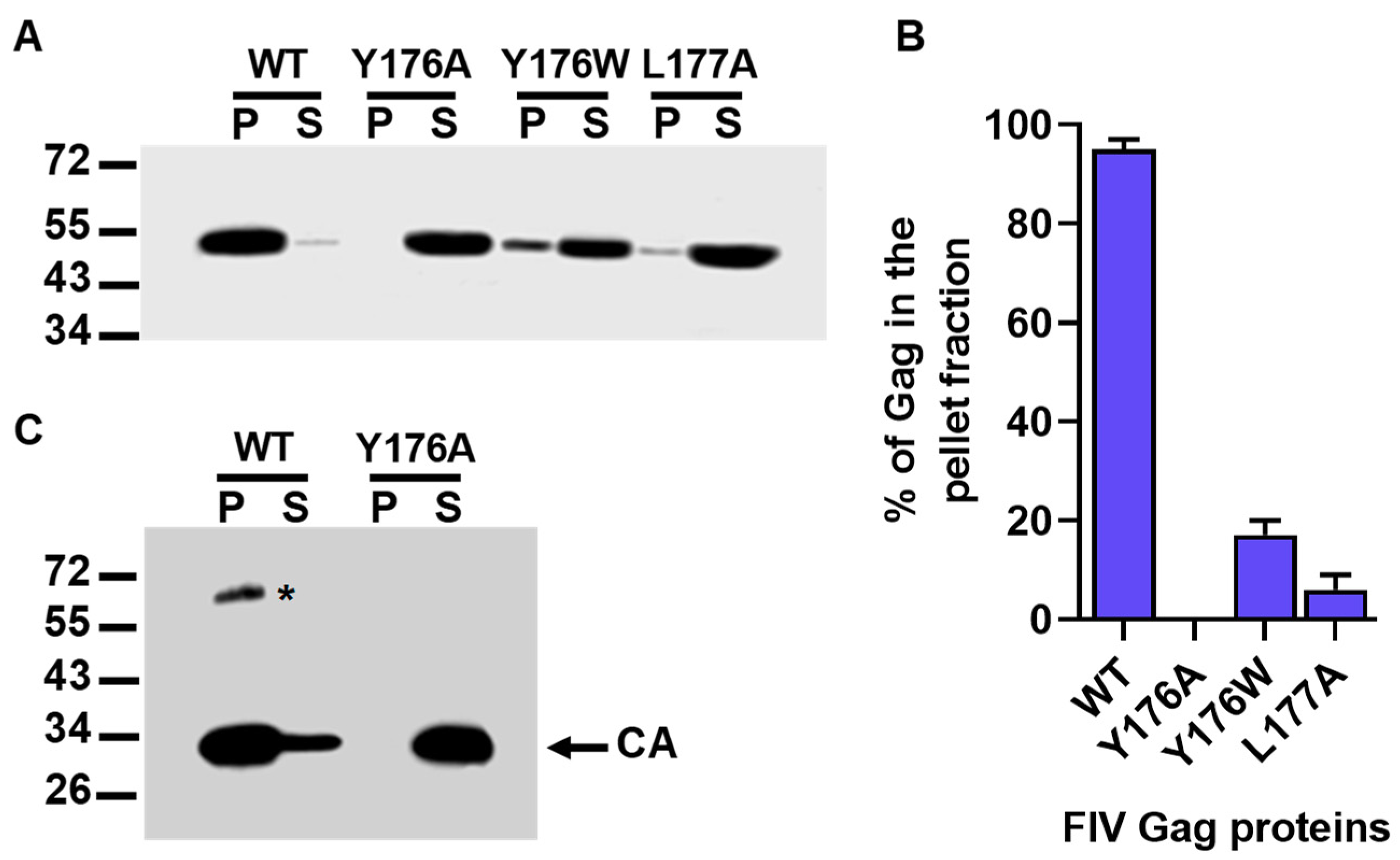
3.3. In Vitro Assembly Phenotype of the Gag Polyproteins Carrying the Y176A, Y176W, and L177A CA Mutations
3.4. Oligomerization Ability of the Y176A FIV CA Mutant
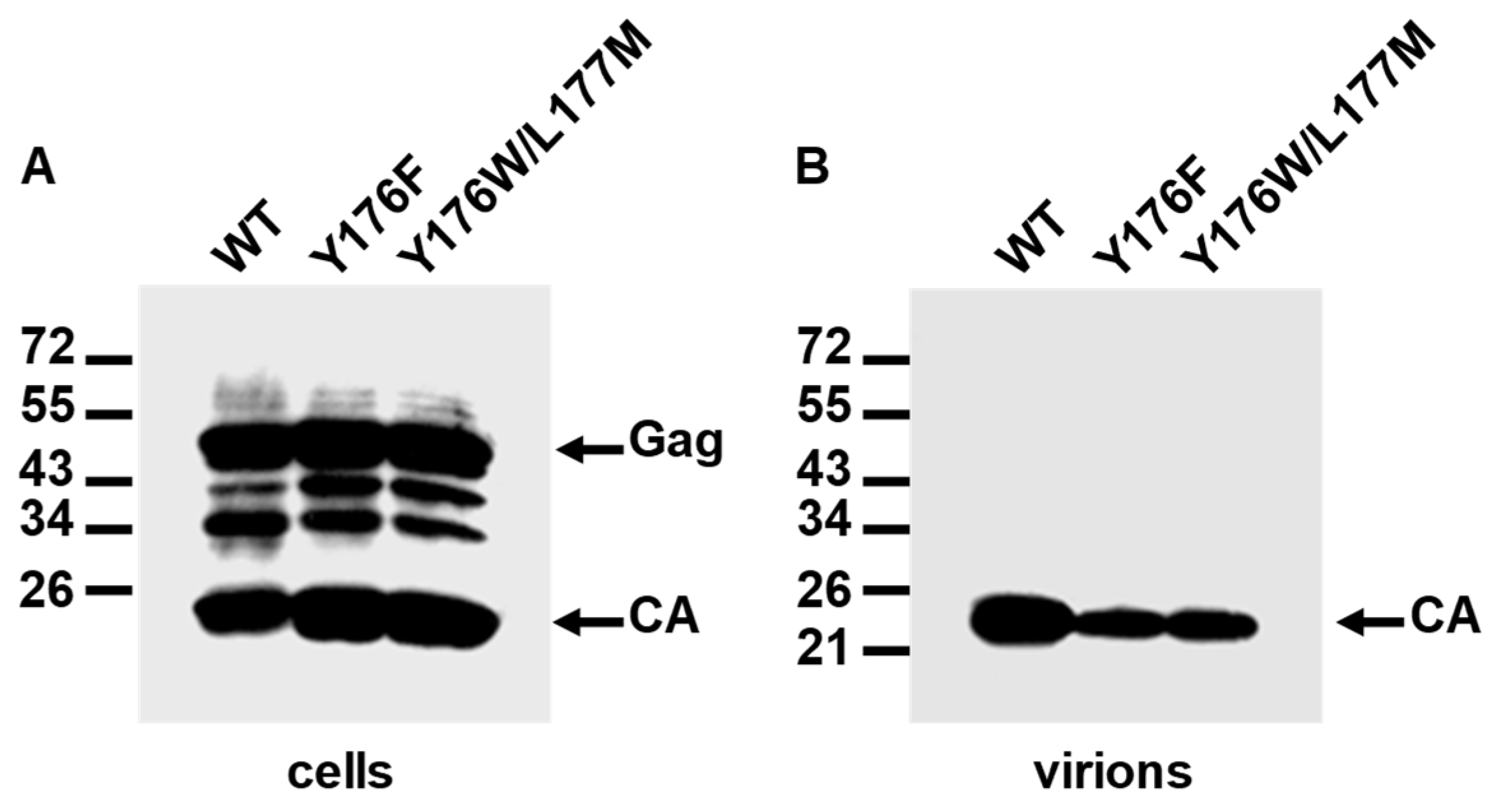
3.5. Assembly and Infectivity Phenotypes of Proviral DNAs Carrying the Y176F or the Y176W/L177M CA Mutation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- González, S.A.; Affranchino, J.L. Properties and functions of feline immunodeficiency virus Gag domains in virion assembly and budding. Viruses 2018, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Manrique, M.L.; Celma, C.C.; González, S.A.; Affranchino, J.L. Mutational analysis of the feline immunodeficiency virus matrix protein. Virus Res. 2001, 76, 103–113. [Google Scholar] [CrossRef]
- Affranchino, J.L.; González, S.A. In vitro assembly of feline immunodeficiency virus Gag polyprotein. Virus Res. 2010, 150, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Delchambre, M.; Gheysen, D.; Thinès, D.; Thiriart, C.; Jacobs, C.; Verdin, E.; Horth, M.; Burny, A.; Bex, F. The Gag precursor of the simian immunodeficiency virus assembles into virus-like particles. EMBO J. 1989, 8, 2653–2660. [Google Scholar] [CrossRef] [PubMed]
- Gheysen, D.; Jacobs, E.; de Foresta, F.; Thiriart, C.; Francotte, M.; Thines, D.; De Wilde, M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell 1989, 59, 103–112. [Google Scholar] [CrossRef]
- Göttlinger, H.G.; Sodroski, J.G.; Haseltine, W.A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 1989, 86, 5781–5785. [Google Scholar] [CrossRef] [PubMed]
- González, S.A.; Affranchino, J.L.; Gelderblom, H.R.; Burny, A. Assembly of the matrix protein of simian immunodeficiency virus into virus-like particles. Virology 1993, 194, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Rein, A. In vitro assembly properties of human immunodeficiency virus type 1 Gag protein lacking the p6 domain. J. Virol. 1999, 73, 2270–2279. [Google Scholar]
- Huseby, D.; Barklis, R.L.; Alfadhli, A.; Barklis, E. Assembly of human immunodeficiency virus precursor Gag proteins. J. Biol. Chem. 2005, 280, 17664–17670. [Google Scholar] [CrossRef] [PubMed]
