Wild Rats, Laboratory Rats, Pet Rats: Global Seoul Hantavirus Disease Revisited
Abstract
1. Introduction
2. Prelude
3. Overview of Chronological SEOV Findings by Continent
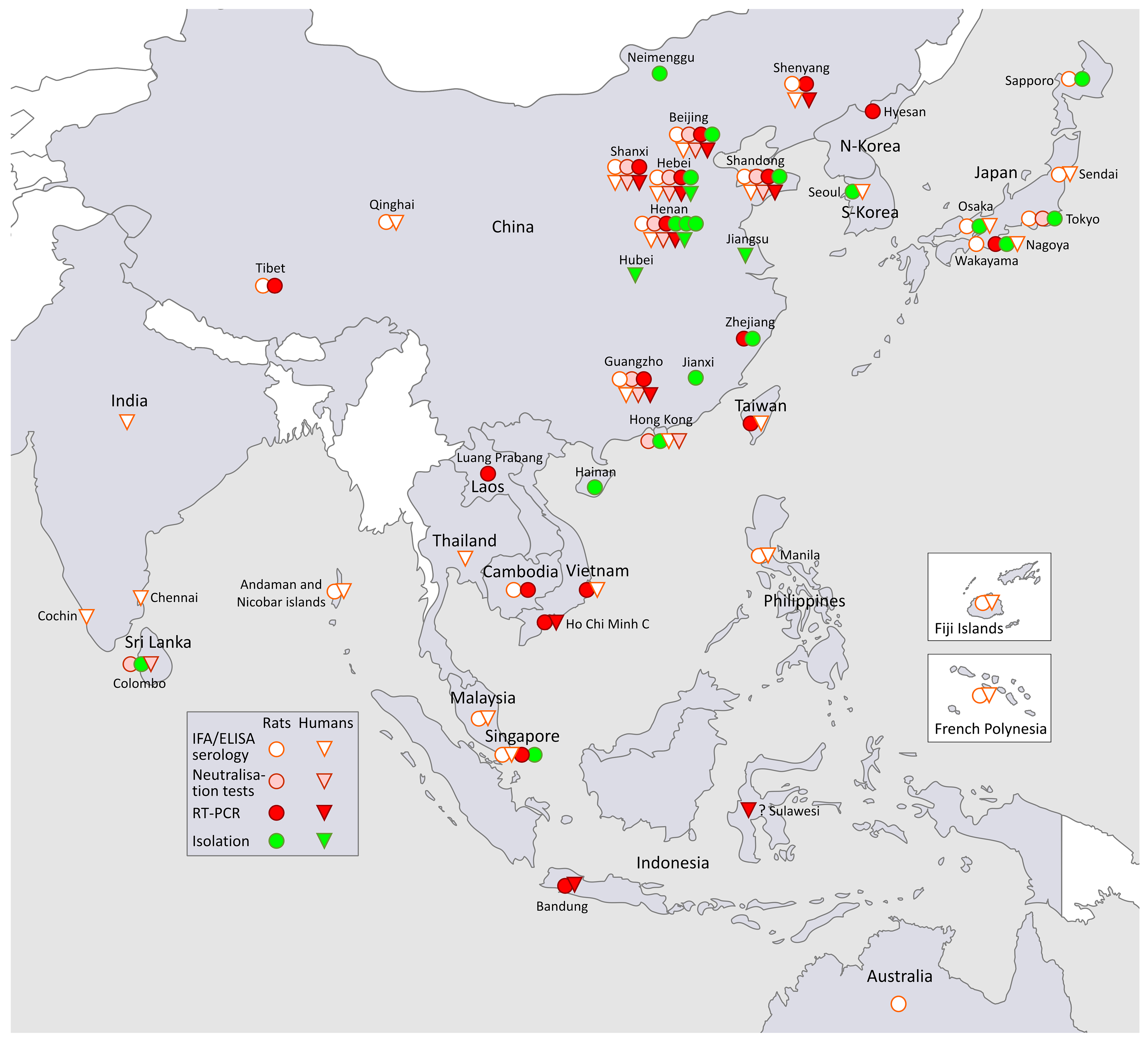
3.1. SEOV in South-East Asia and in the Far East (Figure 1, Asia)
3.1.1. SEOV in Korea and South-East Asia
SEOV in Korea
SEOV in South-East Asia
3.1.2. SEOV in the Far East
SEOV in Japan
SEOV in China
3.1.3. SEOV in South-Asia
3.2. SEOV in Oceania
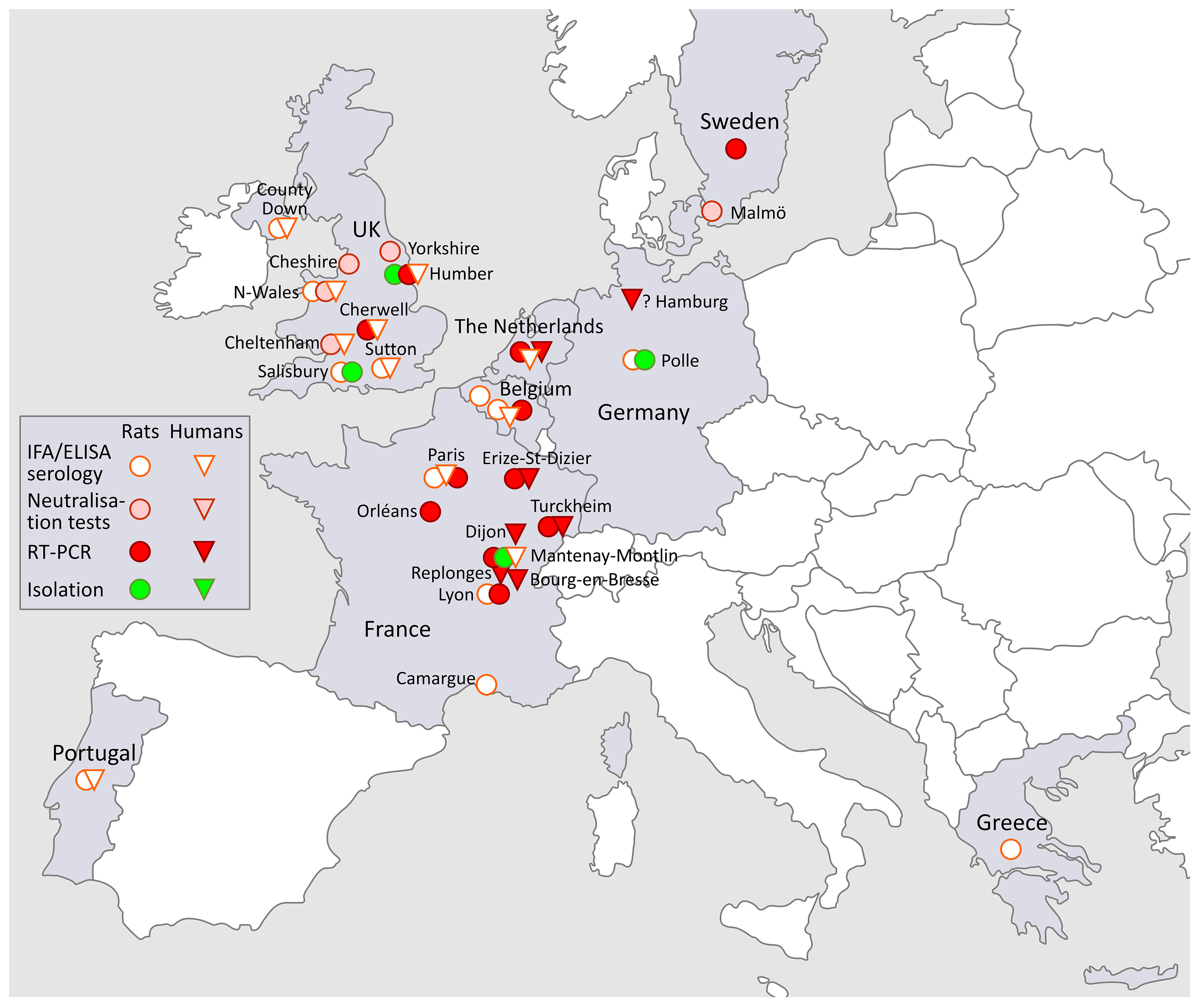
3.3. SEOV in Europe (Figure 2)
3.3.1. Russia
3.3.2. SEOV in Belgium and the Netherlands
3.3.3. SEOV in the UK
3.3.4. SEOV in Germany
3.3.5. SEOV in Portugal, Greece, France and Sweden
3.4. SEOV in the Americas (Figure 3)
3.4.1. SEOV in North America
3.4.2. SEOV in South America
3.4.3. Conclusions For, and Table of American SEOV-HFRS Cases
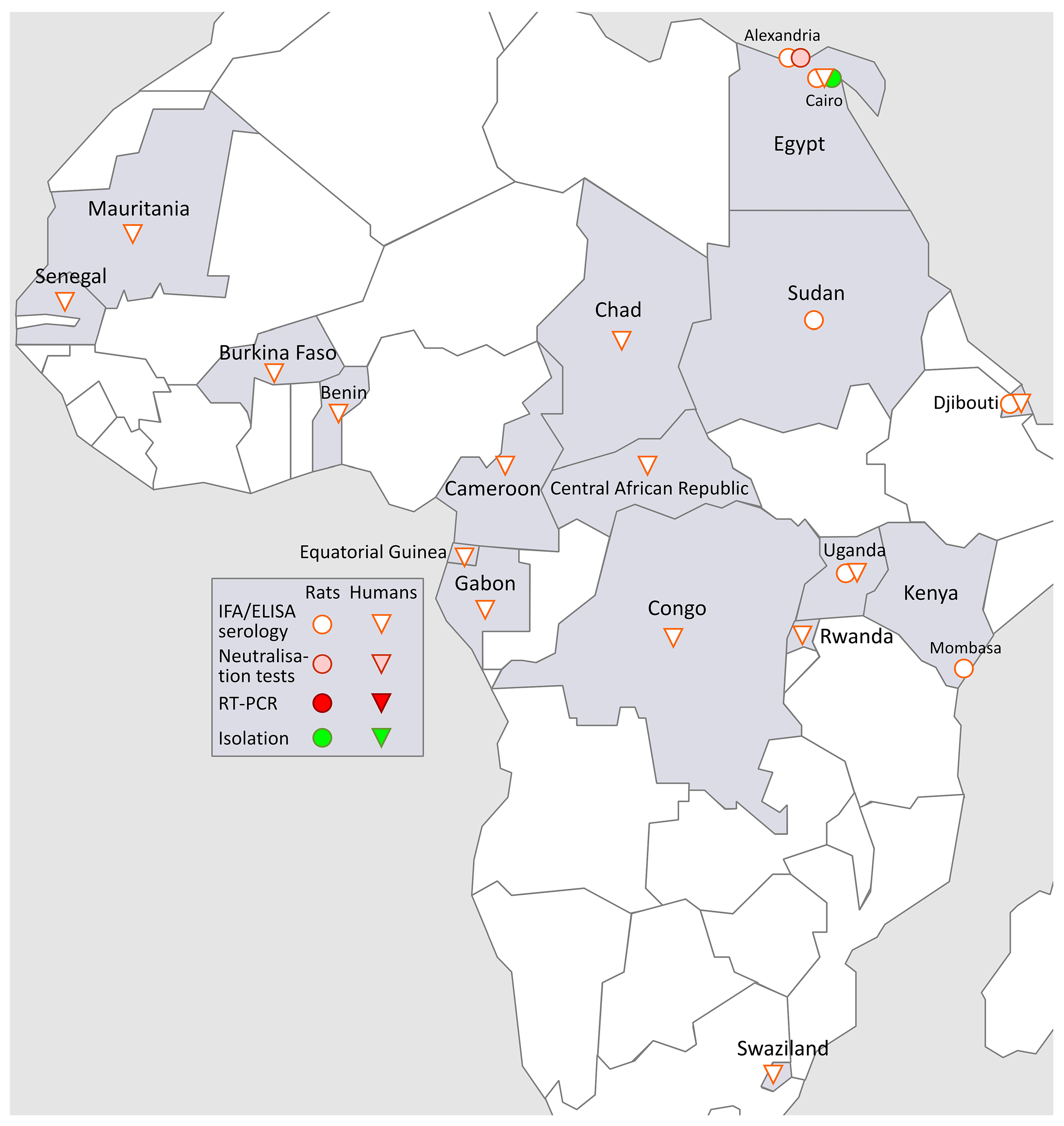
3.5. SEOV in Africa (Figure 4)
4. Current Situation
5. Conclusions
Biographical Sketch
Funding
Acknowledgments
Conflicts of Interest
References
- Clement, J.; LeDuc, J.W.; McElhinney, L.M.; Reynes, J.M.; Van Ranst, M.; Calisher, C.H. Clinical Characteristics of Ratborne Seoul Hantavirus Disease. Emerg. Infect. Dis. 2019, 25, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Kerins, J.L.; Koske, S.E.; Kazmierczak, J.; Austin, C.; Gowdy, K.; Dibernardo, A. Outbreak of Seoul virus among rats and rat owners—United States and Canada, 2017. Can. Commun. Dis. Rep. 2018, 44, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Fill, M.A.; Mullins, H.; May, A.S.; Henderson, H.; Brown, S.M.; Chiang, C.F.; Patel, N.R.; Klena, J.D.; de St Maurice, A.; Knust, B.; et al. Notes from the Field: Multiple Cases of Seoul Virus Infection in a Household with Infected Pet Rats—Tennessee, December 2016-April 2017. Mmwr. Morb. Mortal. Weekly Rep. 2017, 66, 1081–1082. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rubin, R. Pet Rats Infect Daughter, Mother with Hantavirus. JAMA 2017, 318, 1969. [Google Scholar] [CrossRef] [PubMed]
- Jameson, L.J.; Taori, S.K.; Atkinson, B.; Levick, P.; Featherstone, C.A.; van der Burgt, G.; McCarthy, N.; Hart, J.; Osborne, J.C.; Walsh, A.L.; et al. Pet rats as a source of hantavirus in England and Wales, 2013. Euro Surveill. 2013, 18, 20415. [Google Scholar] [PubMed]
- Taori, S.K.; Jameson, L.J.; Campbell, A.; Drew, P.J.; McCarthy, N.D.; Hart, J.; Osborne, J.C.; Sudhanva, M.; Brooks, T.J. UK hantavirus, renal failure, and pet rats. Lancet 2013, 381, 1070. [Google Scholar] [CrossRef]
- Reynes, J.M.; Carli, D.; Bour, J.B.; Boudjeltia, S.; Dewilde, A.; Gerbier, G.; Nussbaumer, T.; Jacomo, V.; Rapt, M.P.; Rollin, P.E.; et al. Seoul Virus Infection in Humans, France, 2014–2016. Emerg. Infect. Dis. 2017, 23, 973–977. [Google Scholar] [CrossRef]
- Lee, H.W.; Baek, L.J.; Johnson, K.M. Isolation of Hantaan virus, the etiologic agent of Korean hemorrhagic fever, from wild urban rats. J. Infect. Dis. 1982, 146, 638–644. [Google Scholar] [CrossRef]
- LeDuc, J.W.; Smith, G.A.; Johnson, K.M. Hantaan-like viruses from domestic rats captured in the United States. Am. J. Trop. Med. Hyg. 1984, 33, 992–998. [Google Scholar] [CrossRef]
- Clement, J.; McKenna, P.; Leirs, H.; Verhagen, R.; Lefevre, A.; Song, G.; Tkachenko, E.; Van der Groen, G. Hantavirus Infections in Rodents. In Virus Infections of Rodents and Lagomorphs/Osterhaus; ADME, Ed.; Elsevier: Amsterdam, The Netherlands, 1994; pp. 295–316. [Google Scholar]
- LeDuc, J.W.; Smith, G.A.; Childs, J.E.; Pinheiro, F.P.; Maiztegui, J.I.; Niklasson, B.; Antoniades, A.; Robinson, D.M.; Khin, M.; Shortridge, K.F.; et al. Global survey of antibody to Hantaan-related viruses among peridomestic rodents. Bull. World Health Organ. 1986, 64, 139–144. [Google Scholar]
- Glass, G.E.; Watson, A.J.; LeDuc, J.W.; Childs, J.E. Domestic cases of hemorrhagic fever with renal syndrome in the United States. Nephron 1994, 68, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.E.; Klein, S.L.; Glass, G.E. A Case Study of Two Rodent-Borne Viruses: Not Always the Same Old Suspects. Front. Ecol. Evol. 2019, 7. [Google Scholar] [CrossRef]
- (World Health Organisation), W.H.O. Haemorrhagic fever with renal syndrome: Memorandum from a WHO meeting. Bull. World Health Organ. 1983, 61, 269–275. [Google Scholar]
- Duchin, J.S.; Koster, F.T.; Peters, C.J.; Simpson, G.L.; Tempest, B.; Zaki, S.R.; Ksiazek, T.G.; Rollin, P.E.; Nichol, S.; Umland, E.T.; et al. Hantavirus pulmonary syndrome: A clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group. N. Engl. J. Med. 1994, 330, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.; Heyman, P.; McKenna, P.; Colson, P.; Avsic-Zupanc, T. The hantaviruses of Europe: From the bedside to the bench. Emerg. Infect. Dis. 1997, 3, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.; Neild, G.; Hinrichsen, S.L.; Crescente, J.A.; Van Ranst, M. Urban leptospirosis versus urban hantavirus infection in Brazil. Lancet 1999, 354, 2003–2004. [Google Scholar] [CrossRef]
