Δccr5 Genotype Is Associated with Mild Form of Nephropathia Epidemica
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethics Statement
2.3. Hantavirus ELISA
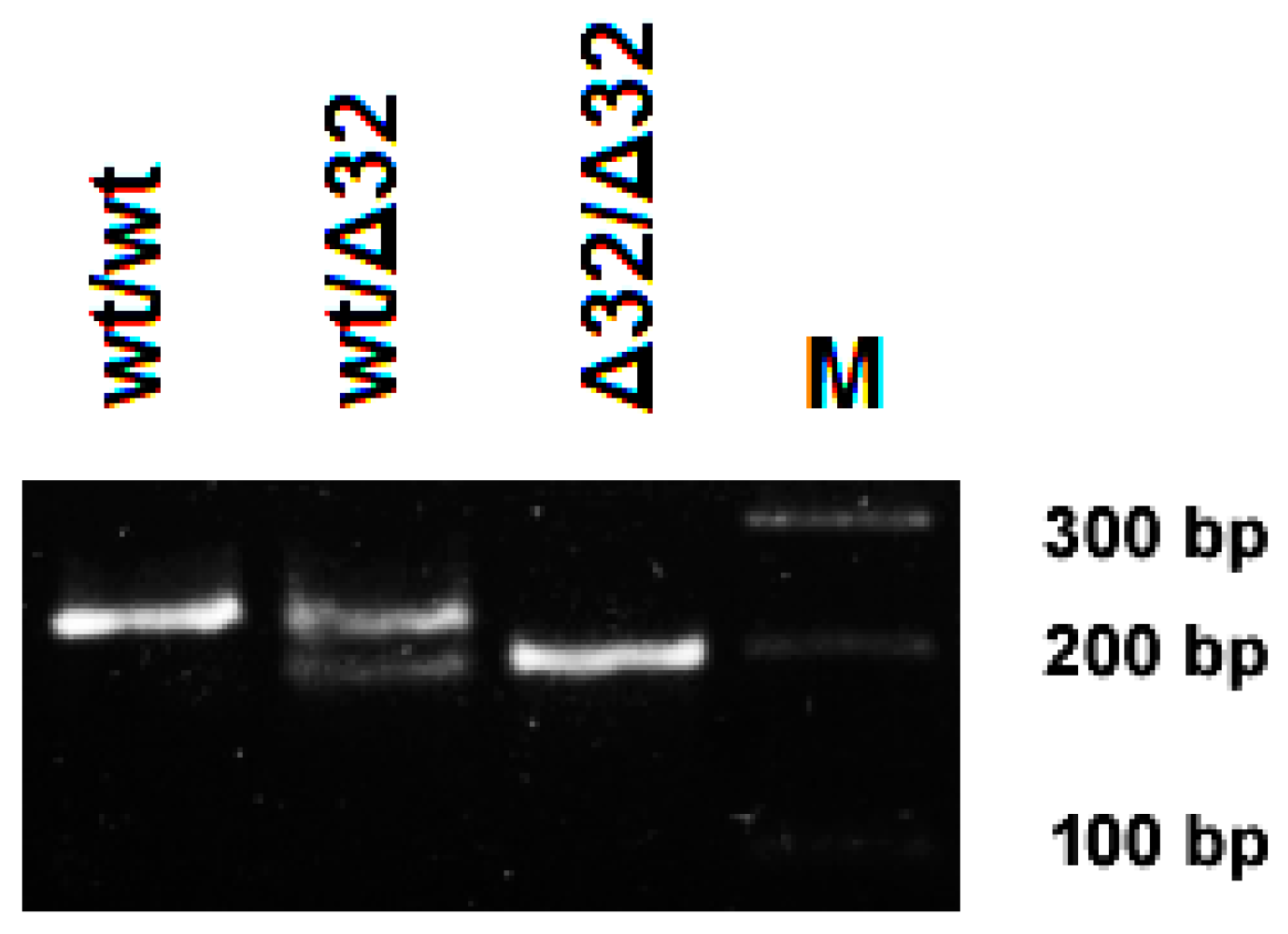
2.4. DNA Extraction and PCR
2.5. Multiplex Analysis
2.6. Statistical Analysis
3. Results
3.1. CCR5 Genetics in NE Cases and Tatarstan Population
3.2. Patient’s Clinical Characteristics
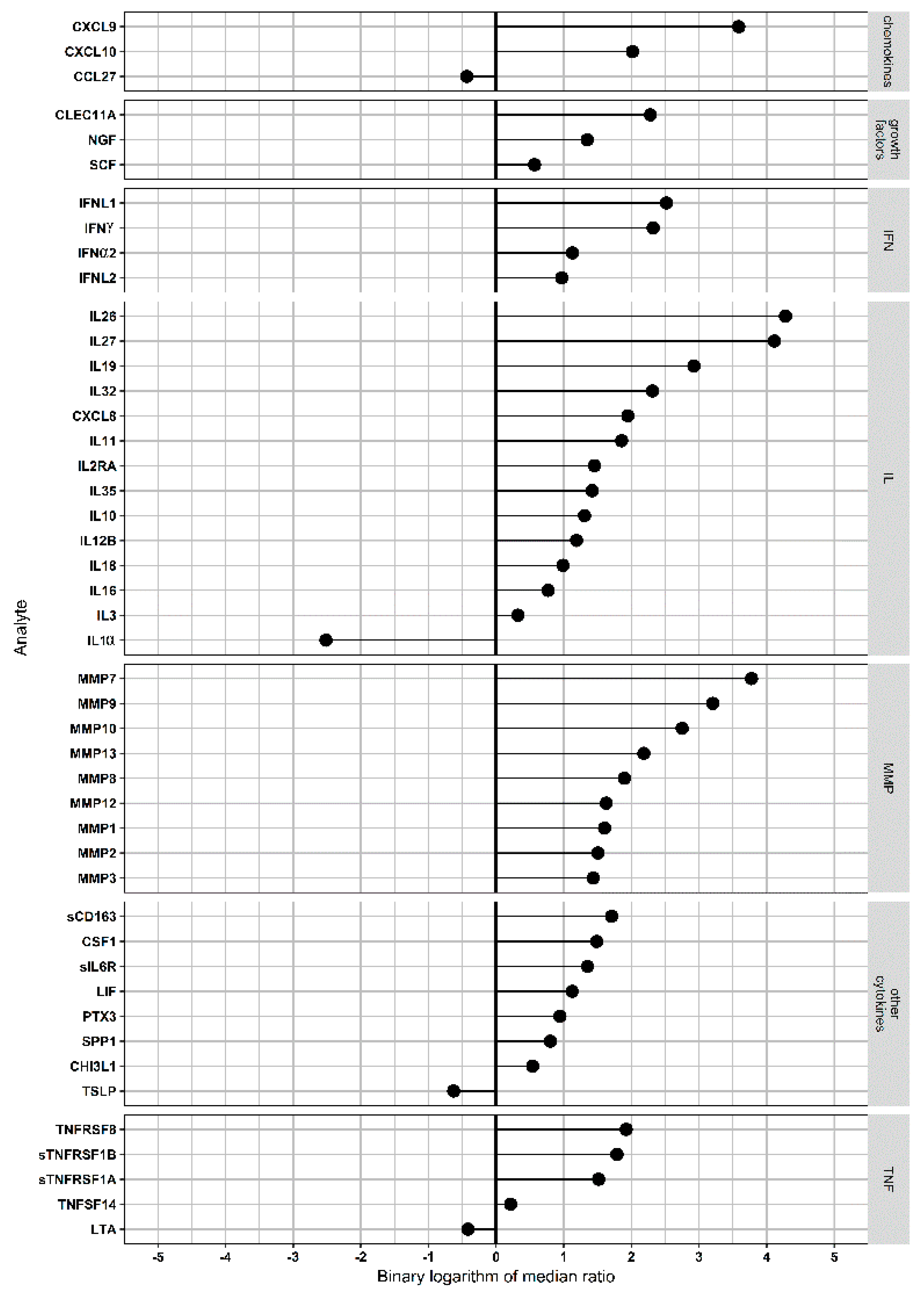
3.3. Assessment of Serum Analytes in NE Cases and Controls
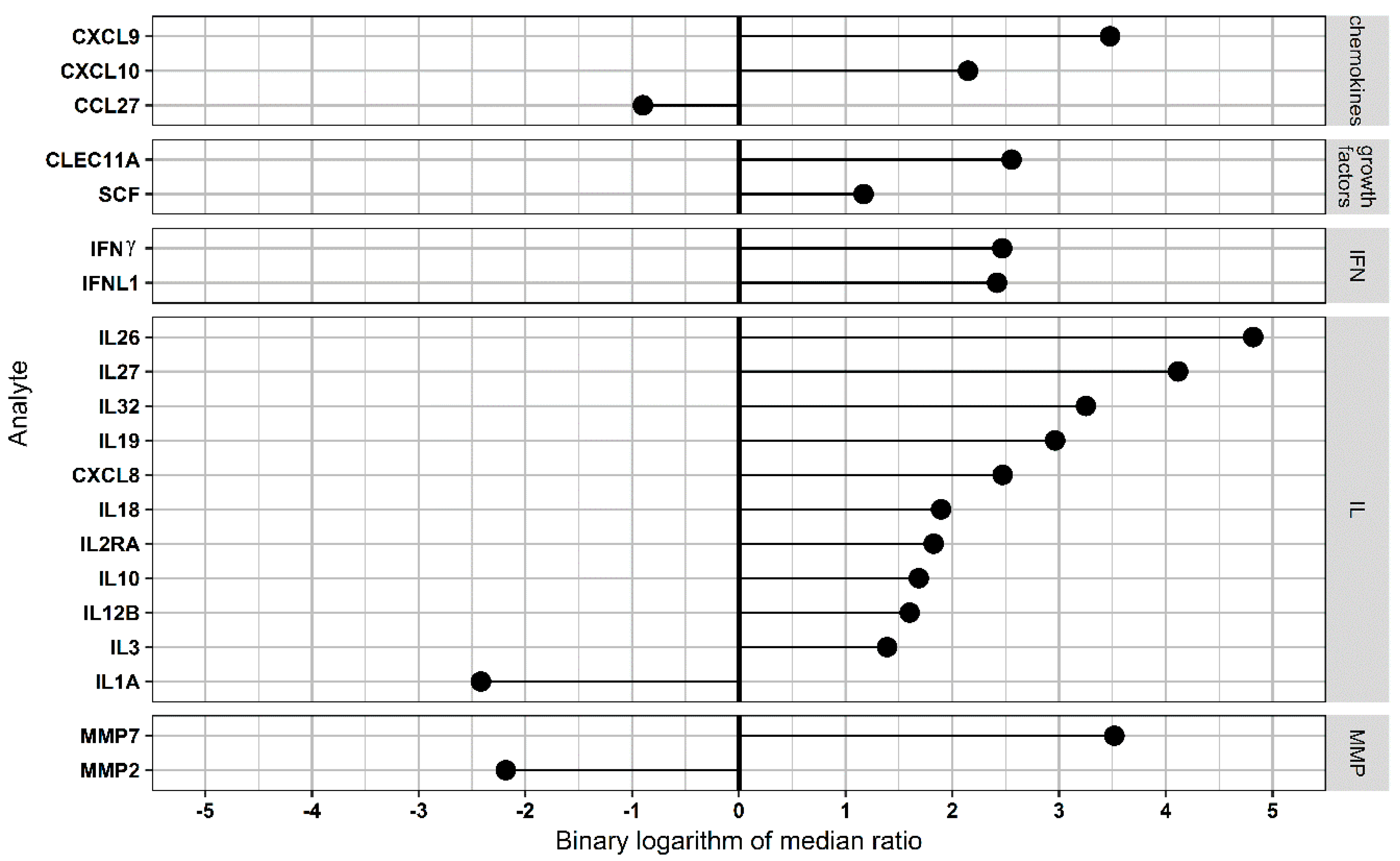
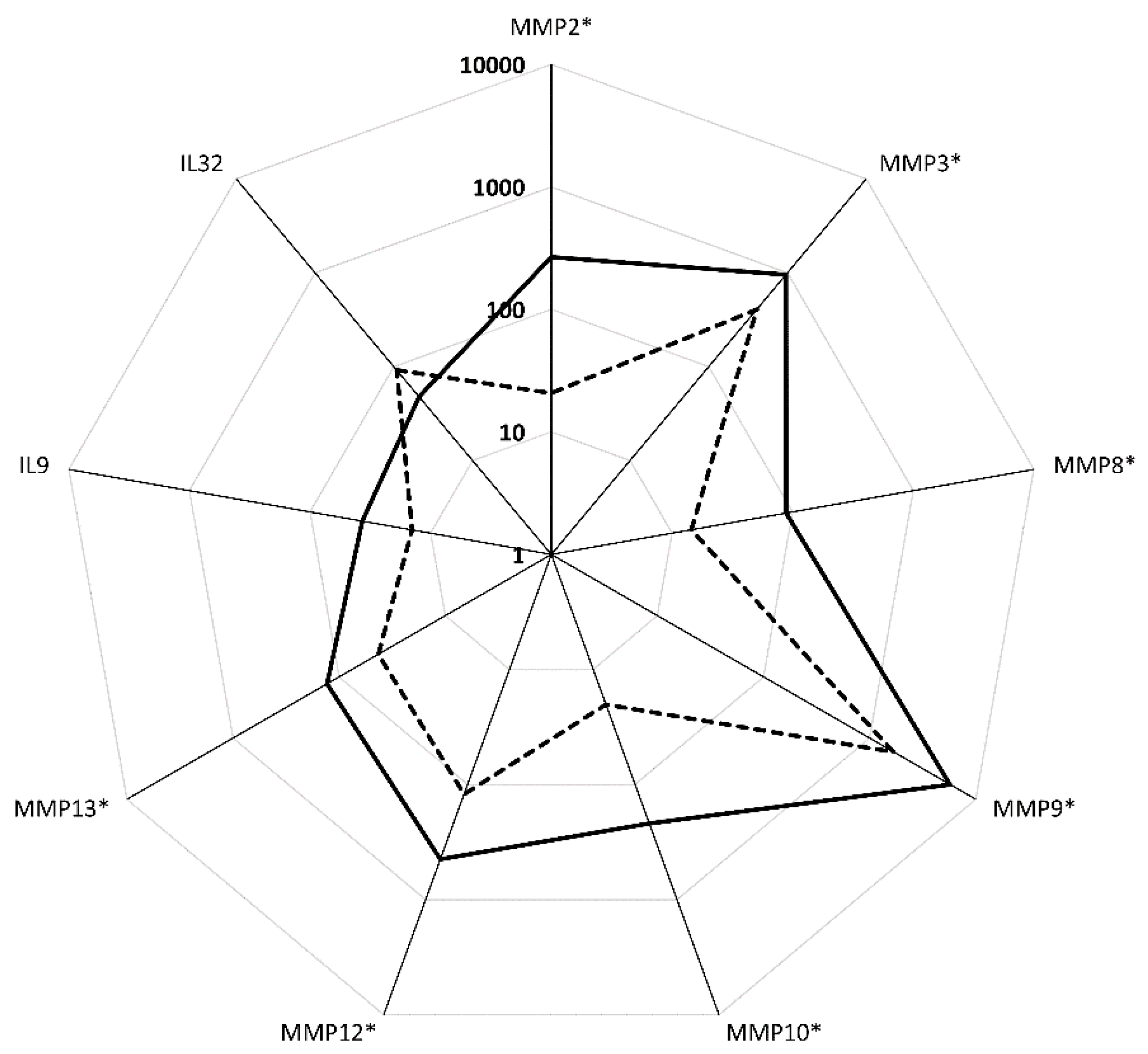
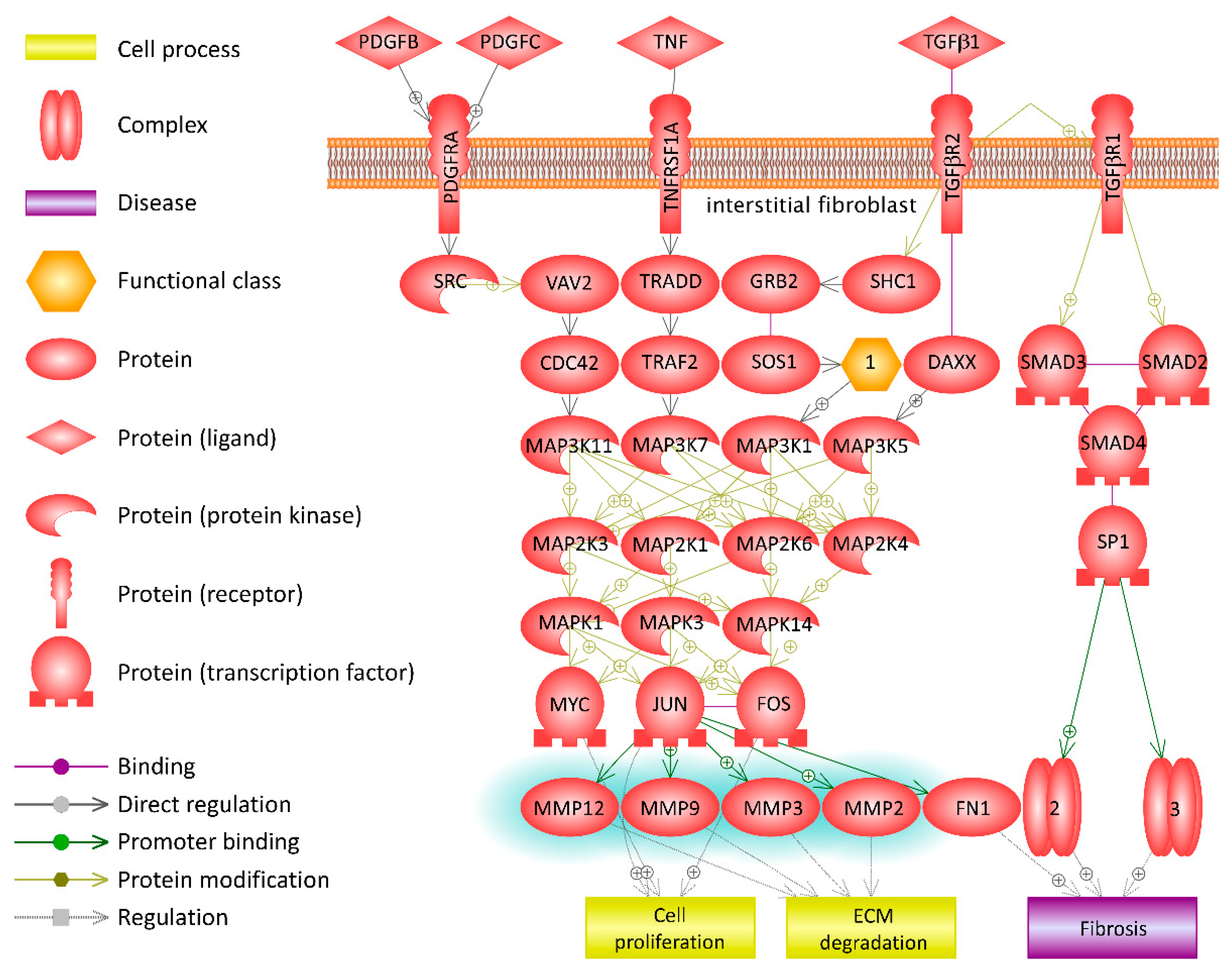
3.4. Pathway Analysis in Functional CCR5 Homozygous and Heterozygous NE Cases
