MiR-125b Suppression Inhibits Apoptosis and Negatively Regulates Sema4D in Avian Leukosis Virus-Transformed Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Plasmid Construction
2.3. Apoptosis Assays
2.4. Small RNA Extraction and Quantification
2.5. RNA Extraction and Quantification
2.6. Bioinformatics Analysis
2.7. Dual Luciferase Assays
2.8. Animal Infection Assay
2.9. DF1 Cell Infection Assay
2.10. Statistical Analysis
3. Results
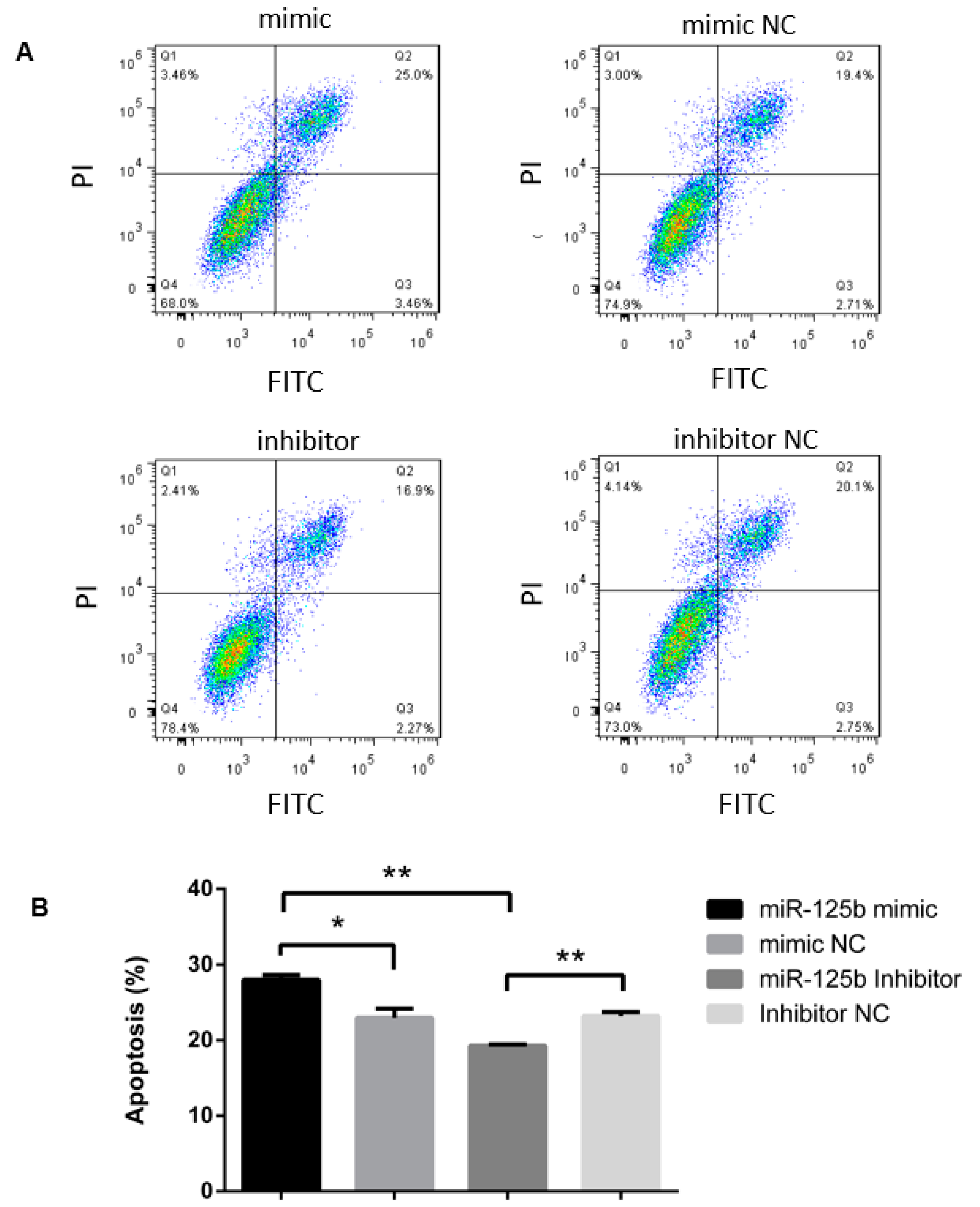
3.1. MiR-125b Positively Associated with Apoptosis Rate
3.2. Prediction of miR-125b Target Genes
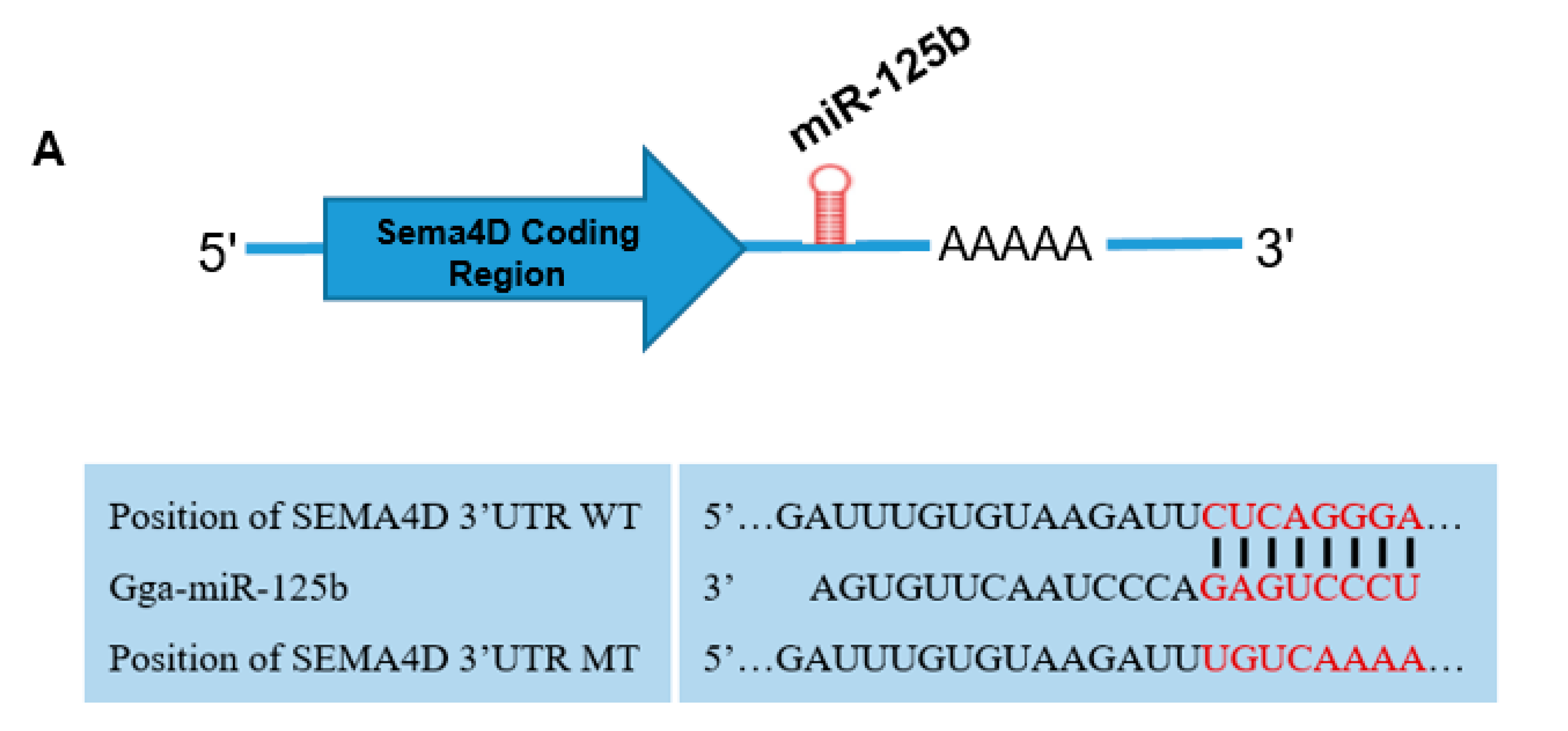
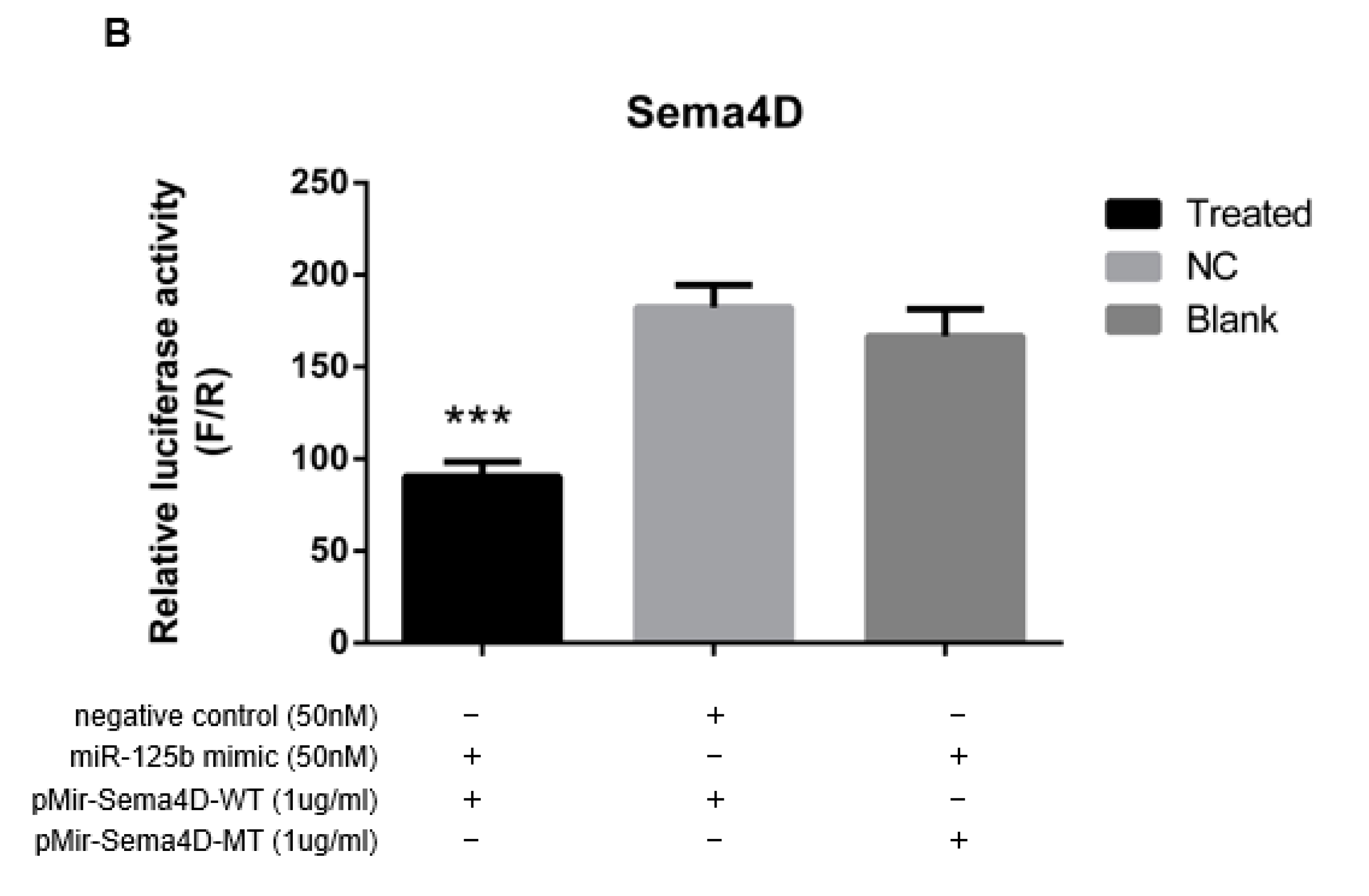
3.3. MiR-125b Binds to the Sema4D 3′ UTR
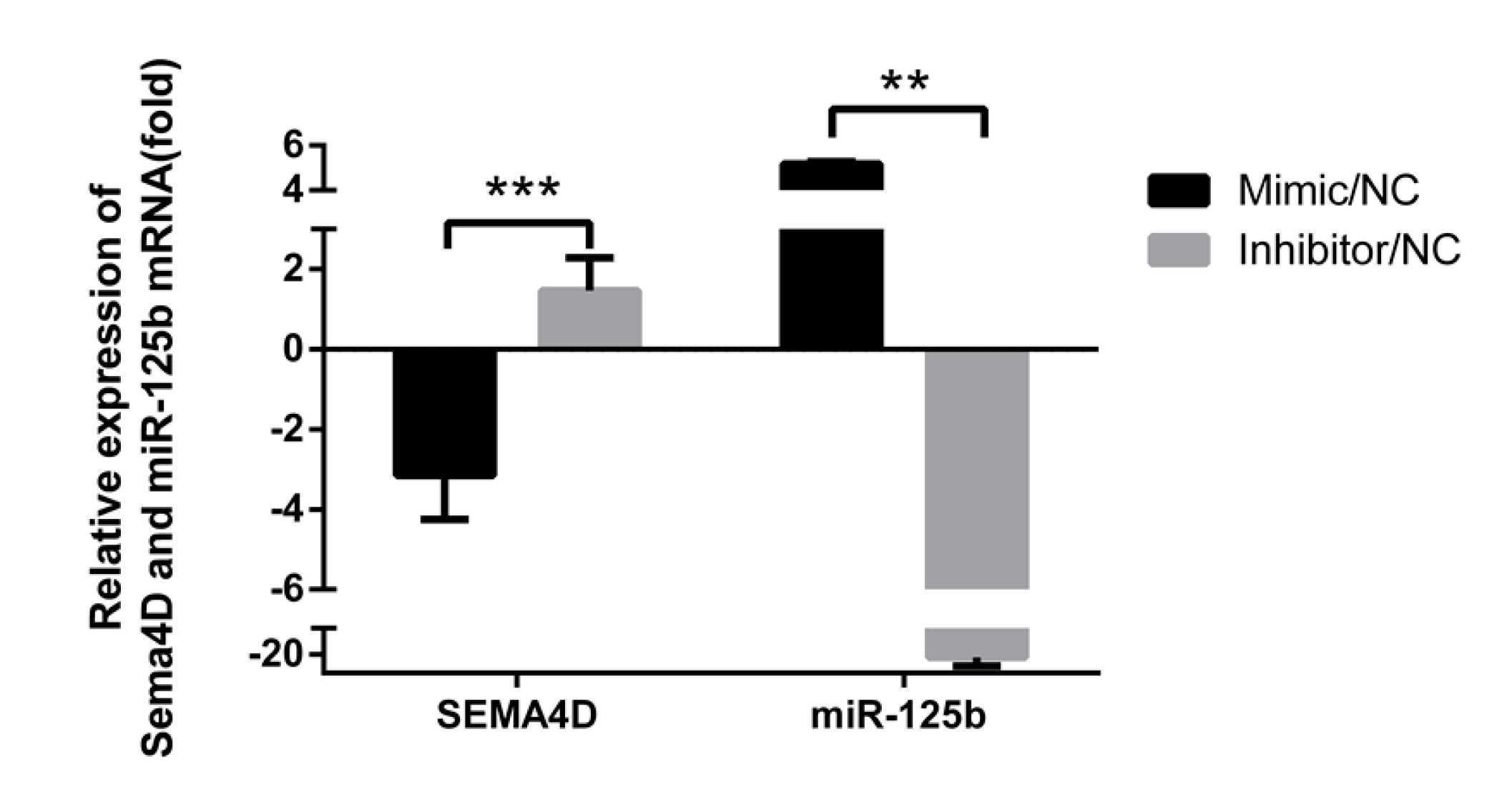
3.4. MiR-125b Suppresses Sema4D Expression
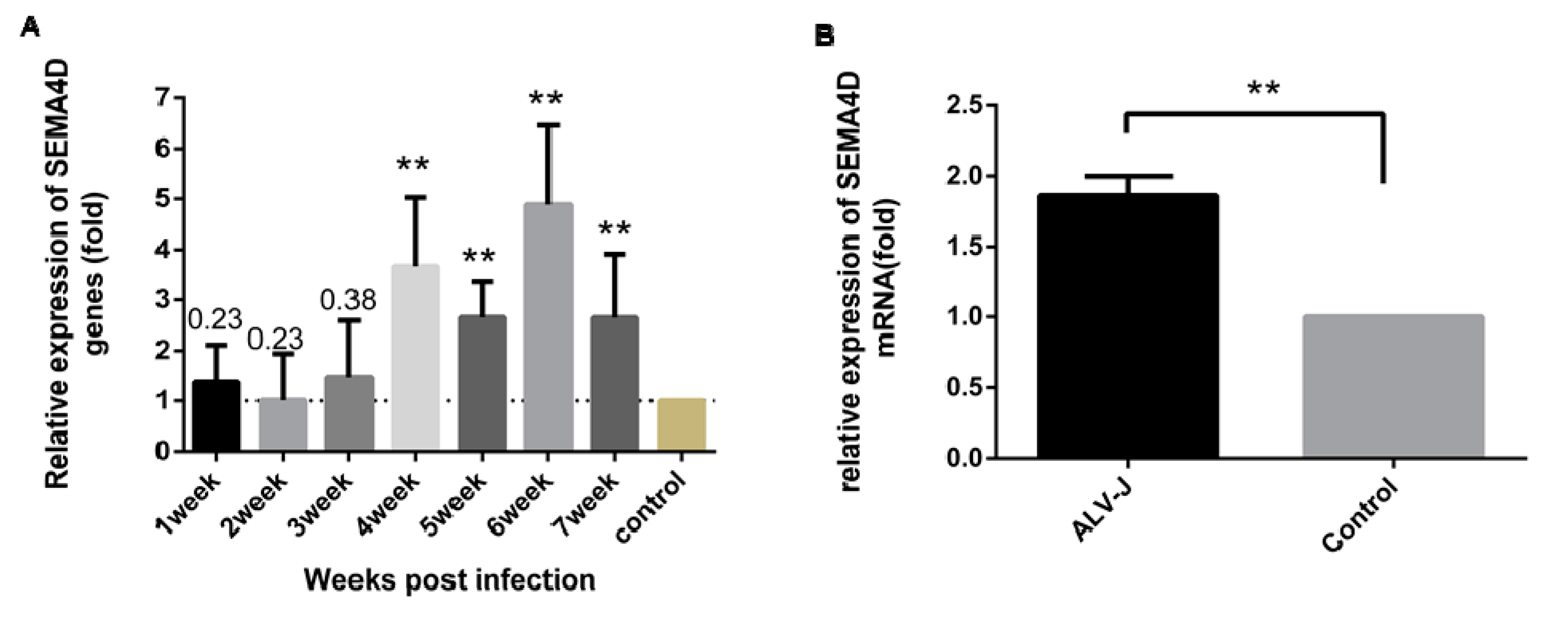
3.5. ALV-J Up-Regulates Sema4D Expression In Vivo and In Vitro
3.6. Sema4D Suppresses HP45 Cell Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Payne, L.N.; Gillespie, A.M.; Howes, K. Induction of myeloid leukosis and other tumors with the HPRS-103 strain of ALV. Vet. Rec. 1991, 129, 447–478. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.N. Developments in avian leukosis research. Leukemia 1992, 6 (Suppl. 3), 150S–152S. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Zhao, Z.; Yuan, L.; Chen, J.; Feng, M.; Zhang, J.; Liao, M.; Cao, W. The construction and application of a cell line resistant to novel subgroup avian leukosis virus (ALV-K) infection. Arch. Virol. 2018, 163, 89–98. [Google Scholar] [CrossRef]
- Du, Y.; Cui, Z.Z.; Qin, A.J.; Silva, R.F.; Lee, L.F. Subgroup J of avian leukosis viruses in China. Chin. J. Virol. 2000, 16, 341–346. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.X.; Cui, Z.Z.; Zhang, J.Y.; Wang, Z.F.; Chen, B.L.; Wang, X. Immunosuppression caused by avian leucosis virus of subgroup J and its coinfection with reticuloendotheliosis virus in broilers. Chin. J. Vet. Sci. 2003, 23, 211–213. (In Chinese) [Google Scholar] [CrossRef]
- Gao, Y.L.; Qin, L.T.; Pan, W.; Wang, Y.Q.; Qi, X.L.; Gao, H.L.; Wang, X.M. Avian leukosis virus subgroup J in layer chickens, China. Emerg. Infect. Dis. 2010, 16, 1637–1638. [Google Scholar] [CrossRef]
- Beemon, K.L.; Bolisetty, M. Mechanisms of oncogenesis by retroviruses. In Retroviruses and Insights into Cancer; Springer: Berlin/Heildelberg, Germany, 2010; pp. 31–52. [Google Scholar]