- Clement, J.; Neild, G.H.; Maes, P.; Leirs, H.; Matthys, P.; Van Ranst, M. Symptomatic human hantavirus in the Americas. Emerg. Infect. Dis. 2007, 13, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.; Maes, P.; Van Ranst, M. Hemorrhagic fever with renal syndrome in the new, and hantavirus pulmonary syndrome in the old world: Paradi (se) gm lost or regained? Virus Res. 2014, 187, 55–58. [Google Scholar] [CrossRef]
- Song, G.; Hang, C.-S.; Liao, X.-X.; Qiu, X.-Z.; Gao, G.-Z.; Du, Y.-L.; Zhao, J.-N.; Xu, J.-K.; Kong, B.-X. Isolation of EHF-related causative agent from Rattus norvegicus captured from patients’ homes in endemic area of the mild type of hemorrhagic fever [in Chinese]. Acta Microbiol. Sin. 1982, 22, 373–377. [Google Scholar]
- Kitamura, T.; Morita, C.; Komatsu, T.; Sugiyama, K.; Arikawa, J.; Shiga, S.; Takeda, H.; Akao, Y.; Imaizumi, K.; Oya, A.; et al. Isolation of virus causing hemorrhagic fever with renal syndrome (HFRS) through a cell culture system. Jpn. J. Med. Sci. Biol. 1983, 36, 17–25. [Google Scholar] [CrossRef]
- Hofmann, J.; Weiss, S.; Kuhns, M.; Zinke, A.; Heinsberger, H.; Kruger, D.H. Importation of Human Seoul Virus Infection to Germany from Indonesia. Emerg. Infect. Dis. 2018, 24, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lee, P.W.; Johnson, K.M. Isolation of the etiologic agent of Korean hemorrhagic fever. J. Infect. Dis. 1978, 190, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Johnson, K.M. Laboratory-acquired infections with Hantaan virus, the etiologic agent of Korean hemorrhagic fever. J. Infect. Dis. 1982, 146, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.W.; Amyx, H.L.; Gibbs, C.J., Jr.; Gajdusek, D.C.; Lee, H.W. Propagation of Korean hemorrhagic fever virus in laboratory rats. Infect. Immun. 1981, 31, 334–338. [Google Scholar]
- Lee, H.W. Hemorrhagic Fever with Renal Syndrome. In Letters and Annual Research Report, 1959–1993; 1994; Tome II; pp. 804, 807. [Google Scholar]
- Nuzum, E.O.; Rossi, C.A.; Stephenson, E.H.; LeDuc, J.W. Aerosol transmission of Hantaan and related viruses to laboratory rats. Am. J. Trop. Med. Hyg. 1988, 38, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ahn, C.; Han, J.S.; Kim, S.; Lee, J.S.; Lee, P.W. Hemorrhagic fever with renal syndrome caused by the Seoul virus. Nephron 1995, 71, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Wong, T.W.; Yap, E.H.; Tan, H.C.; Lee, H.W.; Chu, Y.K.; Lee, P.W. Haemorrhagic fever with renal syndrome involving the liver. Med. J. Aust. 1987, 147, 248–249. [Google Scholar]
- Chan, Y.C.; Wong, T.W.; Yap, E.H. Haemorrhagic fever with renal syndrome: Clinical, virological and epidemiological perspectives. Ann. Acad. Med. Singap. 1987, 16, 696–701. [Google Scholar]
- Wong, T.W.; Chan, Y.C.; Yap, E.H.; Joo, Y.G.; Lee, H.W.; Lee, P.W.; Yanagihara, R.; Gibbs, C.J., Jr.; Gajdusek, D.C. Serological evidence of hantavirus infection in laboratory rats and personnel. Int. J. Epidemiol. 1988, 17, 887–890. [Google Scholar] [CrossRef]
- Wong, T.W.; Chan, Y.C.; Joo, Y.G.; Lee, H.W.; Lee, P.W.; Yanagihara, R. Hantavirus infections in humans and commensal rodents in Singapore. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 248–251. [Google Scholar] [CrossRef]
- Johansson, P.; Yap, G.; Low, H.T.; Siew, C.C.; Kek, R.; Ng, L.C.; Bucht, G. Molecular characterization of two hantavirus strains from different rattus species in Singapore. Virol. J. 2010, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Elwell, M.R.; Ward, G.S.; Tingpalapong, M.; LeDuc, J.W. Serologic evidence of Hantaan-like virus in rodents and man in Thailand. Southeast Asian J. Trop. Med. Public Health 1985, 16, 349–354. [Google Scholar] [PubMed]
- Plyusnina, A.; Ibrahim, I.N.; Plyusnin, A. A newly recognized hantavirus in the Asian house rat (Rattus tanezumi) in Indonesia. J. Gen. Virol. 2009, 90, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Reynes, J.M.; Razafindralambo, N.K.; Lacoste, V.; Olive, M.M.; Barivelo, T.A.; Soarimalala, V.; Heraud, J.M.; Lavergne, A. Anjozorobe hantavirus, a new genetic variant of Thailand virus detected in rodents from Madagascar. Vector Borne Zoonotic Dis. 2014, 14, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Filippone, C.; Castel, G.; Murri, S.; Beaulieux, F.; Ermonval, M.; Jallet, C.; Wise, E.L.; Ellis, R.J.; Marston, D.A.; McElhinney, L.M.; et al. Discovery of hantavirus circulating among Rattus rattus in French Mayotte island, Indian Ocean. J. Gen. Virol. 2016, 97, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.K.; Rossi, C.; Leduc, J.W.; Lee, H.W.; Schmaljohn, C.S.; Dalrymple, J.M. Serological relationships among viruses in the Hantavirus genus, family Bunyaviridae. Virology 1994, 198, 196–204. [Google Scholar] [CrossRef]
- Lee, H.W. Global distribution and molecular biological characteristics of Hantaviruses. J. Kor. Soc. Virol. 1986, 16, 1–5. [Google Scholar]
