Platelet Count in Patients with Mild Disease at Admission is Associated with Progression to Severe Hantavirus Cardiopulmonary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Patients of Interest
2.3. Outcomes
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mertz, G.J.; Hielle, B.L.; Bryan, R.T. Hantavirus infection. Dis. Month 1998, 44, 85–138. [Google Scholar] [CrossRef]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef] [PubMed]
- Manigold, T.; Vial, P. Human hantavirus infections: Epidemiology, clinical features, pathogenesis and immunology. Swiss Med. Wkly. 2014, 144, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.A.; Torres-Perez, F.; Galeno, H.; Navarrete, M.; Vial, P.A.; Palma, R.E.; Ferres, M.; Cook, J.A.; Hjelle, B. Ecology, Genetic Diversity, and Phylogeographic Structure of Andes Virus in Humans and Rodents in Chile. J. Vir. 2009, 83, 2446–2459. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.A.; Bennett, M. Hantavirus infections: Epidemiology and pathogenesis. Microbes Infect. 1999, 1, 1229–1237. [Google Scholar] [CrossRef]
- Sotomayor, V.; Aguilera, X. Epidemiología de la infección humana por hantavirus en Chile. Rev. Chil. Infectología 2000, 17, 220–232. [Google Scholar] [CrossRef]
- Padula, P.J.; Edelstein, A.; Miguel, S.D.; López, N.M.; Rossi, C.M.; Rabinovich, R.D. Hantavirus pulmonary syndrome outbreak in Argentina: Molecular evidence for person-to-person transmission of Andes virus. Virology 1998, 241, 323–330. [Google Scholar] [CrossRef]
- Chaparro, J.; Vega, J.; Terry, W.; Vera, J.L.; Barra, B.; Meyer, R.; Peters, C.J.; Khan, A.S.; Ksiazek, T.G. Assessment of person-to-person transmission of hantavirus pulmonary syndrome in a Chilean hospital setting. J. Hosp. Infect. 1998, 40, 281–285. [Google Scholar] [CrossRef]
- Ferrés, M.; Vial, P.; Marco, C.; Yan, L.; Godoy, P.; Castillo, C.; Hjelle, B.; Delgado, I.; Lee, S.; Mertz, G.J.; et al. Prospective evaluation of household contacts of persons with hantavirus cardiopulmonary syndrome in Chile. J. Infect. Dis. 2007, 195, 1563–1571. [Google Scholar] [CrossRef]
- Martinez-Valdebenito, C.; Calvo, M.; Vial, C.; Mansilla, R.; Marco, C.; Palma, R.E.; Vial, P.A.; Valdivieso, F.; Mertz, G.; Ferrés, M. Person-to-person household and nosocomial transmission of andes hantavirus, Southern Chile, 2011. Emerg. Infect. Dis. 2014, 20, 1629–1636. [Google Scholar] [CrossRef]