- Justice, I.; Malhotra, S.; Ruano, M.; Li, Y.; Zavala, G.; Lee, N.; Morgan, R.; Beemon, K. The MET gene is a common integration target in avian leukosis virus subgroup J-induced chicken hemangiomas. J. Virol. 2015, 89, 4712–4719. [Google Scholar] [CrossRef]
- Rhim, J.S. Viruses, oncogenes, and cancer. Cancer Detect. Prev. 1988, 11, 139–149. [Google Scholar] [CrossRef]
- Sherratt, J.A.; Nowak, M.A. Oncogenes, anti-oncogenes and the immune response to cancer: A mathematical model. Proc. Biol. Sci. 1992, 248, 261. [Google Scholar] [CrossRef]
- Bushati, N.; Cohen, S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007, 23, 175–205. [Google Scholar] [CrossRef]
- Ebert, M.S.; Sharp, P.A. Roles for MicroRNAs in conferring robustness to biological processes. Cell 2012, 149, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; He, X.; Ding, J.; Liang, L.; Zhao, Y.; Zhang, Z.; Yao, X.; Pan, Z.; Zhang, P.; Li, J. Upregulation of miR-23a approximately 27a approximately 24 decreases transforming growth factor-beta-induced tumor-suppressive activities in human hepatocellular carcinoma cells. Int. J. Cancer 2008, 123, 972–978. [Google Scholar] [CrossRef]
- Asangani, I.A.; Rasheed, S.A.; Nikolova, D.A.; Leupold, J.H.; Colburn, N.H.; Post, S.; Allgayer, H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 2008, 27, 2128–2136. [Google Scholar] [CrossRef]
- Strillacci, A.; Griffoni, C.; Sansone, P.; Paterini, P.; Piazzi, G.; Lazzarini, G.; Spisni, E.; Pantaleo, M.A.; Biasco, G.; Tomasi, V. MiR-101 downregulation is involved in cyclooxygenase-2 overexpression in human colon cancer cells. Exp. Cell Res. 2009, 315, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, Y.; Liu, C.; Chen, X.; Qi, Y.; Jiang, Y.; Zou, C.; Zhang, X.; Liu, S.; Wang, X. Analysis of MiR-195 and MiR-497 expression, regulation and role in breast cancer. Clin. Cancer Res. 2011, 17, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Yagi, K.; Tokuzawa, Y.; Kanesaki-Yatsuka, Y.; Suda, T.; Katagiri, T.; Fukuda, T.; Maruyama, M.; Okuda, A.; Amemiya, T. miR-125b inhibits osteoblastic differentiation by down-regulation of cell proliferation. Biochem. Biophys. Res. Commun. 2008, 368, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ding, C.; Chen, C.; Zhang, Z.; Xiao, H.; Xie, F.; Lei, L.; Chen, Y.; Mao, B.; Jiang, M. miR-224 promotion of cell migration and invasion by targeting Homeobox D 10 gene in human hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2014, 29, 835–842. [Google Scholar] [CrossRef]