- Shortridge, K.F.; Lee, H.W.; Le Duc, J.W.; Wong, T.W.; Chau, G.W.; Rosen, L. Serological evidence of Hantaan-related viruses in Hong Kong. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 400–402. [Google Scholar] [CrossRef]
- Reynes, J.M.; Soares, J.L.; Hue, T.; Bouloy, M.; Sun, S.; Kruy, S.L.; Flye Sainte Marie, F.; Zeller, H. Evidence of the presence of Seoul virus in Cambodia. Microbes Infect. 2003, 5, 769–773. [Google Scholar] [CrossRef]
- Henttonen, H.; Buchy, P.; Suputtamongkol, Y.; Jittapalapong, S.; Herbreteau, V.; Laakkonen, J.; Chaval, Y.; Galan, M.; Dobigny, G.; Charbonnel, N.; et al. Recent discoveries of new hantaviruses widen their range and question their origins. Ann. N. Y. Acad. Sci. 2008, 1149, 84–89. [Google Scholar] [CrossRef]
- Truong, T.T.; Yoshimatsu, K.; Araki, K.; Lee, B.H.; Nakamura, I.; Endo, R.; Shimizu, K.; Yasuda, S.P.; Koma, T.; Taruishi, M.; et al. Molecular epidemiological and serological studies of hantavirus infection in northern Vietnam. J. Vet. Med. Sci. 2009, 71, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Huong, V.T.; Yoshimatsu, K.; Luan, V.D.; Tuan le, V.; Nhi, L.; Arikawa, J.; Nguyen, T.M. Hemorrhagic fever with renal syndrome, Vietnam. Emerg. Infect. Dis. 2010, 16, 363–365. [Google Scholar] [CrossRef]
- Luan, V.D.; Yoshimatsu, K.; Endo, R.; Taruishi, M.; Huong, V.T.; Dat, D.T.; Tien, P.C.; Shimizu, K.; Koma, T.; Yasuda, S.P.; et al. Studies on hantavirus infection in small mammals captured in southern and central highland area of Vietnam. J. Vet. Med. Sci. 2012, 74, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Koma, T.; Yoshimatsu, K.; Yasuda, S.P.; Li, T.; Amada, T.; Shimizu, K.; Isozumi, R.; Mai, L.T.; Hoa, N.T.; Nguyen, V.; et al. A survey of rodent-borne pathogens carried by wild Rattus spp. in Northern Vietnam. Epidemiol. Infect. 2013, 141, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Van Cuong, N.; Carrique-Mas, J.; Vo Be, H.; An, N.N.; Tue, N.T.; Anh, N.L.; Anh, P.H.; Phuc, N.T.; Baker, S.; Voutilainen, L.; et al. Rodents and risk in the Mekong Delta of Vietnam: Seroprevalence of selected zoonotic viruses in rodents and humans. Vector Borne Zoonotic Dis. 2015, 15, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Suputthamongkol, Y.; Nitatpattana, N.; Chayakulkeeree, M.; Palabodeewat, S.; Yoksan, S.; Gonzalez, J.P. Hantavirus infection in Thailand: First clinical case report. Southeast Asian J. Trop. Med. Public Health 2005, 36, 700–703. [Google Scholar] [PubMed]
- Pattamadilok, S.; Lee, B.H.; Kumperasart, S.; Yoshimatsu, K.; Okumura, M.; Nakamura, I.; Araki, K.; Khoprasert, Y.; Dangsupa, P.; Panlar, P.; et al. Geographical distribution of hantaviruses in Thailand and potential human health significance of Thailand virus. Am. J. Trop. Med. Hyg. 2006, 75, 994–1002. [Google Scholar] [CrossRef]
- Tamura, M. Occurrence of Epidemic Hemorrhagic Fever in Osaka City: First Cases found in Japan with characteristic Feature of marked Proteinuria. Biken J. 1964, 7, 79–94. [Google Scholar]
- Mantula, P.S.; Outinen, T.K.; Clement, J.P.G.; Huhtala, H.S.A.; Porsti, I.H.; Vaheri, A.; Mustonen, J.T.; Makela, S.M. Glomerular Proteinuria Predicts the Severity of Acute Kidney Injury in Puumala Hantavirus-Induced Tubulointerstitial Nephritis. Nephron 2017, 136, 193–201. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, P.W.; Tamura, M.; Tamura, T.; Okuno, Y. Etiological relation between Korean hemorrhagic fever and epidemic hemorrhagic fever in Japan. Biken J. 1979, 22, 41–45. [Google Scholar]
- Morimoto, Y.; Kishimoto, S.; Yamanouchi, T.; Yamanishi, K.; Takahashi, M.; Kawamata, J.; Tamura, T.; Lee, H.W. Clinical features of hemorrhagic fever with renal syndrome (epidemic hemorrhagic fever) in Japan: A clinical and laboratory study on 27 cases in Osaka in the 1960’s and the 1980’s. Kansenshogaku Zasshi 1985, 59, 439–458. [Google Scholar] [CrossRef][Green Version]
- Morita, C.; Takeuchi, Y.; Matsura, Y.; Kitamura, T. The prevalence of Hantaan viruses among Rattus norvegicus in Tokyo in the 1960’s. Zent. Vet. B 1989, 36, 557–558. [Google Scholar] [CrossRef]
- Lee, H.W. Correspondence on the Discovery and Original Investigations on HFRS and Hantaviruses; Lee, H.-W., Ed.; The Hantaan Life Science Foundation: Seoul, Korea, 2000; Tome I-II; pp. 297–298, 581, 820–821, 826–827, 1747–1748, 1907–1908. [Google Scholar]
- Umenai, T.; Lee, H.W.; Lee, P.W.; Saito, T.; Toyoda, T.; Hongo, M.; Yoshinaga, K.; Nobunaga, T.; Horiuchi, T.; Ishida, N. Korean haemorrhagic fever in staff in an animal laboratory. Lancet 1979, 1, 1314–1316. [Google Scholar] [CrossRef]
- Kawamata, J.; Yamanouchi, T.; Dohmae, K.; Miyamoto, H.; Takahaski, M.; Yamanishi, K.; Kurata, T.; Lee, H.W. Control of laboratory acquired hemorrhagic fever with renal syndrome (HFRS) in Japan. Lab. Anim. Sci. 1987, 37, 431–436. [Google Scholar] [PubMed]