- Vial, P.A.; Valdivieso, F.; Mertz, G.; Castillo, C.; Belmar, E.; Delgado, I.; Tapia, M.; Ferrés, M. Incubation period of hantavirus cardiopulmonary syndrome. Emerg. Infect. Dis. 2006, 12, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.J.; Fritz, C.L.; Knust, B.; Buttke, D.; Enge, B.; Novak, M.G.; Kramer, V.; Osadebe, L.; Messenger, S.; Albariño, C.G.; et al. Hantavirus infections among overnight visitors to Yosemite National Park, California, USA, 2012. Emerg. Infect. Dis. 2014, 20, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Mertz, G.J.; Hjelle, B.; Crowley, M.; Iwamoto, G.; Tomicic, V.; Vial, P.A. Diagnosis and treatment of new world hantavirus infections. Curr. Opin. Infect. Dis. 2006, 19, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, R.; Riquelme, M.; Torres, A.; Rioseco, M.L.; Vergara, J.A.; Scholz, L.; Carriel, A. Hantavirus pulmonary syndrome, southern Chile. Emerg. Infect. Dis. 2003, 9, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Duchin, J.; Koster, F.; Peters, C.; Simpson, G.; Tempest, B.; Zaki, S.R.; Ksiazek, T.G.; Rollin, P.E.; Nichol, S.; Umland, E.T.; et al. Hantavirus Pulmonary Syndrome: A Clinical Description of 17 Patients with newly recognize disease. N. Engl. J. Med. 1994, 330, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Wernly, J.A.; Dietl, C.A.; Tabe, C.E.; Pett, S.B.; Crandall, C.; Milligan, K.; Crowley, M.R. Extracorporeal membrane oxygenation support improves survival of patients with Hantavirus cardiopulmonary syndrome refractory to medical treatment. Eur. J. Cardiothorac. Surg. 2011, 40, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Vial, P.A.; Valdivieso, F.; Calvo, M.; Rioseco, M.L.; Riquelme, R.; Araneda, A.; Tomicic, V.; Graf, J.; Paredes, L.; Florenzano, M.; et al. A non-randomized multicentre trial of human immune plasma for treatment of hantavirus cardiopulmonary syndrome by ANDV. Antivir. Ther. 2014, 20, 377–386. [Google Scholar] [CrossRef]
- Vial, C.; Martinez-Valdebenito, C.; Rios, S.; Martinez, J.; Vial, P.A.; Ferres, M.; Rivera, J.C.; Perez, R.; Valdivieso, F. Molecular method for the detection of Andes hantavirus infection: Validation for clinical diagnostics. Diagn. Microbiol. Infect. Dis. 2015, 84, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Fuenzalida, F.; Otaíza, F.; Valdivieso, F.; Graf, J.; Luco, L.; Ferres, M.; Vial, P.; Bustamante, R.; Tomicic, V.; Sotomayor, V.; et al. Guía Clínica de Prevención, Diagnóstico y Tratamiento del Síndrome Cardiopulmonar por Hantavirus. 2013. Available online: https://www.minsal.cl/sites/default/files/files/HANTA_imprimir.pdf (accessed on 12 July 2019).