- Dai, Z.; Ji, J.; Yan, Y.; Lin, C.; Li, H.; Chen, F.; Liu, Y.; Chen, W.; Bi, Y.; Xie, Q. Role of gga-miR-221 and gga-miR-222 during tumour formation in chickens infected by subgroup j avian leukosis virus. Viruses 2015, 7, 6538–6551. [Google Scholar] [CrossRef]
- Forte, E.; Salinas, R.E.; Chang, C.; Zhou, T.; Linnstaedt, S.D.; Gottwein, E.; Jacobs, C.; Jima, D.; Li, Q.J.; Dave, S.S. The Epstein-Barr virus (EBV)-induced tumor suppressor microRNA MiR-34a is growth promoting in EBV-infected B cells. J. Virol. 2012, 86, 6889–6898. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Y.; Ji, X.; Qi, X.; Qin, L.; Gao, H.; Wang, Y.; Wang, X. Differential expression of microRNAs in avian leukosis virus subgroup J-induced tumors. Vet. Microbiol. 2013, 162, 232. [Google Scholar] [CrossRef]
- Glud, M.; Rossing, M.; Hother, C.; Holst, L.; Hastrup, N.; Nielsen, F.C.; Gniadecki, R.; Drzewiecki, K.T. Downregulation of miR-125b in metastatic cutaneous malignant melanoma. Melanoma Res. 2010, 20, 479–484. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, Y.; Smith, L.P.; Lawrie, C.H.; Saunders, N.J.; Watson, M.; Nair, V. Differential expression of microRNAs in Marek’s disease virus-transformed T-lymphoma cell lines. J. Gen. Virol. 2009, 90, 1551–1559. [Google Scholar] [CrossRef]
- Nazerian, K. An updated list of avian cell lines and transplantable tumours. Avian Pathol. 1987, 16, 527–544. [Google Scholar] [CrossRef][Green Version]
- Ji, X.; Wang, Q.; Gao, Y.; Wang, Y.; Qin, L.; Qi, X.; Gao, H.; Wang, X. Construction of an infectious molecular clone of a subgroup J avian leukosis virus isolation HLJ09SH01 and its pathogenicity. Chin. J. Prev. Vet. Med. 2013, 35, 15–18. (In Chinese) [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, Z.G.; Tian, X.Q.; Sun, D.F.; Liang, Q.C.; Zhang, Y.J.; Lu, R.; Chen, Y.X.; Fang, J.Y. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia 2008, 10, 287–297. [Google Scholar] [CrossRef]
- Wu, N.; Lin, X.; Zhao, X.; Zheng, L.; Xiao, L.; Liu, J.; Ge, L.; Cao, S. MiR-125b acts as an oncogene in glioblastoma cells and inhibits cell apoptosis through p53 and p38MAPK-independent pathways. Br. J. Cancer 2013, 109, 2853. [Google Scholar] [CrossRef]