- Yamanishi, K.; Dantas, J.R.; Takahashi, M.; Yamanouchi, T.; Domae, K.; Kawamata, J.; Kurata, T. Isolation of hemorrhagic fever with renal syndrome (HFRS) virus from a tumor specimen in a rat. Biken J. 1983, 26, 155–160. [Google Scholar]
- Arikawa, J.; Takashima, I.; Hashimoto, N.; Morita, C.; Sugiyama, K.; Matsuura, Y.; Shiga, S.; Kitamura, T. Epidemiological study of hemorrhagic fever with renal syndrome related virus infection among urban rats in two islands in Tokyo Bay, Japan. Acta Virol. 1985, 29, 66–72. [Google Scholar] [PubMed]
- Arikawa, J.; Takashima, I.; Hashimoto, N.; Takahashi, K.; Yagi, K.; Hattori, K. Epidemiological studies of hemorrhagic fever with renal syndrome (HFRS) related virus infection among urban rats in Hokkaido, Japan. Arch. Virol. 1986, 88, 231–240. [Google Scholar] [CrossRef]
- Sugiyama, K.; Minaba, K.; Morita, C.; Kitamura, H.; Yamada, T.; Ogura, K.; Tanaka, R.; Tanikawa, T.; Shiga, S.; Komatsu, T.; et al. Epizootiological study of hantavirus infection among Rattus norvegicus in Tokyo Bay area, Japan. Jpn. J. Med. Sci. Biol. 1993, 46, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Yamamoto, T.; Itoh, M.; Tsukada, K.; Yasue, N.; Lee, H.W. Clinical studies on hemorrhagic fever with renal syndrome found in Nagoya City University Medical School. Kidney Int. Suppl. 1991, 35, S84–S87. [Google Scholar]
- Sugiyama, K.; Takagi, K.; Kinjo, T.; Nakata, Y.; Komatsu, T.; Kitamura, T. Isolation and characterization of a hantavirus from Rattus norvegicus in a residential area of Nagoya City, Japan. J. Vet. Med. Sci. 1995, 57, 51–54. [Google Scholar] [CrossRef][Green Version]
- Kariwa, H.; Isegawa, Y.; Arikawa, J.; Takashima, I.; Ueda, S.; Yamanishi, K.; Hashimoto, N. Comparison of nucleotide sequences of M genome segments among Seoul virus strains isolated from eastern Asia. Virus Res. 1994, 33, 27–38. [Google Scholar] [CrossRef]
- Kariwa, H.; Yoshimatsu, K.; Araki, K.; Chayama, K.; Kumada, H.; Ogino, M.; Ebihara, H.; Murphy, M.E.; Mizutani, T.; Takashima, I.; et al. Detection of hantaviral antibodies among patients with hepatitis of unknown etiology in Japan. Microbiol. Immunol. 2000, 44, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, J.; Yoshimatsu, K.; Kariwa, H. Epidemiology and epizootiology of hantavirus infection in Japan. Jpn. J. Infect. Dis. 2001, 54, 95–102. [Google Scholar] [PubMed]
- Kariwa, H.; Yoshimatsu, K.; Arikawa, J. Hantavirus infection in east Asia. Comp. Immunol. Microbiol. Infect. Dis. 2007, 30, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.; Maes, P. Acute Kidney Injury and Hantavirus Disease. In Oxford Textbook of Clinical Nephrology, 4th ed.; Oxford University Press: Oxford, UK, 2015; pp. 2059–2066. [Google Scholar]
- Jiang, H.; Du, H.; Wang, L.M.; Wang, P.Z.; Bai, X.F. Hemorrhagic Fever with Renal Syndrome: Pathogenesis and Clinical Picture. Front. Cell. Infect. Microbiol. 2016, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.W.; Gajdusek, D.C.; Gibbs, C.J.; Xu, Z.Y. Aetiological relation between Korean haemorrhagic fever with renal syndrome in People’s Republic of China. Lancet 1980, 1, 819–820. [Google Scholar] [CrossRef]
- Song, G.; Hang, C.S.; Liao, H.X.; Fu, J.L.; Gao, G.Z.; Qiu, H.L.; Zhang, Q.F. Antigenic difference between viral strains causing classical and mild types of epidemic hemorrhagic fever with renal syndrome in China. J. Infect. Dis. 1984, 150, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Hang, C.S.; Qui, X.Z.; Ni, D.S.; Liao, H.X.; Gao, G.Z.; Du, Y.L.; Xu, J.K.; Wu, Y.S.; Zhao, J.N.; et al. Etiologic studies of epidemic hemorrhagic fever (hemorrhagic fever with renal syndrome). J. Infect. Dis. 1983, 147, 654–659. [Google Scholar] [CrossRef]
- Song, G. Epidemiological progresses of hemorrhagic fever with renal syndrome in China. Chin. Med. J. 1999, 112, 472–477. [Google Scholar]
- Outinen, T.K.; Mäkelä, S.; Clement, J.; Paakkala, A.; Pörsti, I.; Mustonen, J. Community acquired severe acute kidney injury caused by hantavirus-induced hemorrhagic fever with renal syndrome has a favorable outcome. Nephron 2015, 130, 182–190. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zou, Y.; Fu, Z.F.; Plyusnin, A. Hantavirus infections in humans and animals, China. Emerg. Infect. Dis. 2010, 16, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, S.; Hen, S.; Liu, L.; Yang, W.; Zhang, W. Survey of outbreak of hemorrhagic fever with renal syndrome as a result of experimental white rat infection. Zhonghua Liu Xing Bing Xue Za Zhi 1985, 4, 233–235. [Google Scholar]
- Liu, R.; Chen, H. The risk and prevention of hemorrhagic fever with renal syndrome transmitted by laboratory rats. Chin. J. Vector Bio. Control 1991, 2, 250–254. [Google Scholar]