- Navarrete, M.; Hott, M.; Caroca, J.; Leyton, L.; Venegas, N.; Ismail, K.; Saavedra, F.; Otth, C. Correlación entre criterios clínicos y de laboratorio de casos notificados por sospecha de hantavirosis y el resultado de la técnica de referencia. Rev. Chil. Infectol. 2016, 33, 275–281. [Google Scholar] [CrossRef]
- Koster, F.; Foucar, K.; Hjelle, B.; Scott, A.; Chong, Y.Y.; Larson, R.; McCabe, M. Rapid presumptive diagnosis of Hantavirus cardiopulmonary syndrome by blood smear review. Am. J. Clin. Pathol. 2001, 116, 665–672. [Google Scholar] [CrossRef]
- Vial, P.A.; Valdivieso, F.; Ferres, M.; Riquelme, R.; Rioseco, M.L.; Calvo, M.; Castillo, C.; Díaz, R.; Scholz, L.; Cuiza, A.; et al. High-dose intravenous methylprednisolone for hantavirus cardiopulmonary syndrome in Chile: A double-blind, randomized controlled clinical trial. Clin. Infect. Dis. 2013, 57, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendoça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijis, L.G. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, Z.; Fu, S.; Sang, J.; Deng, H.; Li, F.; Zhang, X.; Li, N.; Han, Q.; Liu, Z. Platelet distribution width at first day of hospital admission in patients with hemorrhagic fever with renal syndrome caused by Hantaan virus may predict disease severity and critical patients’ survival. Dis. Markers 2018, 2018, 9701619. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, J.; Wang, T.; Li, J.; Hui, L.; HA, X. Thrombocytopenia as a predictor of severe acute kidney injury in patients with Hantaan virus infections. PLoS ONE 2013, 8, e53236. [Google Scholar] [CrossRef] [PubMed]
- Outinen, T.K.; Laine, O.K.; Mäkelä, S.; Pörsti, I.; Huhtala, H.; Vaheri, A.; Mustonen, J. Thrombocytopenia associates with the severity of inflammation and variables reflecting capillary leakage in Puumala Hantavirus infection, an analysis of 546 Finnish patients. Infect. Dis. 2016, 48, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Connolly-Andersen, A.M.; Sundberg, E.; Ahlm, C.; Hultdin, J.; Baudin, M.; Larsson, J.; Dunne, E.; Kenny, D.; Lindahl, T.L.; Ramström, S.; et al. Increased thrombopoiesis and platelet activation in hantavirus-infected patients. J. Infect. Dis. 2015, 212, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovskaya, I.N.; Gorbunova, E.E.; Mackow, E.R. Pathogenic hantaviruses direct the adherence of quiescent platelets to infected endothelial cells. J. Virol. 2010, 84, 4832–4839. [Google Scholar] [CrossRef] [PubMed]
- Mackow, E.R.; Gavrilovskaya, I.N. Hantavirus regulation of endothelial cell functions. Thromb. Haemost. 2009, 102, 1030–1041. [Google Scholar] [PubMed]