- Ch’Ng, E.S.; Kumanogoh, A. Roles of Sema4D and Plexin-B1 in tumor progression. Mol. Cancer 2010, 9, 251. [Google Scholar] [CrossRef]
- Yao, Y.; Dou, C.; Lu, Z.; Zheng, X.; Liu, Q. MACC1 suppresses cell apoptosis in hepatocellular carcinoma by targeting the HGF/c-MET/AKT pathway. Cell. Physiol. Biochem. 2015, 35, 983. [Google Scholar] [CrossRef]
- Ito, T.; Bai, T.; Tanaka, T.; Yoshida, K.; Ueyama, T.; Miyajima, M.; Negishi, T.; Kawasaki, T.; Takamatsu, H.; Kikutani, H. Estrogen-dependent proteolytic cleavage of semaphorin 4D and plexin-B1 enhances semaphorin 4D-induced apoptosis during postnatal vaginal remodeling in pubescent mice. PLoS ONE 2014, 9, e97909. [Google Scholar] [CrossRef]
- Ito, T.; Bai, T.; Tanaka, T.; Yoshida, K.; Ueyama, T.; Miyajima, M.; Negishi, T.; Kawasaki, T.; Takamatsu, H.; Kikutani, H. Semaphorin 4D induces vaginal epithelial cell apoptosis to control mouse postnatal vaginal tissue remodeling. Mol. Med. Rep. 2015, 11, 829–836. [Google Scholar] [CrossRef][Green Version]
- Chaoqi, R.; Yulong, G. Screening of miR-125b targets and the functional analysis. In preparation.
- Fung, Y.K.T.; Lewis, W.G.; Crittenden, L.B.; Kung, H.J. Activation of the cellular oncogene c-erbB by Ltr insertion: Molecular basis for induction of erythroblastosis by avian leukosis virus. Cell 1983, 33, 357–368. [Google Scholar] [CrossRef]
- Habbe, N.; Koorstra, J.B.; Mendell, J.T.; Offerhaus, G.J.; Ryu, J.K.; Feldmann, G.; Mullendore, M.E.; Goggins, M.G.; Hong, S.M.; Maitra, A. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol. Ther. 2009, 8, 340–346. [Google Scholar] [CrossRef]
- Kota, J.; Chivukula, R.R.; O’Donnell, K.A.; Wentzel, E.A.; Montgomery, C.L.; Hwang, H.W.; Chang, T.C.; Vivekanandan, P.; Torbenson, M.; Clark, K.R. Therapeutic delivery of miR-26a inhibits cancer cell proliferation and induces tumor-specific apoptosis. Cell 2009, 137, 1005–1017. [Google Scholar] [CrossRef]
- Noteborn, M.H.; Todd, D.; Verschueren, C.A.; de Gauw, H.W.; Curran, W.L.; Veldkamp, S.; Douglas, A.J.; McNulty, M.S.; van der Eb, A.J.; Koch, G. A single chicken anemia virus protein induces apoptosis. J. Virol. 1994, 68, 346–351. [Google Scholar] [CrossRef]