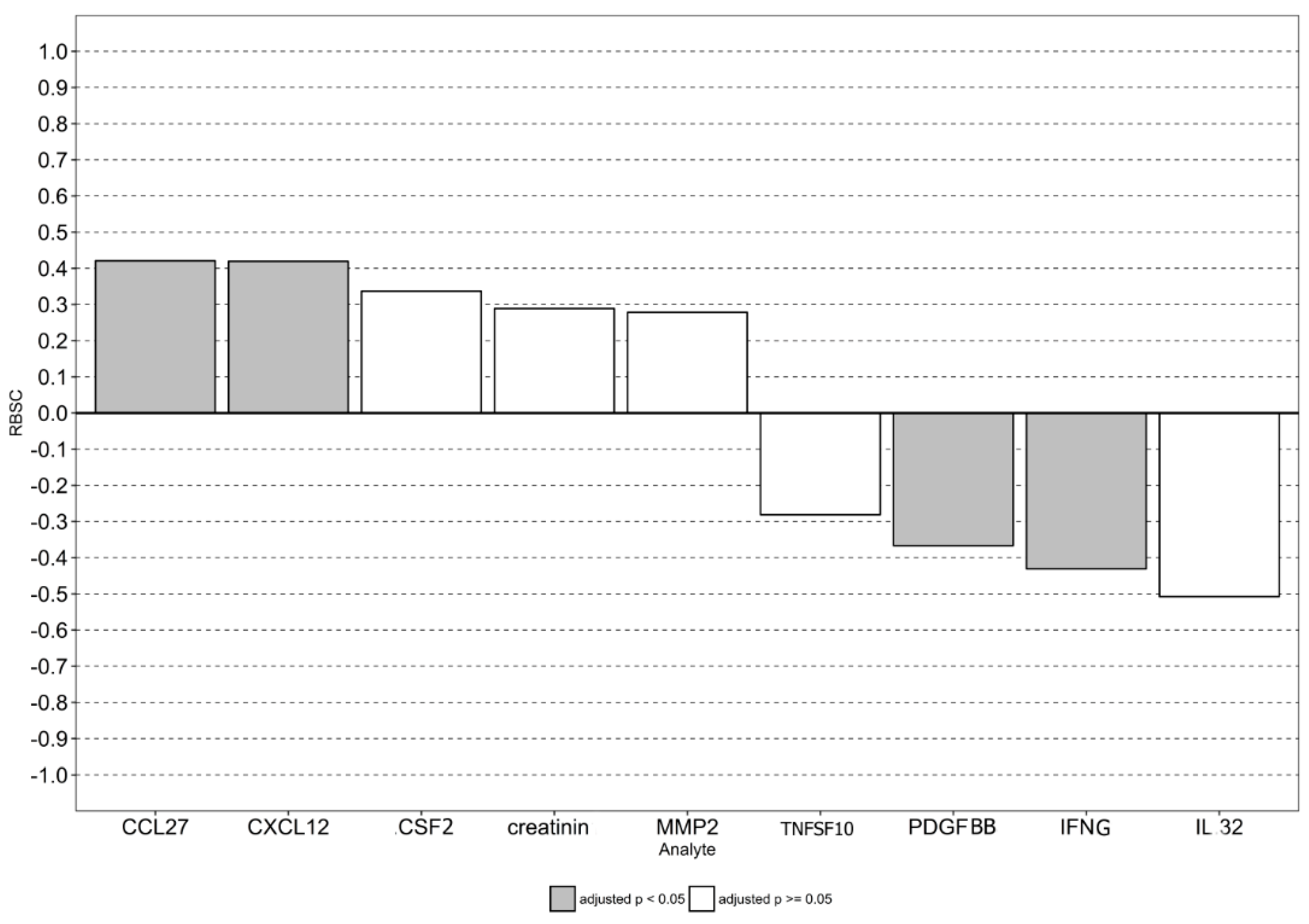
3.5. Prognostic Value of CCR5 Genotype, PDGFBB, CCL27, and CXCL12
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khismatullina, N.A.; Karimov, M.M.; Khaertynov, K.S.; Shuralev, E.A.; Morzunov, S.P.; Khaertynova, I.M.; Ivanov, A.A.; Milova, I.V.; Khakimzyanova, M.B.; Sayfullina, G.; et al. Epidemiological dynamics of nephropathia epidemica in the Republic of Tatarstan, Russia, during the period of 1997–2013. Epidemiol. Infect. 2016, 144, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Rasche, F.M.; Uhel, B.; Kruger, D.H.; Karges, W.; Czock, D.; Hampl, W.; Keller, F.; Meisel, H.; von Muller, L. Thrombocytopenia and acute renal failure