- Zhang, Y.-Z.; Dong, X.; Li, X.; Ma, C.; Xiong, H.-P.; Yan, G.-J.; Gao, N.; Jiang, D.-M.; Li, M.-H.; Li, L.-P. Seoul virus and hantavirus disease, Shenyang, People’s Republic of China. Emerg. Infect. Dis. 2009, 15, 200. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yoshimatsu, K.; Ebihara, H.; Ogino, M.; Araki, K.; Kariwa, H.; Wang, Z.; Luo, Z.; Li, D.; Hang, C.; et al. Genetic diversity of hantaviruses isolated in china and characterization of novel hantaviruses isolated from Niviventer confucianus and Rattus rattus. Virology 2000, 278, 332–345. [Google Scholar] [CrossRef]
- Clement, J. Considerations about a Chinese vaccine preventing a national form of epidemic acute kidney injury (AKI). Infect. Dis. 2016, 48, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Vitarana, T.; Colombage, G.; Bandaranayake, V.; Lee, H.W. Hantavirus disease in Sri Lanka. Lancet 1988, 2, 1263. [Google Scholar]
- Gamage, C.D.; Yasuda, S.P.; Nishio, S.; Kularatne, S.A.; Weerakoon, K.; Rajapakse, J.; Nwafor-Okoli, C.; Lee, R.B.; Obayashi, Y.; Yoshimatsu, K.; et al. Serological evidence of Thailand virus-related hantavirus infection among suspected leptospirosis patients in Kandy, Sri Lanka. Jpn. J. Infect. Dis. 2011, 64, 72–75. [Google Scholar] [PubMed]
- Clement, J.; Maes, P.; Muthusethupathi, M.; Nainan, G.; van Ranst, M. First evidence of fatal hantavirus nephropathy in India, mimicking leptospirosis. Nephrol. Dial. Transpl. 2006, 21, 826–827. [Google Scholar] [CrossRef][Green Version]
- Maes, P.; Clement, J.; Rega Institute, University of Leuven, Belgium. Non-published data, 2009.
- Quelapio, I.D.; Villa, L.; Clarin, S.M.; Bacosa, M.; Tupasi, T.E. Seroepidemiology of Hantavirus in the Philippines. Int. J. Infect. Dis. 2000, 4, 104–107. [Google Scholar] [CrossRef]
- Kosasih, H.; Ibrahim, I.N.; Wicaksana, R.; Alisjahbana, B.; Hoo, Y.; Yo, I.H.; Antonjaya, U.; Widjaja, S.; Winoto, I.; Williams, M.; et al. Evidence of human hantavirus infection and zoonotic investigation of hantavirus prevalence in rodents in western Java, Indonesia. Vector Borne Zoonotic Dis. 2011, 11, 709–713. [Google Scholar] [CrossRef]
- Lie, K.C.; Aziz, M.H.; Kosasih, H.; Neal, A.; Halim, C.L.; Wulan, W.N.; Karyana, M.; Hadi, U. Case report: Two confirmed cases of human Seoul virus infections in Indonesia. BMC Infect. Dis. 2018, 18, 578. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Cameron, S.; Higgins, G.; Burrell, C. Are humans infected by Hantaviruses in Australia? Intern. Med. J. 2005, 35, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Chungue, E.; Rollin, P.E.; Saluzzo, J.F.; Gentile, B.; Roux, J.F.; Sureau, P. Serological survey of Hantavirus infection in French Polynesia. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 258. [Google Scholar] [CrossRef]
- Lloyd, G.; Jones, N. Infection of laboratory workers with hantavirus acquired from immunocytomas propagated in laboratory rats. J. Infect. 1986, 12, 117–125. [Google Scholar] [CrossRef]
- Pilaski, J.; Ellerich, C.; Kreutzer, T.; Lang, A.; Benik, W.; Pohl-Koppe, A.; Bode, L.; Vanek, E.; Autenrieth, I.B.; Bigos, K.; et al. Haemorrhagic fever with renal syndrome in Germany. Lancet 1991, 337, 111. [Google Scholar] [CrossRef]
- Desmyter, J.; LeDuc, J.W.; Johnson, K.M.; Brasseur, F.; Deckers, C.; van Ypersele de Strihou, C. Laboratory rat associated outbreak of haemorrhagic fever with renal syndrome due to Hantaan-like virus in Belgium. Lancet 1983, 2, 1445–1448. [Google Scholar] [CrossRef]
- De Strihou, C.v.Y. Acute oliguric interstitial nephritis. Kidney Int. 1979, 16, 751–765. [Google Scholar] [CrossRef]
- McKenna, P.; Clement, J.; Matthys, P.; Coyle, P.V.; McCaughey, C. Serological evidence of Hantavirus disease in Northern Ireland. J. Med. Virol. 1994, 43, 33–38. [Google Scholar] [CrossRef]
- McElhinney, L.; Marston, D.A.; Pounder, K.C.; Goharriz, H.; Wise, E.L.; Verner-Carlsson, J.; Jennings, D.; Johnson, N.; Civello, A.; Nunez, A.; et al. High prevalence of Seoul hantavirus in a breeding colony of pet rats. Epidemiol. Infect. 2017, 145, 3115–3124. [Google Scholar] [CrossRef]
- Lee, P.W.; Gibbs, C.J.; Gajdusek, D.C.; Xu, Z.Y. Evidence for Respiratory Infection and Airborne Dissemination of Hantavirus. In Proceedings of the 10th International Congress on tropical Medicine and Malaria, Manila, Philippines, 9–15 November 1980; p. 41. [Google Scholar]
- Osterhaus, A.; Spijkers, I. Hantavirus infections in the Netherlands. Ned. Tijdschr. Voor Geneeskd. 1984, 128, 2461–2462. [Google Scholar]
- Lloyd, G.; Bowen, E.T.; Jones, N.; Pendry, A. HFRS outbreak associated with laboratory rats in UK. Lancet 1984, 1, 1175–1176. [Google Scholar] [CrossRef]
- McKenna, P.; Van der Groen, G.; Hoofd, G.; Beelaert, G.; Leirs, H.; Verhagen, R.; Kints, J.; Cormont, F.; Nisol, F.; Bazin, H. Eradication of hantavirus infection among laboratory rats by application of caesarian section and a foster mother technique. J. Infect. 1992, 25, 181–190. [Google Scholar] [CrossRef]