- Hay, S.; Kannourakis, G. A time to kill: Viral manipulation of the cell death program. J. Gen. Virol. 2002, 83, 1547–1564. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, E.J.; Esau, C.; Schmittgen, T.D. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas 2009, 38, 190–199. [Google Scholar] [CrossRef]
- He, X.; Dong, Y.; Wu, C.W.; Zhao, Z.R.; Ng, S.S.M.; Chan, F.K.L.; Sung, J.Y.; Yu, J. MicroRNA-218 inhibits cell cycle progression and promotes apoptosis in colon cancer cells by downregulating oncogene BMI. Gastroenterology 2012, 142, 1491–1498. [Google Scholar] [CrossRef]
- Nie, J.; Liu, L.; Zheng, W.; Chen, L.; Wu, X.; Xu, Y.; Du, X.; Han, W. microRNA-365, down-regulated in colon cancer, inhibits cell cycle progression and promotes apoptosis of colon cancer cells by probably targeting Cyclin D1 and Bcl-2. Carcinogenesis 2012, 33, 220–225. [Google Scholar] [CrossRef]
- Xia, H.F.; He, T.Z.; Liu, C.M.; Cui, Y.; Song, P.P.; Jin, X.H.; Ma, X. MiR-125b expression affects the proliferation and apoptosis of human glioma cells by targeting Bmf. Cell Physiol. Biochem. 2009, 23, 347–358. [Google Scholar] [CrossRef]
- Cui, F.; Li, X.; Zhu, X.; Huang, L.; Huang, Y.; Mao, C.; Yan, Q.; Zhu, J.; Zhao, W.; Shi, H. MiR-125b Inhibits tumor growth and promotes apoptosis of cervical cancer cells by targeting phosphoinositide 3-kinase catalytic subunit delta. Cell Physiol. Biochem. 2012, 30, 1310–1318. [Google Scholar] [CrossRef]
- Honoré, C.; Hummelshoj, T.; Hansen, B.E.; Madsen, H.O.; Eggleton, P.; Garred, P. The innate immune component ficolin 3 (Hakata antigen) mediates the clearance of late apoptotic cells. Arthritis Rheum. 2007, 56, 1598–1607. [Google Scholar] [CrossRef]
- Litvack, M.L.; Djiadeu, P.; Renganathan, S.D.; Sy, S.; Post, M.; Palaniyar, N. Natural IgM and innate immune collectin SP-D bind to late apoptotic cells and enhance their clearance by alveolar macrophages in vivo. Mol. Immunol. 2010, 48, 37. [Google Scholar] [CrossRef]
- Shang, Y.L.; Liu, S.D.; Ding, B.J.; Xiao, Y.H.; Cheng, Z.Q.; Sun, X.M.; Chu, Y.L. Immunosuppressive mechanism of broilers infected with avian leukosis virus subgroup J (ALV-J). Chin. J. Vet. 2005, 25, 573–577. [Google Scholar] [CrossRef]
- Krueger, G.R.F.; Buja, L.M. Abnormal variation of the immune response as related to cancer. In Selected Aspects of Cancer Progression: Metastasis, Apoptosis and Immune Response; Springer: Berlin/Heidelberg, Germany, 2008; pp. 193–222. [Google Scholar]