- Rauddi, M.L.; Mac Donald, C.L.; Affranchino, J.L.; González, S.A. Mapping of the self-interaction domains in the simian immunodeficiency virus Gag polyprotein. AIDS Res. Hum. Retrovir. 2011, 27, 303–316. [Google Scholar] [CrossRef]
- Sundquist, W.I.; Kräusslich, H.G. HIV-1 assembly, budding, and maturation. Cold Spring Harb. Perspect. Med. 2012, 2, a006924. [Google Scholar] [CrossRef] [PubMed]
- Bell, N.M.; Lever, A.M. HIV Gag polyprotein: Processing and early viral particle assembly. Trends Microbiol. 2013, 21, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.H.; Schnölzer, M.; Hasselkus-Light, C.S.; Henson, M.; Lerner, D.A.; Phillips, T.R.; Wagaman, P.C.; Kent, S.B. Identification of proteolytic processing sites within the Gag and Pol polyproteins of feline immunodeficiency virus. J. Virol. 1993, 67, 1869–1876. [Google Scholar] [PubMed]
- Von Schwedler, U.K.; Stray, K.M.; Garrus, J.E.; Sundquist, W.I. Functional surfaces of the human immunodeficiency virus type 1 capsid protein. J. Virol. 2003, 77, 5439–5450. [Google Scholar] [CrossRef] [PubMed]
- Abdusetir Cerfoglio, J.C.; González, S.A.; Affranchino, J.L. Structural elements in the Gag polyprotein of feline immunodeficiency virus involved in Gag self-association and assembly. J. Gen. Virol. 2014, 95, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.R.; Schooler, J.B.; Ding, J.H.; Kieffer, C.; Fillmore, C.; Sundquist, W.I.; Grant, J.J. Electron cryotomography of immature HIV-1 virions reveals the structure of the CA and SP1 Gag shells. EMBO J. 2007, 26, 2218–2226. [Google Scholar] [CrossRef]
- Briggs, J.A.; Riches, J.D.; Glass, B.; Bartonova, V.; Zanetti, G.; Kräusslich, H.G. Structure and assembly of immature HIV. Proc. Natl. Acad. Sci. USA 2009, 106, 11090–11095. [Google Scholar] [CrossRef]
- de Marco, A.; Davey, N.E.; Ulbrich, P.; Phillips, J.M.; Lux, V.; Riches, J.D.; Fuzik, T.; Ruml, T.; Kräusslich, H.G.; Vogt, V.M.; et al. Conserved and variable features of Gag structure and arrangement in immature retrovirus particles. J. Virol. 2010, 84, 11729–11736. [Google Scholar] [CrossRef]
- Bharat, T.A.; Castillo Menendez, L.R.; Hagen, W.J.; Lux, V.; Igonet, S.; Schorb, M.; Schur, F.K.; Kräusslich, H.G.; Briggs, J.A. Cryo-electron microscopy of tubular arrays of HIV-1 Gag resolves structures essential for immature virus assembly. Proc. Natl. Acad. Sci. USA 2014, 111, 8233–8238. [Google Scholar] [CrossRef]