- Groen, J.; Jordans, H.G.; Clement, J.P.; Rooijakkers, E.J.; UytdeHaag, F.G.; Dalrymple, J.; Van der Groen, G.; Osterhaus, A.D. Identification of Hantavirus serotypes by testing of post-infection sera in immunofluorescence and enzyme-linked immunosorbent assays. J. Med. Virol. 1991, 33, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Heyman, P.; Baert, K.; Plyusnina, A.; Cochez, C.; Lundkvist, A.; Esbroeck, M.V.; Goossens, E.; Vandenvelde, C.; Plyusnin, A.; Stuyck, J. Serological and genetic evidence for the presence of Seoul hantavirus in Rattus norvegicus in Flanders, Belgium. Scand. J. Infect. Dis. 2009, 41, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Verner-Carlsson, J.; Lohmus, M.; Sundstrom, K.; Strand, T.M.; Verkerk, M.; Reusken, C.; Yoshimatsu, K.; Arikawa, J.; van de Goot, F.; Lundkvist, A. First evidence of Seoul hantavirus in the wild rat population in the Netherlands. Infect. Ecol. Epidemiol. 2015, 5, 27215. [Google Scholar] [CrossRef][Green Version]
- Swanink, C.; Reimerink, J.; Gisolf, J.; de Vries, A.; Claassen, M.; Martens, L.; Waegemaekers, T.; Rozendaal, H.; Valkenburgh, S.; Hoornweg, T.; et al. Autochthonous Human Case of Seoul Virus Infection, the Netherlands. Emerg. Infect. Dis. 2018, 24, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Van der Groen, G.; Yamanishi, K.; McCormick, J.; Lloyd, G.; Tkachenko, E. Characterization of Hantaviruses using monoclonal antibodies. Acta Virol. 1987, 31, 499–503. [Google Scholar]
- Clement, J. Unpublished pioneer hantavirus serology 1987-1992 on suspected clinical cases. Queen Astrid Military Hospital: Brussels, Belgium.
- Shi, X.; McCaughey, C.; Elliott, R.M. Genetic characterisation of a Hantavirus isolated from a laboratory-acquired infection. J. Med. Virol. 2003, 71, 105–109. [Google Scholar] [CrossRef] [PubMed]
- McCaughey, C.; Montgomery, W.I.; Twomey, N.; Addley, M.; O’Neill, H.J.; Coyle, P.V. Evidence of hantavirus in wild rodents in Northern Ireland. Epidemiol. Infect. 1996, 117, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.; Jameson, L.; Meigh, R.; Brooks, T. Hantavirus: An infectious cause of acute kidney injury in the UK. BMJ Case Rep. 2014, 2014, bcr2014205529. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.G.; Williams, N.J.; Bennett, M.; Jennings, D.; Chantrey, J.; McElhinney, L.M. Detection of Seoul virus in wild brown rats (Rattus norvegicus) from pig farms in Northern England. Vet. Rec. 2019, 184, 525. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.; Mesquita, M.; Alves, M.; Filipi, A. Febre Hemorragica com Sindroma Renal-Primeiro caso clinico diagnosticado em Portugal. Sep. Rev. Port. Doenas Infecc. 1993, 16, 209–214. [Google Scholar]
- Filipe, A.R.; Andrade, H.R.; Sommer, A.I.; Traavik, T. Hantaviral antigens and antibodies in wild rodents in Portugal. Acta Virol 1991, 35, 287–291. [Google Scholar] [PubMed]
- Papa, A.; Mills, J.N.; Kouidou, S.; Ma, B.; Papadimitriou, E.; Antoniadis, A. Preliminary characterization and natural history of hantaviruses in rodents in northern Greece. Emerg. Infect. Dis. 2000, 6, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Dournon, E.; Moriniere, B.; Matheron, S.; Girard, P.M.; Gonzalez, J.P.; Hirsch, F.; McCormick, J.B. HFRS after a wild rodent bite in the Haute-Savoie—And risk of exposure to Hantaan-like virus in a Paris laboratory. Lancet 1984, 1, 676–677. [Google Scholar] [CrossRef]
- Ragnaud, J.M.; Lamouliatte, H.; Paix, M.A.; Fleury, H.; Roy, J.; Magnol, R. Hemorrhagic fever with renal syndrome: A case with jaundice. Gastroenterol. Clin. Biol. 1986, 10, 686. [Google Scholar]
- Le Guenno, B. Les hantavirus. Méd. Mal. Infect. 1997, 27, 703–710. [Google Scholar] [CrossRef]
- Bour, A.; Reynes, J.M.; Plaisancie, X.; Dufour, J.F. Seoul hantavirus infection-associated hemorrhagic fever with renal syndrome in France: A case report. Rev. Med. Interne 2016, 37, 493–496. [Google Scholar] [CrossRef]
- Mace, G.; Feyeux, C.; Mollard, N.; Chantegret, C.; Audia, S.; Rebibou, J.M.; Spagnolo, G.; Bour, J.B.; Denoyel, G.A.; Sagot, P.; et al. Severe Seoul hantavirus infection in a pregnant woman, France, October 2012. Euro Surveill. 2013, 18, 20464. [Google Scholar]
- Clement, J.; Vergote, V.; Laenen, L.; Van Ranst, M. Distinguishing between hantavirus-induced haemorrhagic fever with renal syndrome and pregnancy-induced liver pathologies (AFLP and HELLP syndromes. Euro Surveill. 2013, 18, 20493. [Google Scholar] [PubMed]
- Heyman, P.; Plyusnina, A.; Berny, P.; Cochez, C.; Artois, M.; Zizi, M.; Pirnay, J.P.; Plyusnin, A. Seoul hantavirus in Europe: First demonstration of the virus genome in wild Rattus norvegicus captured in France. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Dupinay, T.; Pounder, K.C.; Ayral, F.; Laaberki, M.H.; Marston, D.A.; Lacote, S.; Rey, C.; Barbet, F.; Voller, K.; Nazaret, N.; et al. Detection and genetic characterization of Seoul virus from commensal brown rats in France. Virol. J. 2014, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Reynes, J.M.; Inst. Pasteur, Paris, France. Unpublished data, 2018.