- Guo, Y.E.; Steitz, J.A. Virus meets host microRNA: The destroyer, the booster, the hijacker. Mol. Cell. Biol. 2014, 34, 3780–3787. [Google Scholar] [CrossRef]
- Cazalla, D.; Yario, T.; Steitz, J.A. Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science 2010, 328, 1563–1566. [Google Scholar] [CrossRef]
- Tam, W.; Ben-Yehuda, D.; Hayward, W.S. Bic, a novel gene activated by proviral insertions in avian leukosis virus-induced lymphomas, is likely to function through its noncoding RNA. Mol. Cell. Biol. 1997, 17, 1490–1502. [Google Scholar] [CrossRef]
- Masotti, A.; Donninelli, G.; Da, S.L.; Varano, B.; Del Cornò, M.; Gessani, S. HIV-1 gp120 influences the expression of microRNAs in human monocyte-derived dendritic cells via STAT3 activation. BMC Genom. 2015, 16, 480. [Google Scholar] [CrossRef]
- Jopling, C.L.; Yi, M.K.; Lancaster, A.M.; Lemon, S.M.; Sarnow, P. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science 2005, 309, 1577–1581. [Google Scholar] [CrossRef]
- Xie, L.; Liao, Y.; Shen, L.; Hu, F.; Yu, S.; Zhou, Y.; Zhang, Y.; Yang, Y.; Li, D.; Ren, M. Identification of the miRNA-mRNA regulatory network of small cell osteosarcoma based on RNA-seq. Oncotarget 2017, 8, 42525–42536. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Ho, H.L.; Lin, S.C. Upregulation of miR-125b, miR-181d, and miR-221 predicts poor prognosis in MGMT promoter-unmethylated glioblastoma patients. Am. J. Clin. Pathol. 2018, 149, 412–417. [Google Scholar] [CrossRef]
- Sui, M.H.; Jiao, A.H.; Zhai, H.Y.; Wang, Y.; Wang, Y.; Sun, D.J.; Li, P. Upregulation of miR-125b is associated with poor prognosis and trastuzumab resistance in HER2-positive gastric cancer. Exp. Ther. Med. 2017, 14, 657–663. [Google Scholar] [CrossRef]
- Le, S.C.; Nagel, R.; Egan, D.A.; Schrier, M.; Mesman, E.; Mangiola, A.; Anile, C.; Maira, G.; Mercatelli, N.; Ciafrè, S.A. Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J. 2007, 26, 3699–3708. [Google Scholar] [CrossRef]
- Yu, B.; Zhou, S.; Wang, Y.; Qian, T.; Ding, G.; Ding, F.; Gu, X. miR-221 and miR-222 promote Schwann cell proliferation and migration by targeting LASS2 after sciatic nerve injury. J. Cell Sci. 2012, 125, 2675–2683. [Google Scholar] [CrossRef]