- Schur, F.K.; Hagen, W.J.; Rumlova, M.; Ruml, T.; Müller, B.; Kräusslich, H.G.; Briggs, J.A. Structure of the immature HIV-1 capsid in intact virus particles at 8.8 Å resolution. Nature 2015, 517, 505–508. [Google Scholar] [CrossRef]
- Ganser-Pornillos, B.K.; Cheng, A.; Yeager, M. Structure of full-length HIV-1 CA: A model for the mature capsid lattice. Cell 2007, 131, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Pornillos, O.; Ganser-Pornillos, B.K.; Yeager, M. Atomic-level modelling of the HIV capsid. Nature 2011, 469, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Perilla, J.R.; Yufenyuy, E.L.; Meng, X.; Chen, B.; Ning, J.; Ahn, J.; Gronenborn, A.M.; Schulten, K.; Aiken, C.; et al. Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature 2013, 497, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Murakami, T.; Agresta, B.E.; Campbell, S.; Freed, E.O.; Levin, J.G. Human immunodeficiency virus type 1 N-terminal capsid mutants that exhibit aberrant core morphology and are blocked in initiation of reverse transcription in infected cells. J. Virol. 2001, 75, 9357–9366. [Google Scholar] [CrossRef]
- Forshey, B.M.; von Schwedler, U.; Sundquist, W.I.; Aiken, C. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J. Virol. 2002, 76, 5667–5677. [Google Scholar] [CrossRef] [PubMed]
- Mattei, S.; Schur, F.K.; Briggs, J.A. Retrovirus maturation-an extraordinary structural transformation. Curr. Opin. Virol. 2016, 18, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ambrose, Z.; Martin, T.D.; Oztop, I.; Mulky, A.; Julias, J.G.; Vandegraaff, N.; Baumann, J.G.; Wang, R.; Yuen, W.; et al. Flexible use of nuclear import pathways by HIV-1. Cell Host Microbe 2010, 7, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Logue, E.C.; Taylor, K.T.; Goff, P.H.; Landau, N.R. The cargo-binding domain of transportin 3 is required for lentivirus nuclear import. J. Virol. 2011, 85, 12950–12961. [Google Scholar] [CrossRef]
- Fassati, A. Multiple roles of the capsid protein in the early steps of HIV-1 infection. Virus Res. 2012, 170, 15–24. [Google Scholar] [CrossRef]
- Jacques, D.A.; McEwan, W.A.; Hilditch, L.; Price, A.J.; Towers, G.J.; James, L.C. HIV-1 uses dynamic capsid pores to import nucleotides and fuel encapsidated DNA synthesis. Nature 2016, 536, 349–353. [Google Scholar] [CrossRef]