- Macneil, A.; Ksiazek, T.G.; Rollin, P.E. Hantavirus Pulmonary Syndrome, United States, 1993–2009. Emerg. Infect. Dis. 2012, 17, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
| Variables | Value | Reference Value |
|---|---|---|
| Age, years, median (IQR) | 35 (23–46) | |
| Male, N (%) | 123 (70) | |
| Prodromal time, days, median (IQR) | 5 (4–7) | |
| SP, mmHg, median (IQR) | 108 (99–120) | |
| DP, mmHg, median (IQR) | 66 (60–74) | |
| P/F ratio, median (IQR) | 167 (114–248) | |
| Lactate, mmol/L, median (IQR) | 1.8 (0.3–3.3) | 0.8–1.7 |
| Hematocrit, %, median (IQR) | 44 (39–49) | 37–47 |
| Leukocytes, x1000/mL, median (IQR) | 11.5(8.1–17.4) | 4–10 |
| Platelets, x1000/mL, median (IQR) | 56 (37–86) | 150–400 |
| LDH, U/L, median (IQR) | 756 (477–1100) | 105–333 |
| Blood pH, median (IQR) | 7.42 (7.34–7.45) | 7.35–7.45 |
| Serun creatinine, mg/dL, median (IQR) | 0.9 (0.7–1.4) | 0.6–1.2 |
| ALT, U/L, median (IQR) | 66 (44–117) | 5–37 |
| AST, U/L, median (IQR) | 110 (71–197) | 10–41 |
| Amylase, U/L, median (IQR) | 44 (34–71) | 0–125 |
| Respiratory failure, N (%) | 133 (77) | |
| Invasive mechanical ventilation, N (%) | 87 (51) | |
| Circulatory failure, N (%) | 71 (41) | |
| Severity at Admission | ||
| Mild disease, N (%) | 40 (23) | |
| Moderate disease, N (%) | 86 (49) | |
| Severe Disease, N (%) | 49 (28) | |
| Severity during Hospital Stay | ||
| Mild disease, N (%) | 26 (14) | |
| Moderate disease, N (%) | 56 (32) | |
| Severe Disease, N (%) | 94 (54) | |
| Hospital LOS, Days, Median (IQR) | 10 (7–16) | |
| In-hospital Mortality, N (%) | 36 (21) |
| Variables | All N = 40 | Progression N = 14 | Non-Progression N = 26 | Significance |
|---|---|---|---|---|
| Age, years, median (IQR) | 38 (26–46) | 36 (27–46) | 39 (28–46) | 0.827 |
| Male, N (%) | 32 (80) | 12 (86) | 20 (77) | 0.412 |
| Prodomal time, days, median (IQR) | 4 (3–5) | 3 (3–6) | 4 (4–5) | 0.278 |
| SP, mmHg, median (IQR) | 113 (100–128) | 105 (100–122) | 117 (100–129) | 0.406 |
| DP, mmHg, median (IQR) | 67 (60–71) | 65 (60–76) | 70 (60–70) | 0.944 |
| Lactate, mmol/L, median (IQR) | 1.8 (1.1–2.3) | 1.9 (1.6–3.0) | 1.4 (1.1–2.2) | 0.299 |
| Hematocrit, %, median (IQR) | 44 (44–48) | 44 (40–47) | 43 (38–48) | 0.285 |
| Leukocytes, x1000/mL, median (IQR) | 9.8 (6.7–12.4) | 10.9 (9.4–16.1) | 8.7 (6.3–11.8) | 0.104 |
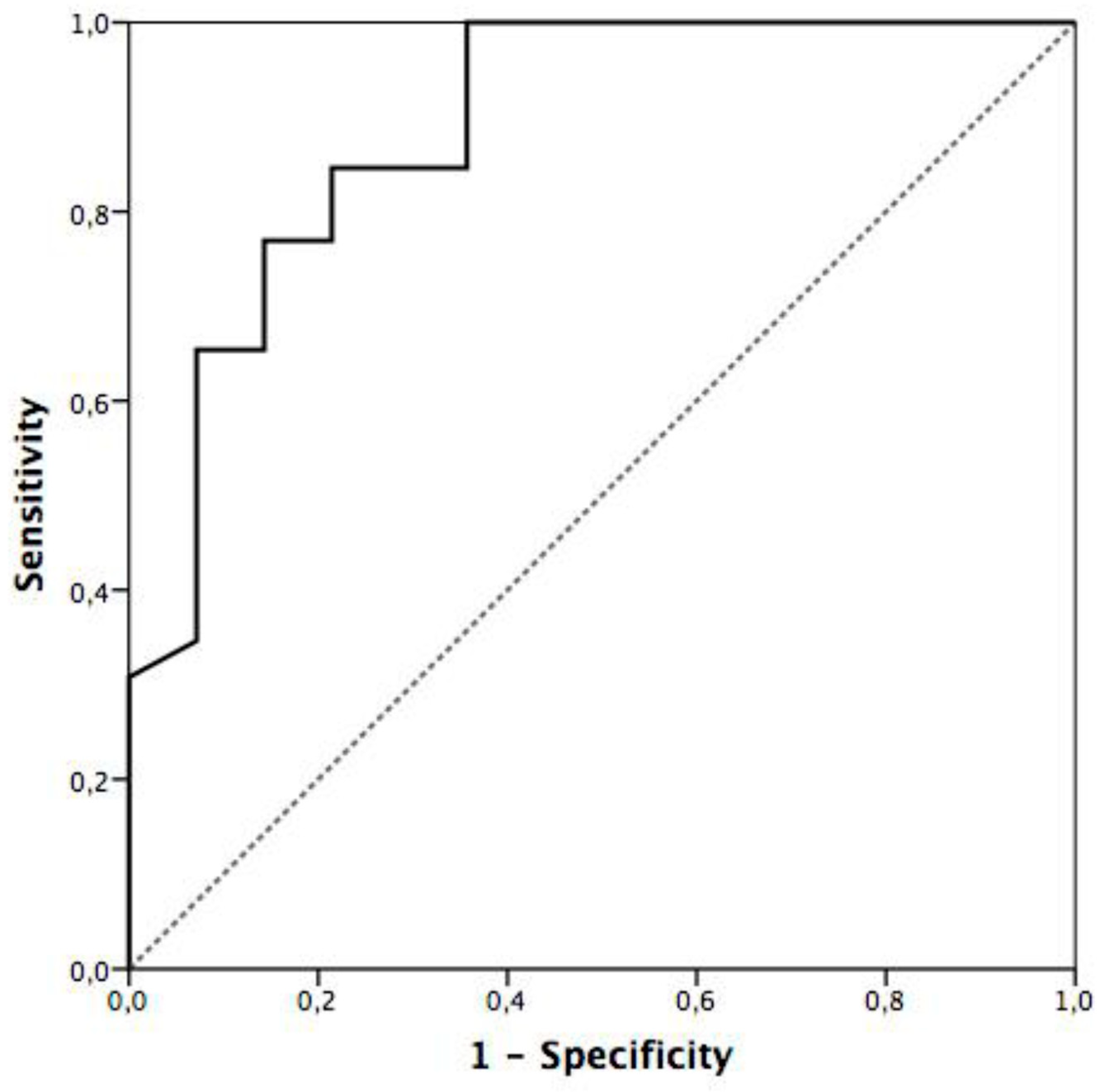
| Platelets, x1000/mL, median (IQR) | 71 (44–110) | 37 (34–58) | 83 (64–127) | <0.001 |