- Lundkvist, A.; Verner-Carlsson, J.; Plyusnina, A.; Forslund, L.; Feinstein, R.; Plyusnin, A. Pet rat harbouring Seoul hantavirus in Sweden, June 2013. Euro Surveill. 2013, 18. [Google Scholar] [CrossRef]
- Hindrichsen, S.; De Andrade, A.M.; Clement, J.; Leirs, H.; Mc Kenna, P.; Matthys, P.; Neild, G. Hantavirus infection in Brazilian patients from Recife with suspected leptospirosis. Lancet 1993, 341, 50. [Google Scholar] [CrossRef]
- Tsai, T.; Bauer, S.; Sasso, D.; McCormick, J.; Bradford, H.; Caraway, C.; McFarland, L.; Medrano, O.; Soulie, G. Preliminary evidence that Hantaan or a closely related virus is enzootic in domestic rodents. N. Engl. J. Med. 1982, 307, 623–624. [Google Scholar]
- LeDuc, J.W.; Smith, G.A.; Bagley, L.R.; Hasty, S.E.; Johnson, K.M. Preliminary evidence that Hantaan or a closely related virus is enzootic in domestic rodents. N. Engl. J. Med. 1982, 307, 624. [Google Scholar]
- Gibbs, C.J., Jr.; Takenaka, A.; Franko, M.; Gajdusek, D.C.; Griffin, M.D.; Chields, J.; Korch, G.W.; Wartzok, D. Seroepidemiology of Hantaan virus. Lancet 1982, 2, 1406–1407. [Google Scholar] [CrossRef]
- Stone, R. A rogues’ gallery of hantaviruses. Science 1993, 262, 835. [Google Scholar] [CrossRef]
- Tsai, T.F.; Bauer, S.P.; Sasso, D.R.; Whitfield, S.G.; McCormick, J.B.; Caraway, T.C.; McFarland, L.; Bradford, H.; Kurata, T. Serological and virological evidence of a Hantaan virus-related enzootic in the United States. J. Infect. Dis. 1985, 152, 126–136. [Google Scholar] [CrossRef]
- LeDuc, J.W.; Smith, G.A.; Macy, M.; Hay, R.J. Certified cell lines of rat origin appear free of infection with Hantavirus. J. Infect. Dis. 1985, 152, 1082–1083. [Google Scholar] [CrossRef]
- Yanagihara, R.; Chin, C.T.; Weiss, M.B.; Gajdusek, D.C.; Diwan, A.R.; Poland, J.B.; Kleeman, K.T.; Wilfert, C.M.; Meiklejohn, G.; Glezen, W.P. Serological evidence of Hantaan virus infection in the United States. Am. J. Trop. Med. Hyg. 1985, 34, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.E.; Korch, G.W.; Glass, G.E.; LeDuc, J.W.; Shah, K.V. Epizootiology of Hantavirus infections in Baltimore: Isolation of a virus from Norway rats, and characteristics of infected rat populations. Am. J. Epidemiol. 1987, 126, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.E.; Glass, G.E.; Korch, G.W.; Arthur, R.R.; Shah, K.V.; Glasser, D.; Rossi, C.; Leduc, J.W. Evidence of human infection with a rat-associated Hantavirus in Baltimore, Maryland. Am. J. Epidemiol. 1988, 127, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.E.; Glass, G.E.; Ksiazek, T.G.; Rossi, C.A.; Oro, J.G.; Leduc, J.W. Human-rodent contact and infection with lymphocytic choriomeningitis and Seoul viruses in an inner-city population. Am. J. Trop. Med. Hyg. 1991, 44, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Hinson, E.R.; Shone, S.M.; Zink, M.C.; Glass, G.E.; Klein, S.L. Wounding: The primary mode of Seoul virus transmission among male Norway rats. Am. J. Trop. Med. Hyg. 2004, 70, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Easterbrook, J.D.; Shields, T.; Klein, S.L.; Glass, G.E. Norway rat population in Baltimore, Maryland, 2004. Vector Borne Zoonotic Dis. 2005, 5, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Easterbrook, J.D.; Kaplan, J.B.; Vanasco, N.B.; Reeves, W.K.; Purcell, R.H.; Kosoy, M.Y.; Glass, G.E.; Watson, J.; Klein, S.L. A survey of zoonotic pathogens carried by Norway rats in Baltimore, Maryland, USA. Epidemiol. Infect. 2007, 135, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, R. Hantavirus infection in the United States: Epizootiology and epidemiology. Rev. Infect. Dis. 1990, 12, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Firth, C.; Bhat, M.; Firth, M.A.; Williams, S.H.; Frye, M.J.; Simmonds, P.; Conte, J.M.; Ng, J.; Garcia, J.; Bhuva, N.P.; et al. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. mBio 2014, 5, e01933-14. [Google Scholar] [CrossRef]
- Childs, J.E.; McLafferty, S.L.; Sadek, R.; Miller, G.L.; Khan, A.S.; DuPree, E.R.; Advani, R.; Mills, J.N.; Glass, G.E. Epidemiology of rodent bites and prediction of rat infestation in New York City. Am. J. Epidemiol. 1998, 148, 78–87. [Google Scholar] [CrossRef]
- Cross, R.W.; Waffa, B.; Freeman, A.; Riegel, C.; Moses, L.M.; Bennett, A.; Safronetz, D.; Fischer, E.R.; Feldmann, H.; Voss, T.G.; et al. Old World hantaviruses in rodents in New Orleans, Louisiana. Am. J. Trop. Med. Hyg. 2014, 90, 897–901. [Google Scholar] [CrossRef] [PubMed]
- LeDuc, J.W.; Smith, G.A.; Pinheiro, F.P.; Vasconcelos, P.F.; Rosa, E.S.; Maiztegui, J.I. Isolation of a Hantaan-related virus from Brazilian rats and serologic evidence of its widespread distribution in South America. Am. J. Trop. Med. Hyg. 1985, 34, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Iversson, L.B.; da Rosa, A.P.; Rosa, M.D.; Lomar, A.V.; Sasaki Mda, G.; LeDuc, J.W. Human infection by Hantavirus in southern and southeastern Brazil. Rev. Assoc. Med. Bras. (1992) 1994, 40, 85–92. [Google Scholar]
- Clement, J.; Hinrichsen, S.; Crescente, J.; Bigaignon, G.; Yersin, C.; Muthusethupathi, M.; Nainan, G.; Hartskeerl, R.; Terpstra, W.; Lundkvist, A. Hantavirus-induced hemorrhagic fever with renal syndrome (HFRS) has to be considered in the differential diagnosis of leptospirosis-suspected cases in the New and the Old World. Am. J. Trop. Med. Hyg. 1999, 61, 406–407. [Google Scholar]
- Costa, F.; Porter, F.H.; Rodrigues, G.; Farias, H.; de Faria, M.T.; Wunder, E.A.; Osikowicz, L.M.; Kosoy, M.Y.; Reis, M.G.; Ko, A.I.; et al. Infections by Leptospira interrogans, Seoul virus, and Bartonella spp. among Norway rats (Rattus norvegicus) from the urban slum environment in Brazil. Vector Borne Zoonotic Dis. 2014, 14, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.I.; Galvao Reis, M.; Ribeiro Dourado, C.M.; Johnson, W.D., Jr.; Riley, L.W. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet 1999, 354, 820–825. [Google Scholar] [CrossRef]
- Sunil-Chandra, N.; Clement, J.; Maes, P.; De Silva, H.; Van Esbroeck, M.; Van Ranst, M. Concomitant leptospirosis-hantavirus co-infection in acute patients hospitalized in Sri Lanka: Implications for a potentially worldwide underestimated problem. Epidemiol. Infect. 2015, 143, 2081–2093. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.; Van Esbroeck, M.; Lagrou, K.; Verschueren, J.; Sunil-Chandra, N.P.; Van Ranst, M. Leptospirosis versus hantavirus infections in the Netherlands and in Belgium, 2000 to 2014. Euro Surveill. 2014, 19. [Google Scholar] [CrossRef]
- Weissenbacher, M.C.; Merani, M.S.; Hodara, V.L.; de Villafane, G.; Gajdusek, D.C.; Chu, Y.K.; Lee, H.W. Hantavirus infection in laboratory and wild rodents in Argentina. Medicina 1990, 50, 43–46. [Google Scholar]
- Cueto, G.R.; Cavia, R.; Bellomo, C.; Padula, P.J.; Suarez, O.V. Prevalence of hantavirus infection in wild Rattus norvegicus and R. rattus populations of Buenos Aires City, Argentina. Trop. Med. Int. Health 2008, 13, 46–51. [Google Scholar] [CrossRef]