- Jin, Z.; Jin, L.; Peterson, D.L.; Lawson, C.L. Model for lentivirus capsid core assembly based on crystal dimers of EIAV p26. J. Mol. Biol. 1999, 286, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Campos-Olivas, R.; Newman, J.L.; Summers, M.F. Solution structure and dynamics of the Rous sarcoma virus capsid protein and comparison with capsid proteins of other retroviruses. J. Mol. Biol. 2000, 296, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Folio, C.; Sierra, N.; Dujardin, M.; Alvarez, G.; Guillon, C. Crystal structure of the full-length feline immunodeficiency virus capsid protein shows an N-terminal β-hairpin in the absence of N-terminal proline. Viruses 2017, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Mammano, F.; Ohagen, A.; Höglund, S.; Göttlinger, H.G. Role of the major homology region of human immunodeficiency virus type 1 in virion morphogenesis. J. Virol. 1994, 68, 4927–4936. [Google Scholar] [PubMed]
- Craven, R.C.; Leure-duPree, A.E.; Weldon, R.A., Jr.; Wills, J.W. Genetic analysis of the major homology region of the Rous sarcoma virus Gag protein. J. Virol. 1995, 69, 4213–4227. [Google Scholar] [PubMed]
- Schur, F.K.; Obr, M.; Hagen, W.J.; Wan, W.; Jakobi, A.J.; Kirkpatrick, J.M.; Sachse, C.; Kräusslich, H.G.; Briggs, J.A. An atomic model of HIV-1 capsid-SP1 reveals structures regulating assembly and maturation. Science 2016, 353, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.M.; Zadrozny, K.K.; Chrustowicz, J.; Purdy, M.D.; Yeager, M.; Ganser-Pornillos, B.K.C.; Pornillos, O. Crystal structure of an HIV assembly and maturation switch. Elife 2016, 5, e17063. [Google Scholar] [CrossRef] [PubMed]
- Gamble, T.R.; Yoo, S.; Vajdos, F.F.; von Schwedler, U.K.; Worthylake, D.K.; Wang, H.; McCutcheon, J.P.; Sundquist, W.I.; Hill, C.P. Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science 1997, 278, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.A.; Wilk, T.; Welker, R.; Kräusslich, H.G.; Fuller, S.D. Structural organization of authentic, mature HIV-1 virions and cores. EMBO J. 2003, 22, 1707–1715. [Google Scholar] [CrossRef]