- Yao, Y.; Reheman, A.; Xu, Y.; Li, Q. miR-125b contributes to ovarian granulosa cell apoptosis through targeting BMPR1B, a major gene for sheep prolificacy. Reprod. Sci. 2018, 1, 193371911877054. [Google Scholar] [CrossRef]
- Fornari, F.; Gramantieri, L.; Ferracin, M.; Veronese, A.; Sabbioni, S.; Calin, G.A.; Grazi, G.L.; Giovannini, C.; Croce, C.M.; Bolondi, L. miR-125b controls CDKN1C/p57 and Sema4D/p27 expression in human hepatocellular carcinoma. Oncogene 2008, 27, 5651–5661. [Google Scholar] [CrossRef]
- Yukawa, K.; Tanaka, T.; Kishino, M.; Yoshida, K.; Takeuchi, N.; Ito, T.; Takamatsu, H.; Kikutani, H.; Kumanogoh, A. Deletion of Sema4D gene reduces intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Int. J. Mol. Med. 2010, 26, 39–44. [Google Scholar] [CrossRef][Green Version]
- Ji, J.; Xu, X.; Wang, X.; Yao, L.G.; Shang, H.Q.; Li, H.G.; Ma, J.Y.; Bi, Y.Z.; Xie, Q.M. Expression of dysregulated miRNA in vivo in DF-1cells during the course of subgroup J avian leukosis virus infection. Microbial. Pathog. 2019, 126, 40–44. [Google Scholar] [CrossRef]
- Masuda, K.; Furuyama, T.; Takahara, M.; Fujioka, S.; Kurinami, H.; Inagaki, S. Sema4D stimulates axonal outgrowth of embryonic DRG sensory neurones. Genes Cells 2004, 9, 821–829. [Google Scholar] [CrossRef]
- Okuno, T.; Nakatsuji, Y.; Moriya, M.; Takamatsu, H.; Nojima, S.; Takegahara, N.; Toyofuku, T.; Nakagawa, Y.; Kang, S.; Friedel, R.H. Roles of Sema4D-plexin-B1 interactions in the central nervous system for pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 2010, 184, 1499–1506. [Google Scholar] [CrossRef]
- Suzuki, K.; Kumanogoh, A.; Kikutani, H. CD100/Sema4D, a lymphocyte semaphorin involved in the regulation of humoral and cellular immune responses. Cytokine Growth Factor Rev. 2003, 14, 17–24. [Google Scholar] [CrossRef]
- Pettigrew, C.A.; Cotter, T.G. Deregulation of cell death (apoptosis): Implications for tumor development. Discov. Med. 2009, 8, 61–63. [Google Scholar]
- Finlay, D.; Teriete, P.; Vamos, M.; Cosford, N.D.P.; Vuori, K. Inducing death in tumor cells: Roles of the inhibitor of apoptosis proteins. F1000Research 2017, 6, 587. [Google Scholar] [CrossRef]

| Pathway ID | Gene ID | Gene Name | KEGG and GO |
|---|---|---|---|
| ko: K16794 | 374224 | Platelet activating factor acetylhydrolase 1b regulatory subunit 1 (PAFAH1B1) | Metabolic pathways Ether lipid metabolism |
