Transport via Macropinocytic Vesicles Is Crucial for Productive Infection with Bombyx Mori Nucleopolyhedrovirus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Construction of Transient Expression Vector and Immunofluorescence Assay
2.3. Syncytium Formation Assay
2.4. Reversible Conformational Change Assay
2.5. Fluid Uptake Assay
2.6. Ammonium Chloride Treatment and DMF Inducement
2.7. Flow Cytometry Analysis
2.8. DMF Comparison between AcBac-GFP and BmBac-GFP Infection
2.9. Construction of Recombinant Labeled Virus
2.10. Tracing of Viral Particles during Infection
3. Results
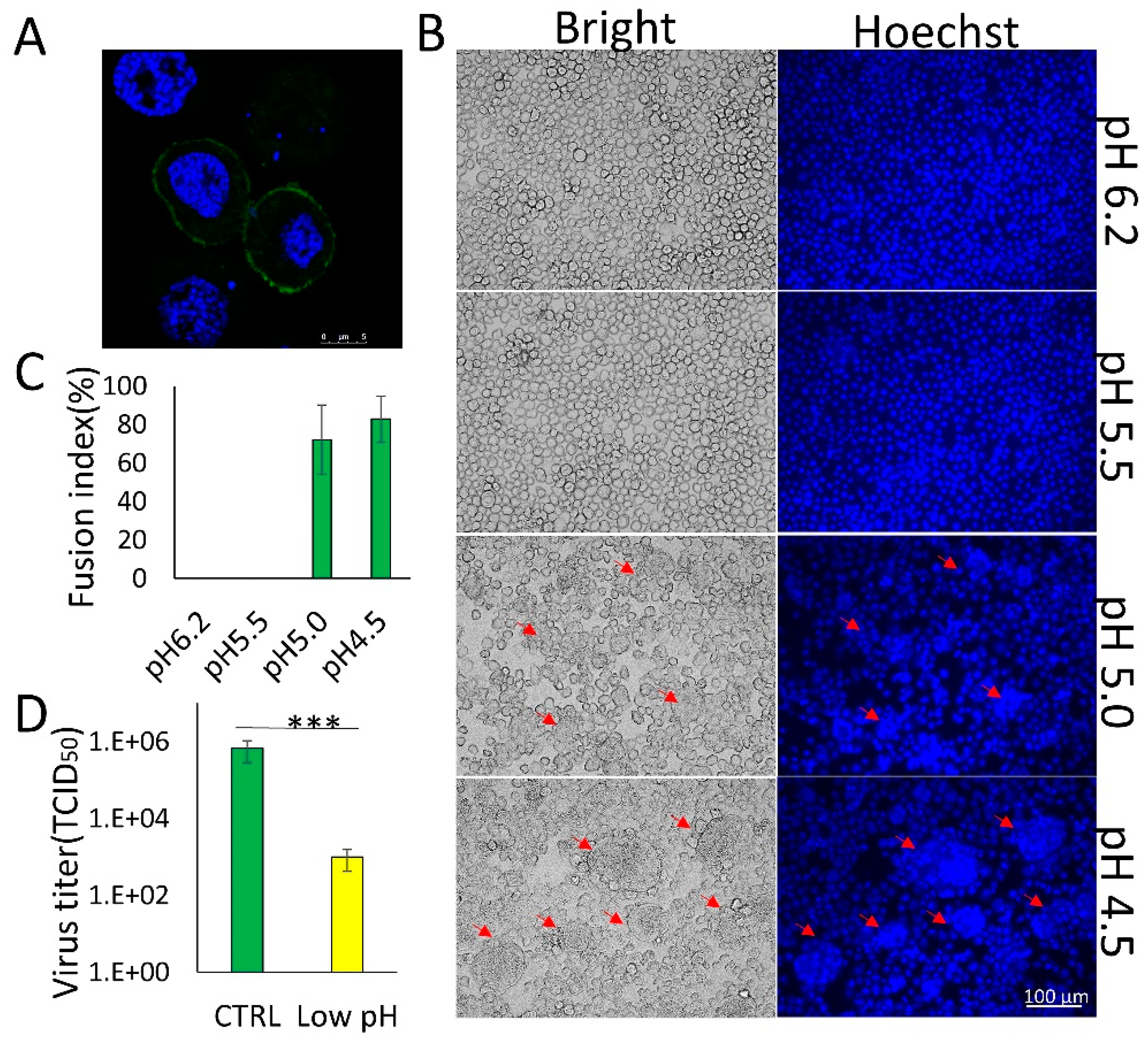
3.1. Membrane Fusion Induced by BmNPV GP64 is Triggered by Low pH
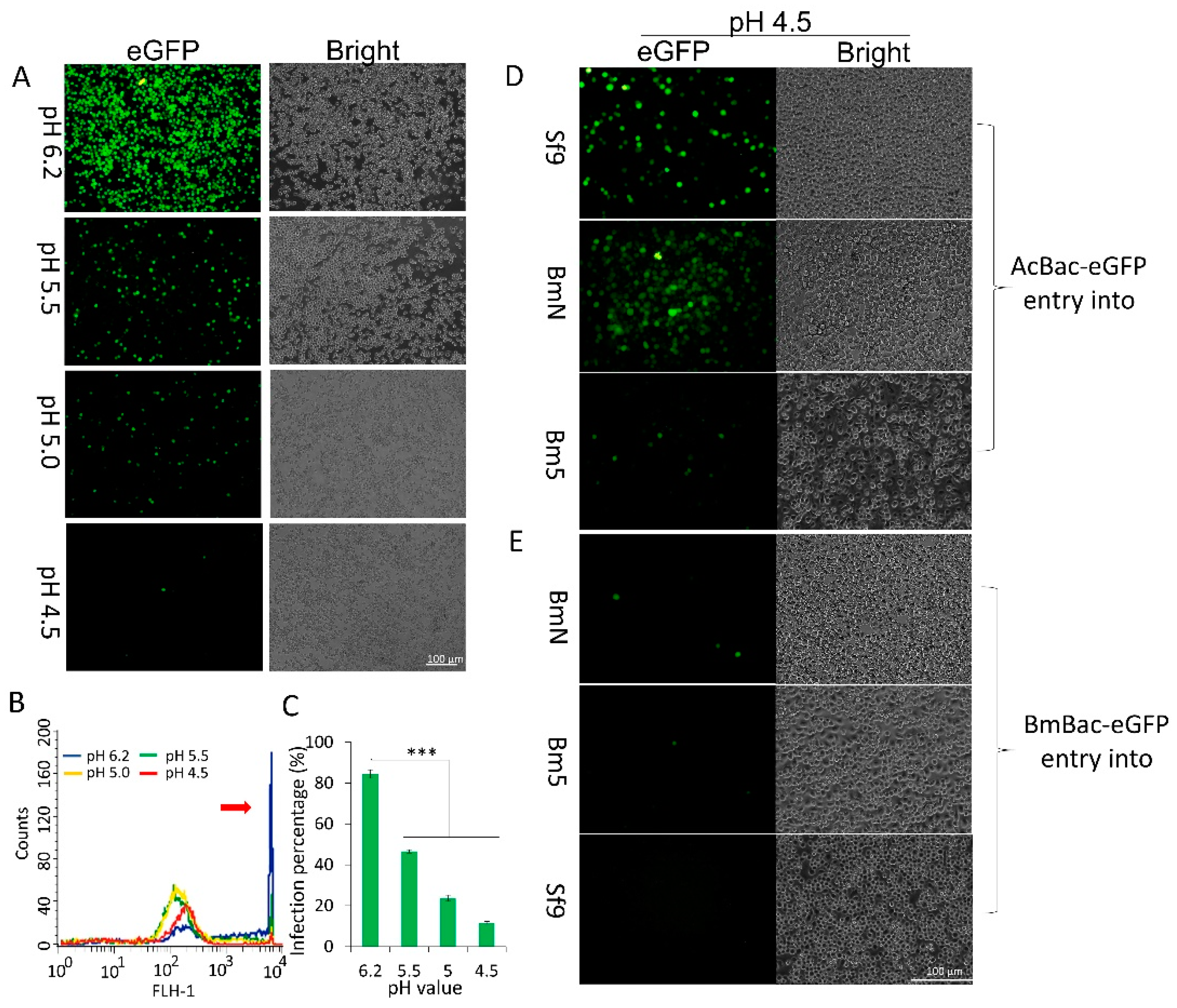
3.2. Virus-Activated Fluid Uptake Mediates BmNPV Entry into Host Cells
3.3. DMF Results in a Productive Infection for AcMNPV but an Abortive Infection for BmNPV
3.4. DMF-Mediated Abortive Infection of BmNPV Results from a Failure of Nucleocapsid Transport to the Nucleus
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rohrmann, G.F. Baculovirus Molecular Biology, 3rd ed.; Bethesda: Rockville, MD, USA, 2013; pp. 1–15. [Google Scholar]
- Wang, M.; Yin, F.; Shen, S.; Tan, Y.; Deng, F.; Vlak, J.M.; Hu, Z.; Wang, H. Partial functional rescue of Helicoverpa armigera single nucleocapsid nucleopolyhedrovirus infectivity by replacement of F protein with GP64 from Autographa californica multicapsid nucleopolyhedrovirus. J. Virol. 2010, 84, 11505–11514. [Google Scholar] [CrossRef] [PubMed]
- Gomi, S.; Majima, K.; Maeda, S. Sequence analysis of the genome of Bombyx mori nucleopolyhedrovirus. J. Gen. Virol. 1999, 80 Pt 5, 1323–1337. [Google Scholar] [CrossRef]
- Dong, S.; Wang, M.; Qiu, Z.; Deng, F.; Vlak, J.M.; Hu, Z.; Wang, H. Autographa californica multicapsid nucleopolyhedrovirus efficiently infects Sf9 cells and transduces mammalian cells via direct fusion with the plasma membrane at low pH. J. Virol. 2010, 84, 5351–5359. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, J.; Loureiro, S.; Abrescia, N.G.; Stuart, D.I.; Jones, I.M. The postfusion structure of baculovirus gp64 supports a unified view of viral fusion machines. Nat. Struct Mol. Biol. 2008, 15, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, T.; Volkman, L.E.; Welch, M.D. Actin-based motility drives baculovirus transit to the nucleus and cell surface. J. Cell. Biol. 2010, 190, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, C.; Kaname, Y.; Taguwa, S.; Abe, T.; Fukuhara, T.; Tani, H.; Moriishi, K.; Matsuura, Y. Baculovirus GP64-mediated entry into mammalian cells. J. Virol. 2012, 86, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hao, B.; Cheng, C.; Liang, F.; Shen, X.; Cheng, X. Entry of Bombyx mori nucleopolyhedrovirus into BmN cells by cholesterol-dependent macropinocytic endocytosis. Biochem. Biophys. Res. Commun. 2014, 453, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Virus entry by macropinocytosis. Nature. Cell Biol. 2009, 11, 510–520. [Google Scholar] [CrossRef]
- Townsley, A.C.; Moss, B. Two distinct low-pH steps promote entry of vaccinia virus. J. Virol. 2007, 81, 8613–8620. [Google Scholar] [CrossRef]
- Rizopoulos, Z.; Balistreri, G.; Kilcher, S.; Martin, C.K.; Syedbasha, M.; Helenius, A.; Mercer, J. Vaccinia Virus Infection Requires Maturation of Macropinosomes. Traffic 2015, 16, 814–831. [Google Scholar] [CrossRef]
- Xu, Y.P.; Cheng, R.L.; Xi, Y.; Zhang, C.X. Genomic diversity of Bombyx mori nucleopolyhedrovirus strains. Genomics 2013, 102, 63–71. [Google Scholar] [CrossRef]
- Blissard, G.W.; Wenz, J.R. Baculovirus gp64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J. Virol. 1992, 66, 6829–6835. [Google Scholar]
- White, J.; Matlin, K.; Helenius, A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J. Cell. Biol. 1981, 89, 674–679. [Google Scholar] [CrossRef]
- Kielian, M.C.; Marsh, M.; Helenius, A. Kinetics of endosome acidification detected by mutant and wild-type Semliki Forest virus. EMBO J. 1986, 5, 3103–3109. [Google Scholar] [CrossRef]
- Katou, Y.; Yamada, H.; Ikeda, M.; Kobayashi, M. A single amino acid substitution modulates low-pH-triggered membrane fusion of GP64 protein in Autographa californica and Bombyx mori nucleopolyhedroviruses. Virology 2010, 404, 204–214. [Google Scholar] [CrossRef]