| LDH, U/L, median (IQR) | 779 (485–1008) | 726 (486–1593) | 798 (483–978) | 0.432 |
| Blood pH, median (IQR) | 7.43 (7.39–7.47) | 7.40 (7.33–7.45) | 7.45 (7.41–7.48) | 0.149 |
| Serum creatinine, mg/dL, median (IQR) | 0.9 (0.5–1.2) | 1.0 (0.4–1.3) | 0.9 (0.4–1.0) | 0.860 |
| ALT, U/L, median (IQR) | 51 (33–90) | 46 (30–82) | 53 (33–129) | 0.225 |
| AST, U/L, median (IQR) | 99 (54–177) | 135 (52–175) | 87 (56–186) | 0.731 |
| Amylase, U/L, median (IQR) | 42 (33–66) | 38 (31–57) | 44 (34–69) | 0.521 |
| Hospital LOS, days, median (IQR) | 9 (6–14) | 12 (7–18) | 9 (6–12) | 0.154 |
| In-hospital mortality, N (%) | 4 (10) | 4 (29) | 0 (0) | 0.011 |
| Variables | R | P Value |
|---|---|---|
| Prodomal time | −0.172 | 0.481 |
| Lactate | −0.461 | 0.153 |
| Hematocrit | −0.394 | 0.012 |
| Leukocyte count | −0.064 | 0.697 |
| LDH | −0.345 | 0.084 |
| Blood pH | −0.006 | 0.974 |
| Serum creatinine | 0.14 | 0.417 |
| Disease during Hospital Stay | ||||
|---|---|---|---|---|
| Progression | Non-Progression | Total | ||
| Platelets | <115K | 14 | 18 | 32 |
| >115K | 0 | 8 | 8 | |
| 14 | 26 | 40 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, R.; Vial, C.; Graf, J.; Calvo, M.; Ferrés, M.; Mertz, G.; Cuiza, A.; Agüero, B.; Aguilera, D.; Araya, D.; et al. Platelet Count in Patients with Mild Disease at Admission is Associated with Progression to Severe Hantavirus Cardiopulmonary Syndrome. Viruses 2019, 11, 693. https://doi.org/10.3390/v11080693
López R, Vial C, Graf J, Calvo M, Ferrés M, Mertz G, Cuiza A, Agüero B, Aguilera D, Araya D, et al. Platelet Count in Patients with Mild Disease at Admission is Associated with Progression to Severe Hantavirus Cardiopulmonary Syndrome. Viruses. 2019; 11(8):693. https://doi.org/10.3390/v11080693
Chicago/Turabian StyleLópez, René, Cecilia Vial, Jerónimo Graf, Mario Calvo, Marcela Ferrés, Gregory Mertz, Analía Cuiza, Begonia Agüero, Dante Aguilera, Diego Araya, and et al. 2019. "Platelet Count in Patients with Mild Disease at Admission is Associated with Progression to Severe Hantavirus Cardiopulmonary Syndrome" Viruses 11, no. 8: 693. https://doi.org/10.3390/v11080693
APA StyleLópez, R., Vial, C., Graf, J., Calvo, M., Ferrés, M., Mertz, G., Cuiza, A., Agüero, B., Aguilera, D., Araya, D., Pailamilla, I., Paratori, F., Torres-Torres, V., Vial, P. A., & Hantavirus Study Group in Chile. (2019). Platelet Count in Patients with Mild Disease at Admission is Associated with Progression to Severe Hantavirus Cardiopulmonary Syndrome. Viruses, 11(8), 693. https://doi.org/10.3390/v11080693