- Garcia, P.M.; Percy, S.; Herrera, A.L.; Donaires, F.; Alvarez, C.; Arrasco, J.; Cabezas, C.; Rodriguez, H.; Mamani, E. Etiologic confirmation of the first two cases of human hantavirosis in Peru. Rev. Peru Med. Exp. Salud Publica 2011, 28, 566–567. [Google Scholar] [PubMed]
- Woods, C.; Palekar, R.; Kim, P.; Blythe, D.; de Senarclens, O.; Feldman, K.; Farnon, E.C.; Rollin, P.E.; Albarino, C.G.; Nichol, S.T.; et al. Domestically acquired Seoul virus causing hemorrhagic fever with renal syndrome-Maryland, 2008. Clin. Infect. Dis. 2009, 49, e109–e112. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.F.; Sethi, V.; Petroll, A.E.; Kazmierczak, J.; Erickson, B.R.; Nichol, S.T.; Rollin, P.E.; Davis, J.P. Seoul virus infection in a Wisconsin patient with recent travel to China, March 2009: First documented case in the Midwestern United States. Am. J. Trop. Med. Hyg. 2010, 83, 1266–1268. [Google Scholar] [CrossRef] [PubMed]
- Roig, I.L.; Musher, D.M.; Tweardy, D.J. Severe pulmonary involvement in a case attributed to domestically acquired Seoul hantavirus in the United States. Clin. Infect. Dis. 2012, 54, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.; Colson, P.; Saegeman, V.; Lagrou, K.; Van Ranst, M. ‘Bedside assessment’of acute hantavirus infections and their possible classification into the spectrum of haemophagocytic syndromes. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.; Colson, P.; Saegeman, V.; Lagrou, K.; Van Ranst, M. Are hantavirus infections also part of the rapidly growing spectrum of an infection-triggered reactive haemophagocytic syndrome? Clin. Microbiol. Infect. 2016, 22, 745–746. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.; Saluzzo, J.; van der Groen, G. Evidence for hantavirus disease (HVD) in Africa? Kidney Int. 1987, 32, 603–604. [Google Scholar]
- Rodier, G.; Soliman, A.K.; Bouloumie, J.; Kremer, D. Presence of antibodies to Hantavirus in rat and human populations of Djibouti. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 160–161. [Google Scholar] [CrossRef][Green Version]
- Botros, B.A.; Sobh, M.; Wierzba, T.; Arthur, R.R.; Mohareb, E.W.; Frenck, R.; El Refaie, A.; Mahmoud, I.; Chapman, G.D.; Graham, R.R. Prevalence of hantavirus antibody in patients with chronic renal disease in Egypt. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 331–336. [Google Scholar] [CrossRef]
- Gonzalez, J.; McCormick, J.; Baudon, D.; Gautun, J.; Meunier, D.; Dournon, E.; Georges, A. Serological evidence for Hantaan-related virus in Africa. Lancet 1984, 324, 1036–1037. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; Josse, R.; Johnson, E.D.; Merlin, M.; Georges, A.J.; Abandja, J.; Danyod, M.; Delaporte, E.; Dupont, A.; Ghogomu, A.; et al. Antibody prevalence against haemorrhagic fever viruses in randomized representative Central African populations. Res. Virol 1989, 140, 319–331. [Google Scholar] [CrossRef]
- Klempa, B.; Koivogui, L.; Sylla, O.; Koulemou, K.; Auste, B.; Kruger, D.H.; ter Meulen, J. Serological evidence of human hantavirus infections in Guinea, West Africa. J. Infect. Dis. 2010, 201, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, P.; Tia, M.; Alabi, A.; Anon, J.C.; Auste, B.; Essbauer, S.; Gnionsahe, A.; Kigninlman, H.; Klempa, B.; Kraef, C.; et al. Human Infections by Non-Rodent-Associated Hantaviruses in Africa. J. Infect. Dis. 2016, 214, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, P.T.; Klempa, B.; Ithete, N.L.; Auste, B.; Mfune, J.K.; Hoveka, J.; Matthee, S.; Preiser, W.; Kruger, D.H. Hantaviruses in Africa. Virus Res. 2014, 187, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Duggan, J.M.; Close, R.; McCann, L.; Wright, D.; Keys, M.; McCarthy, N.; Mannes, T.; Walsh, A.; Charlett, A.; Brooks, T.J.G. A seroprevalence study to determine the frequency of hantavirus infection in people exposed to wild and pet fancy rats in England. Epidemiol. Infect. 2017, 145, 2458–2465. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.; McKenna, P.; Van Der Groen, G.; Vaheri, A.; Peters, C. Hantaviruses. In Zoonoses; Biology, Clinical Practice, and Public Health Control; Oxford University Press: Oxford, UK; New York, NY, USA; Tokyo, Japan, 1998; pp. 331–351. [Google Scholar]
- Clement, J.; Maes, P.; Saegeman, V.; Lagrou, K.; Van Ranst, M.; Lundkvist, A. Comment on “A Cluster of Three Cases of Hantavirus Pulmonary Syndrome among Canadian Military Personnel”. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 7458409. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; No, J.S.; Lee, S.H.; Song, D.H.; Lee, D.; Kim, J.A.; Gu, S.H.; Park, S.; Jeong, S.T.; Kim, H.C.; et al. Multiplex PCR-Based Next-Generation Sequencing and Global Diversity of Seoul Virus in Humans and Rats. Emerg. Infect. Dis. 2018, 24, 249–257. [Google Scholar] [CrossRef] [PubMed]

| Case no. | Reference | Location USA | Peak S. Creat. | Peak Proteinuria | Nadir Platelets | Transaminitis | Lab Confirmation | ICU Treatment | Special Features |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1994, Glass [12] | Baltimore, MD | 3 mg/dL | 1700 mg/24hrs | N.R. | +++ | PRNT | N.R. | Dyspnea and pleural effusions |
| 2 | 1994, Glass [12] | Baltimore, MD | N.R. | 954 mg/24hrs | N.R. | + | PRNT | N.R. | Dyspnea |
| 3 | 1994, Glass [12] | Baltimore, MD | 1.4 mg/dL | 550 mg/24hrs | N.R. | + | PRNT | N.R. | Pleural effusions and hemoptysis |
| 4 | 2009, Woods [151] | Baltimore, MD | 12.3 mg/dL | 600 mg/dL 3+ | 36,000/mm3 | +++ | RT-PCR | 6 x Dialysis | Cough, high CK, myoglobulinuria |
| 5 | 2010, Nielsen [152] | Milwaukee WI | 1.53 mg/dL | 2 + | 26,000/mm3 | +++ | RT-PCR | none | Hypoxia, pleural effusions, ferritin 38,394 ng/mL. Liver biopsy |
| 6 | 2012, Roig [153] | Brazoria, TX | 6 mg/dL | 2 + | 114,000/mm3 | ++ | ELISA | Ventilation | Fatal ARDS |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clement, J.; LeDuc, J.W.; Lloyd, G.; Reynes, J.-M.; McElhinney, L.; Van Ranst, M.; Lee, H.-W. Wild Rats, Laboratory Rats, Pet Rats: Global Seoul Hantavirus Disease Revisited. Viruses 2019, 11, 652. https://doi.org/10.3390/v11070652
Clement J, LeDuc JW, Lloyd G, Reynes J-M, McElhinney L, Van Ranst M, Lee H-W. Wild Rats, Laboratory Rats, Pet Rats: Global Seoul Hantavirus Disease Revisited. Viruses. 2019; 11(7):652. https://doi.org/10.3390/v11070652
Chicago/Turabian StyleClement, Jan, James W. LeDuc, Graham Lloyd, Jean-Marc Reynes, Lorraine McElhinney, Marc Van Ranst, and Ho-Wang Lee. 2019. "Wild Rats, Laboratory Rats, Pet Rats: Global Seoul Hantavirus Disease Revisited" Viruses 11, no. 7: 652. https://doi.org/10.3390/v11070652
APA StyleClement, J., LeDuc, J. W., Lloyd, G., Reynes, J.-M., McElhinney, L., Van Ranst, M., & Lee, H.-W. (2019). Wild Rats, Laboratory Rats, Pet Rats: Global Seoul Hantavirus Disease Revisited. Viruses, 11(7), 652. https://doi.org/10.3390/v11070652