- Hefferon, K.L.; Oomens, A.G.; Monsma, S.A.; Finnerty, C.M.; Blissard, G.W. Host cell receptor binding by baculovirus GP64 and kinetics of virion entry. Virology 1999, 258, 455–468. [Google Scholar] [CrossRef]
- White, J.M.; Whittaker, G.R. Fusion of Enveloped Viruses in Endosomes. Traffic 2016, 17, 593–614. [Google Scholar] [CrossRef]
- Mercer, J.; Schelhaas, M.; Helenius, A. Virus entry by endocytosis. Annu. Rev. Biochem. 2010, 79, 803–833. [Google Scholar] [CrossRef]
- Galloway, S.E.; Reed, M.L.; Russell, C.J.; Steinhauer, D.A. Influenza HA subtypes demonstrate divergent phenotypes for cleavage activation and pH of fusion: implications for host range and adaptation. PLoS. Pathog 2013, 9, e1003151. [Google Scholar] [CrossRef]
- Lozach, P.Y.; Huotari, J.; Helenius, A. Late-penetrating viruses. Curr. Opin. Virol. 2011, 1, 35–43. [Google Scholar] [CrossRef]
- Johannsdottir, H.K.; Mancini, R.; Kartenbeck, J.; Amato, L.; Helenius, A. Host cell factors and functions involved in vesicular stomatitis virus entry. J. Virol. 2009, 83, 440–453. [Google Scholar] [CrossRef]
- Qin, F.; Xu, C.; Hu, J.; Lei, C.; Zheng, Z.; Peng, K.; Wang, H.; Sun, X. Dissecting the cell entry pathway of baculovirus by single particle tracking and quantitative electron microscopic analysis. J. Virol. 2019. [Google Scholar] [CrossRef]
- Marsh, M.; Bron, R. SFV infection in CHO cells: cell-type specific restrictions to productive virus entry at the cell surface. J. Cell Sci. 1997, 110 Pt 1, 95–103. [Google Scholar]
- Racoosin, E.L.; Swanson, J.A. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J. Cell Biol. 1993, 121, 1011–1020. [Google Scholar] [CrossRef]
- Katou, Y.; Ikeda, M.; Kobayashi, M. Abortive replication of Bombyx mori nucleopolyhedrovirus in Sf9 and High Five cells: defective nuclear transport of the virions. Virology 2006, 347, 455–465. [Google Scholar] [CrossRef]
- Au, S.; Wu, W.; Zhou, L.; Theilmann, D.A.; Pante, N. A new mechanism for nuclear import by actin-based propulsion used by a baculovirus nucleocapsid. J. Cell Sci. 2016, 129, 2905–2911. [Google Scholar] [CrossRef]