in Puumala hantavirus infections. Emerg. Infect. Dis. 2004, 10, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Temonen, M.; Mustonen, J.; Helin, H.; Pasternack, A.; Vaheri, A.; Holthofer, H. Cytokines, adhesion molecules, and cellular infiltration in nephropathia epidemica kidneys: An immunohistochemical study. Clin. Immunol. Immunopathol. 1996, 78, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Martynova, E.V.; Valiullina, A.H.; Gusev, O.A.; Davidyuk, Y.N.; Garanina, E.E.; Shakirova, V.G.; Khaertynova, I.; Anokhin, V.A.; Rizvanov, A.A.; Khaiboullina, S.F. High Triglycerides Are Associated with Low Thrombocyte Counts and High VEGF in Nephropathia Epidemica. J. Immunol. Res. 2016, 2016, 8528270. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; LaRosa, G.; Kassam, N.; Gordon, C.J.; Heath, H.; Ruffing, N.; Chen, H.; Humblias, J.; Samson, M.; Parmentier, M.; et al. Interaction of chemokine receptor CCR5 with its ligands: Multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J. Exp. Med. 1997, 186, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Schall, T.J.; Bacon, K.; Toy, K.J.; Goeddel, D.V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 1990, 347, 669–671. [Google Scholar] [CrossRef]
- Glass, W.G.; Lim, J.K.; Cholera, R.; Pletnev, A.G.; Gao, J.L.; Murphy, P.M. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J. Exp. Med. 2005, 202, 1087–1098. [Google Scholar] [CrossRef]