- Pornillos, O.; Ganser-Pornillos, B.K.; Kelly, B.N.; Hua, Y.; Whitby, F.G.; Stout, C.D.; Sundquist, W.I.; Hill, C.P.; Yeager, M. X-ray structures of the hexameric building block of the HIV capsid. Cell 2009, 137, 1282–1292. [Google Scholar] [CrossRef]
- Gres, A.T.; Kirby, K.A.; KewalRamani, V.N.; Tanner, J.J.; Pornillos, O.; Sarafianos, S.G. STRUCTURAL VIROLOGY. X-ray crystal structures of native HIV-1 capsid protein reveal conformational variability. Science 2015, 349, 99–103. [Google Scholar] [CrossRef]
- Esteva, M.J.; Affranchino, J.L.; González, S.A. Lentiviral Gag assembly analyzed through the functional characterization of chimeric simian immunodeficiency viruses expressing different domains of the feline immunodeficiency virus capsid protein. PLoS ONE 2014, 9, e114299. [Google Scholar] [CrossRef] [PubMed]
- Olmsted, R.A.; Hirsch, V.M.; Purcell, R.H.; Johnson, P.R. Nucleotide sequence analysis of feline immunodeficiency virus: Genome organization and relationship to other lentiviruses. Proc. Natl. Acad. Sci. USA 1989, 86, 8088–8092. [Google Scholar] [CrossRef]
- González, S.A.; Affranchino, J.L. Processing, fusogenicity, virion incorporation and CXCR4-binding activity of a feline immunodeficiency virus envelope glycoprotein lacking the two conserved N-glycosylation sites at the C-terminus of the V3 domain. Arch. Virol. 2016, 161, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Celma, C.C.P.; Paladino, M.G.; González, S.A.; Affranchino, J.L. Importance of the short cytoplasmic domain of feline immunodeficiency virus transmembrane glycoprotein for fusion activity and envelope glycoprotein incorporation into virions. Virology 2007, 366, 405–414. [Google Scholar] [CrossRef] [PubMed]
- González, S.A.; Paladino, M.G.; Affranchino, J.L. Palmitoylation of the feline immunodeficiency virus envelope glycoprotein and its effect on fusion activity and envelope incorporation into virions. Virology 2012, 428, 1–10. [Google Scholar] [CrossRef]
- Manrique, J.M.; Affranchino, J.L.; González, S.A. In vitro binding of the simian immunodeficiency virus matrix protein to the cytoplasmic domain of the envelope glycoprotein. Virology 2008, 373, 273–279. [Google Scholar] [CrossRef]
- Manrique, M.L.; Rauddi, M.L.; González, S.A.; Affranchino, J.L. Functional domains in the feline immunodeficiency virus nucleocapsid protein. Virology 2004, 327, 83–92. [Google Scholar] [CrossRef]