| ko: K09208 | 427493 | Krueppel-like factor 13 (KLF13) | Regulation of transcription from RNA polymerase II promoter |
| ko: K06521 | 396331 | Semaphorin 4D/CD100 (Sema4D) | Axon guidance cell adhesion molecules |
| ko: no | 445340 | PR domain zinc finger protein 1 (PRDM1) | Regulation of transcription |
| ko: K03211 | 395750 | ETS variant 6 (ETV6) | Transcriptional activator |
| ko: no | 421301 | Limb bud and heart development (LBH) | Regulation of stem cell differentiation regulation of transcription |
| ko: K11584 | 423460 | Protein phosphatase 2 regulatory subunit B’gamma (PPP2R5C) | Cellular Processes mRNA surveillance pathway |
| ko: K04678 | 416487 | SMAD specific E3 ubiquitin protein ligase 1 (SMURF1) | TGF-beta signaling pathway endocytosis |
| ko: K22040 | 420289 | Transcriptional repressor GATA binding 1 (TRPS1) | Negative regulation of transcription |
| ko: K11850 | 424216 | Ubiquitin specific peptidase 37 (USP37) | Ubiquitin system |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, C.; Xie, R.; Yao, Y.; Yu, M.; Chang, F.; Xing, L.; Zhang, Y.; Liu, Y.; Wang, S.; Farooque, M.; et al. MiR-125b Suppression Inhibits Apoptosis and Negatively Regulates Sema4D in Avian Leukosis Virus-Transformed Cells. Viruses 2019, 11, 728. https://doi.org/10.3390/v11080728
Ren C, Xie R, Yao Y, Yu M, Chang F, Xing L, Zhang Y, Liu Y, Wang S, Farooque M, et al. MiR-125b Suppression Inhibits Apoptosis and Negatively Regulates Sema4D in Avian Leukosis Virus-Transformed Cells. Viruses. 2019; 11(8):728. https://doi.org/10.3390/v11080728
Chicago/Turabian StyleRen, Chaoqi, Ruyu Xie, Yongxiu Yao, Mengmeng Yu, Fangfang Chang, Lixiao Xing, Yao Zhang, Yongzhen Liu, Suyan Wang, Muhammad Farooque, and et al. 2019. "MiR-125b Suppression Inhibits Apoptosis and Negatively Regulates Sema4D in Avian Leukosis Virus-Transformed Cells" Viruses 11, no. 8: 728. https://doi.org/10.3390/v11080728
APA StyleRen, C., Xie, R., Yao, Y., Yu, M., Chang, F., Xing, L., Zhang, Y., Liu, Y., Wang, S., Farooque, M., Wang, Y., Qi, X., Liu, C., Zhang, Y., Cui, H., Li, K., Gao, L., Pan, Q., Nair, V., ... Gao, Y. (2019). MiR-125b Suppression Inhibits Apoptosis and Negatively Regulates Sema4D in Avian Leukosis Virus-Transformed Cells. Viruses, 11(8), 728. https://doi.org/10.3390/v11080728