- Hull, J.; Rowlands, K.; Lockhart, E.; Moore, C.; Sharland, M.; Kwiatkowski, D. Variants of the chemokine receptor CCR5 are associated with severe bronchiolitis caused by respiratory syncytial virus. J. Infect. Dis. 2003, 188, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Rothman, A.L.; Kurane, I.; Montoya, J.M.; Nolte, K.B.; Norman, J.E.; Waite, D.C.; Koster, F.T.; Ennis, F.A. High levels of cytokine-producing cells in the lung tissues of patients with fatal hantavirus pulmonary syndrome. J. Infect. Dis. 1999, 179, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Sironen, T.; Klingstrom, J.; Vaheri, A.; Andersson, L.C.; Lundkvist, A.; Plyusnin, A. Pathology of Puumala hantavirus infection in macaques. PLoS ONE 2008, 3, e3035. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.R.; Greer, P.W.; Coffield, L.M.; Goldsmith, C.S.; Nolte, K.B.; Foucar, K.; Feddersen, R.M.; Zumwalt, R.E.; Miller, G.L.; Khan, A.S.; et al. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am. J. Pathol. 1995, 146, 552–579. [Google Scholar] [PubMed]
- Rugeles, M.T.; Solano, F.; Diaz, F.J.; Bedoya, V.I.; Patino, P.J. Molecular characterization of the CCR 5 gene in seronegative individuals exposed to human immunodeficiency virus (HIV). J. Clin. Virol. 2002, 23, 161–169. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 1 April 2017).
- RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2015.
- The “Comprehensive R Archive Network” (CRAN). Available online: https://CRAN.R-project.org/ (accessed on 23 July 2019).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag New York: New York, NY, USA, 2009. [Google Scholar]
- Dick, J.M. Chemical composition and the potential for proteomic transformation in cancer, hypoxia, and hyperosmotic stress. PeerJ 2017, 5, e3421. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Jed Wing, S.W.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B.; Benesty, M.; the R Core Team; et al. Misc Functions for Training and Plotting Classification and Regression Models. The “Comprehensive R Archive Network” (CRAN). Available online: https://CRAN.R-project.org/ (accessed on 22 July 2019).
- Caret: Classification and Regression Training. R Package Version 6.0-78. Available online: https://CRAN.R-project.org/package=caret (accessed on 1 April 2017).
- Wickham, H. Reshaping Data with the reshape Package. J. Stat. Softw. 2017, 21, 1–20. [Google Scholar]
- Outinen, T.K.; Laine, O.K.; Makela, S.; Porsti, I.; Huhtala, H.; Vaheri, A.; Mustonen, J. Thrombocytopenia associates with the severity of inflammation and variables reflecting capillary leakage in Puumala Hantavirus infection, an analysis of 546 Finnish patients. Infect. Dis. (Lond.) 2016, 48, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.K.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef]
- Doni, A.; Peri, G.; Chieppa, M.; Allavena, P.; Pasqualini, F.; Vago, L.; Romani, L.; Garlanda, C.; Mantovani, A. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur. J. Immunol. 2003, 33, 2886–2893. [Google Scholar] [CrossRef]
- Marsac, D.; García, S.; Fournet, A.; Aguirre, A.; Pino, K.; Ferres, M.; Kalergis, A.M.; Lopez-Lastra, M.; Veas, F. Infection of human monocyte-derived dendritic cells by ANDES Hantavirus enhances pro-inflammatory state, the secretion of active MMP-9 and indirectly enhances endothelial permeability. Virol. J. 2011, 8, 223. [Google Scholar] [CrossRef]
- Easterbrook, J.D.; Klein, S.L. Corticosteroids modulate Seoul virus infection, regulatory T cell responses, and MMP-9 expression in male, but not female, Norway rats. J. Gen. Virol. 2008, 89, 2723. [Google Scholar] [CrossRef]
- Khaiboullina, S.F.; Levis, S.; Morzunov, S.P.; Martynova, E.V.; Anokhin, V.A.; Gusev, O.A.; St Jeor, S.C.; Lombardi, V.C.; Rizvanov, A.A. Serum cytokine profiles differentiating hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Front. Immunol. 2017, 8, 567. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, J.; Wang, T.; Li, J.; Hui, L.; Ha, X. Thrombocytopenia as a predictor of severe acute kidney injury in patients with Hantaan virus infections. PLoS ONE 2013, 8, e53236. [Google Scholar] [CrossRef] [PubMed]
- Pothapregada, S.; Kamalakannan, B.; Thulasingam, M. Role of platelet transfusion in children with bleeding in dengue fever. J. Vector Borne Dis. 2015, 52, 304–308. [Google Scholar] [PubMed]