- Manrique, J.M.; Celma, C.C.; Hunter, E.; Affranchino, J.L.; González, S.A. Positive and negative modulation of virus infectivity and envelope glycoprotein incorporation into virions by amino acid substitutions at the N terminus of the simian immunodeficiency virus matrix protein. J. Virol. 2003, 77, 10881–10888. [Google Scholar] [CrossRef]
- Manrique, M.L.; González, S.A.; Affranchino, J.L. Functional relationship between the matrix proteins of feline and simian immunodeficiency viruses. Virology 2004, 329, 157–167. [Google Scholar] [CrossRef]
- Du, S.; Betts, L.; Yang, R.; Shi, H.; Concel, J.; Ahn, J.; Aiken, C.; Zhang, P.; Yeh, J.I. Structure of the HIV-1 full-length capsid in a conformationally-trapped unassembled state induced by small-molecule binding. J. Mol. Biol. 2011, 406, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C.; Westergreen, N.; Barajas, B.C.; Ressler, D.T.B.; Phuong, D.J.; Swain, J.V.; Lingappa, V.R.; Lingappa, J.R. The formation of RNA granule-derived capsid assembly intermediates appears to be conserved between HIV-1 and the non-primate lentivirus FIV. J. Virol. 2018, 92, e01761-17. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovejero, C.A.; González, S.A.; Affranchino, J.L. The Conserved Tyr176/Leu177 Motif in the α-Helix 9 of the Feline Immunodeficiency Virus Capsid Protein Is Critical for Gag Particle Assembly. Viruses 2019, 11, 816. https://doi.org/10.3390/v11090816
Ovejero CA, González SA, Affranchino JL. The Conserved Tyr176/Leu177 Motif in the α-Helix 9 of the Feline Immunodeficiency Virus Capsid Protein Is Critical for Gag Particle Assembly. Viruses. 2019; 11(9):816. https://doi.org/10.3390/v11090816
Chicago/Turabian StyleOvejero, César A., Silvia A. González, and José L. Affranchino. 2019. "The Conserved Tyr176/Leu177 Motif in the α-Helix 9 of the Feline Immunodeficiency Virus Capsid Protein Is Critical for Gag Particle Assembly" Viruses 11, no. 9: 816. https://doi.org/10.3390/v11090816
APA StyleOvejero, C. A., González, S. A., & Affranchino, J. L. (2019). The Conserved Tyr176/Leu177 Motif in the α-Helix 9 of the Feline Immunodeficiency Virus Capsid Protein Is Critical for Gag Particle Assembly. Viruses, 11(9), 816. https://doi.org/10.3390/v11090816