- Nanbo, A.; Imai, M.; Watanabe, S.; Noda, T.; Takahashi, K.; Neumann, G.; Halfmann, P.; Kawaoka, Y. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS. Pathog. 2010, 6, e1001121. [Google Scholar] [CrossRef]
- Kaletsky, R.L.; Simmons, G.; Bates, P. Proteolysis of the Ebola virus glycoproteins enhances virus binding and infectivity. J. Virol. 2007, 81, 13378–13384. [Google Scholar] [CrossRef]
- Miller, E.H.; Obernosterer, G.; Raaben, M.; Herbert, A.S.; Deffieu, M.S.; Krishnan, A.; Ndungo, E.; Sandesara, R.G.; Carette, J.E.; Kuehne, A.I.; et al. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J. 2012, 31, 1947–1960. [Google Scholar] [CrossRef]
- Marechal, V.; Prevost, M.C.; Petit, C.; Perret, E.; Heard, J.M.; Schwartz, O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 2001, 75, 11166–11177. [Google Scholar] [CrossRef]
- Meier, O.; Boucke, K.; Hammer, S.V.; Keller, S.; Stidwill, R.P.; Hemmi, S.; Greber, U.F. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 2002, 158, 1119–1131. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Li, C.; Tang, X.; Liu, L.; Nan, W.; Shen, X.; Hao, B. Transport via Macropinocytic Vesicles Is Crucial for Productive Infection with Bombyx Mori Nucleopolyhedrovirus. Viruses 2019, 11, 668. https://doi.org/10.3390/v11070668
Huang J, Li C, Tang X, Liu L, Nan W, Shen X, Hao B. Transport via Macropinocytic Vesicles Is Crucial for Productive Infection with Bombyx Mori Nucleopolyhedrovirus. Viruses. 2019; 11(7):668. https://doi.org/10.3390/v11070668
Chicago/Turabian StyleHuang, Jinshan, Chenya Li, Xudong Tang, Lin Liu, Wenbin Nan, Xingjia Shen, and Bifang Hao. 2019. "Transport via Macropinocytic Vesicles Is Crucial for Productive Infection with Bombyx Mori Nucleopolyhedrovirus" Viruses 11, no. 7: 668. https://doi.org/10.3390/v11070668
APA StyleHuang, J., Li, C., Tang, X., Liu, L., Nan, W., Shen, X., & Hao, B. (2019). Transport via Macropinocytic Vesicles Is Crucial for Productive Infection with Bombyx Mori Nucleopolyhedrovirus. Viruses, 11(7), 668. https://doi.org/10.3390/v11070668