- Perrier, P.; Martinez, F.O.; Locati, M.; Bianchi, G.; Nebuloni, M.; Vago, G.; Bazzoni, F.; Sozzani, S.; Allavena, P.; Mantovani, A. Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: Induction of the B cell-activating chemokine, CXC chemokine ligand 13. J. Immunol. 2004, 172, 7031–7042. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Luster, A.D. Chemokines and their receptors: Drug targets in immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 171–197. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, J.E.; Miller, S.C.; Smith, J.; Lu, B.; Gerard, C.; Cookenham, T.; Roberts, A.D.; Woodland, D.L. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity 2008, 29, 101–113. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell. Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Whitelock, J.M.; Murdoch, A.D.; Iozzo, R.V.; Underwood, P.A. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J. Biol. Chem. 1996, 271, 10079–10086. [Google Scholar] [CrossRef]
- Abraham, M.; Shapiro, S.; Karni, A.; Weiner, H.L.; Miller, A. Gelatinases (MMP-2 and MMP-9) are preferentially expressed by Th1 vs. Th2 cells. J. Neuroimmunol. 2005, 163, 157–164. [Google Scholar] [CrossRef]
- Bini, A.; Itoh, Y.; Kudryk, B.J.; Nagase, H. Degradation of cross-linked fibrin by matrix metalloproteinase 3 (stromelysin 1): Hydrolysis of the gamma Gly 404-Ala 405 peptide bond. Biochemistry 1996, 35, 13056–13063. [Google Scholar] [CrossRef]
- Krampert, M.; Bloch, W.; Sasaki, T.; Bugnon, P.; Rulicke, T.; Wolf, E.; Aumailley, M.; Parks, W.C.; Werner, S. Activities of the matrix metalloproteinase stromelysin-2 (MMP-10) in matrix degradation and keratinocyte organization in wounded skin. Mol. Biol. Cell. 2004, 15, 5242–5254. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, D.V.; di Battista, J.A.; Martel-Pelletier, J.; Reboul, P.; He, Y.; Jolicoeur, F.C.; Pelletier, J.P. Modulation of TIMP-1 synthesis by antiinflammatory cytokines and prostaglandin E2 in interleukin 17 stimulated human monocytes/macrophages. J. Rheumatol. 2001, 28, 712–718. [Google Scholar] [PubMed]
- Van Hamburg, J.P.; Asmawidjaja, P.S.; Davelaar, N.; Mus, A.M.; Colin, E.M.; Hazes, J.M.; Dolhain, R.J.; Lubberts, E. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011, 63, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Homey, B.; Alenius, H.; Muller, A.; Soto, H.; Bowman, E.P.; Yuan, W.; McEvoy, L.; Lauerma, A.I.; Assmann, T.; Bunemann, E.; et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat. Med. 2002, 8, 157–165. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kletenkov, K.; Martynova, E.; Davidyuk, Y.; Kabwe, E.; Shamsutdinov, A.; Garanina, E.; Shakirova, V.; Khaertynova, I.; Anokhin, V.; Tarlinton, R.; et al. Δccr5 Genotype Is Associated with Mild Form of Nephropathia Epidemica. Viruses 2019, 11, 675. https://doi.org/10.3390/v11070675
Kletenkov K, Martynova E, Davidyuk Y, Kabwe E, Shamsutdinov A, Garanina E, Shakirova V, Khaertynova I, Anokhin V, Tarlinton R, et al. Δccr5 Genotype Is Associated with Mild Form of Nephropathia Epidemica. Viruses. 2019; 11(7):675. https://doi.org/10.3390/v11070675
Chicago/Turabian StyleKletenkov, Konstantin, Ekaterina Martynova, Yuriy Davidyuk, Emmanuel Kabwe, Anton Shamsutdinov, Ekaterina Garanina, Venera Shakirova, Ilsiyar Khaertynova, Vladimir Anokhin, Rachael Tarlinton, and et al. 2019. "Δccr5 Genotype Is Associated with Mild Form of Nephropathia Epidemica" Viruses 11, no. 7: 675. https://doi.org/10.3390/v11070675
APA StyleKletenkov, K., Martynova, E., Davidyuk, Y., Kabwe, E., Shamsutdinov, A., Garanina, E., Shakirova, V., Khaertynova, I., Anokhin, V., Tarlinton, R., Rizvanov, A., Khaiboullina, S., & Morzunov, S. (2019). Δccr5 Genotype Is Associated with Mild Form of Nephropathia Epidemica. Viruses, 11(7), 675. https://doi.org/10.3390/v11070675







