Antiviral Efficacy of Flavonoids against Enterovirus 71 Infection in Vitro and in Newborn Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds, Cells, Viruses, and Animals
2.2. Cytopathic Effect (CPE) Inhibition Assay
2.3. Cytotoxicity Assay
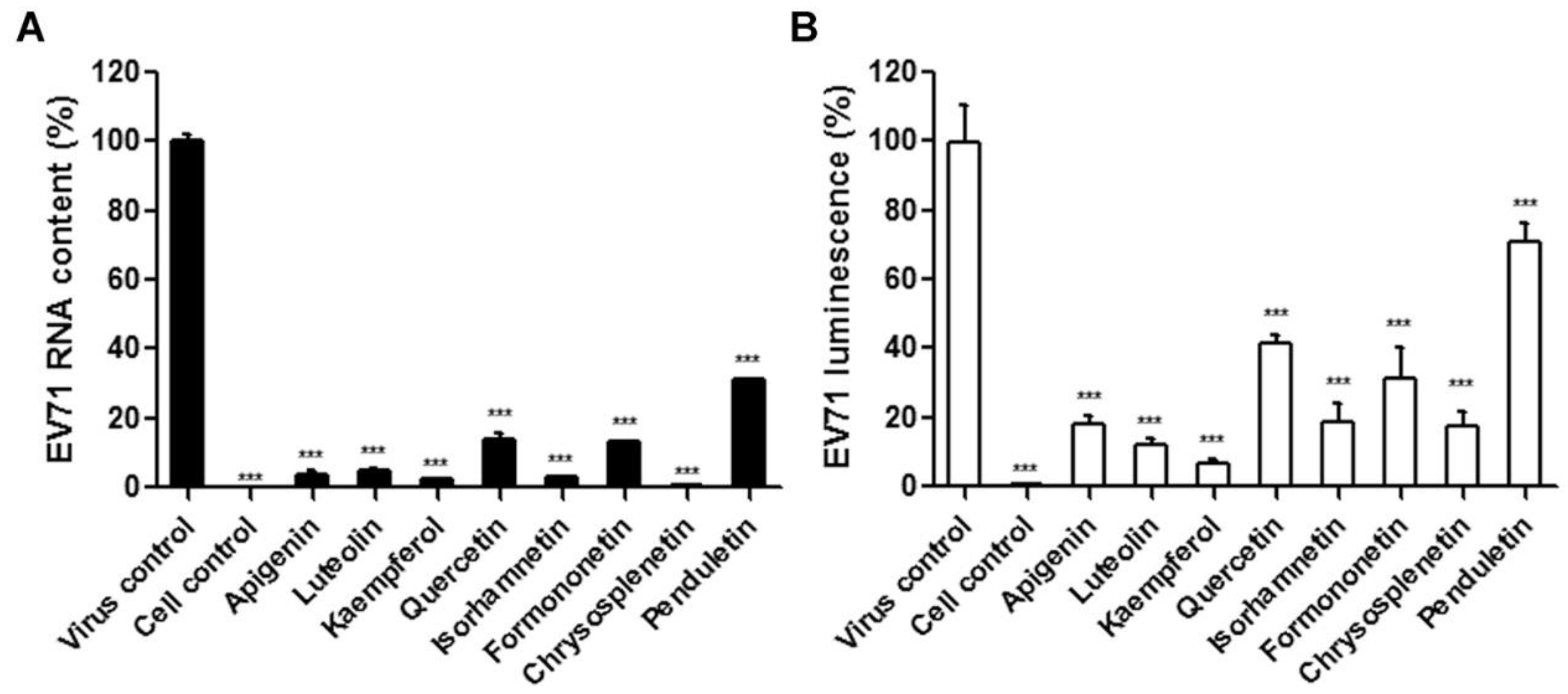
2.4. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.5. EV71-luc Based Viral Protein Synthesis Assay
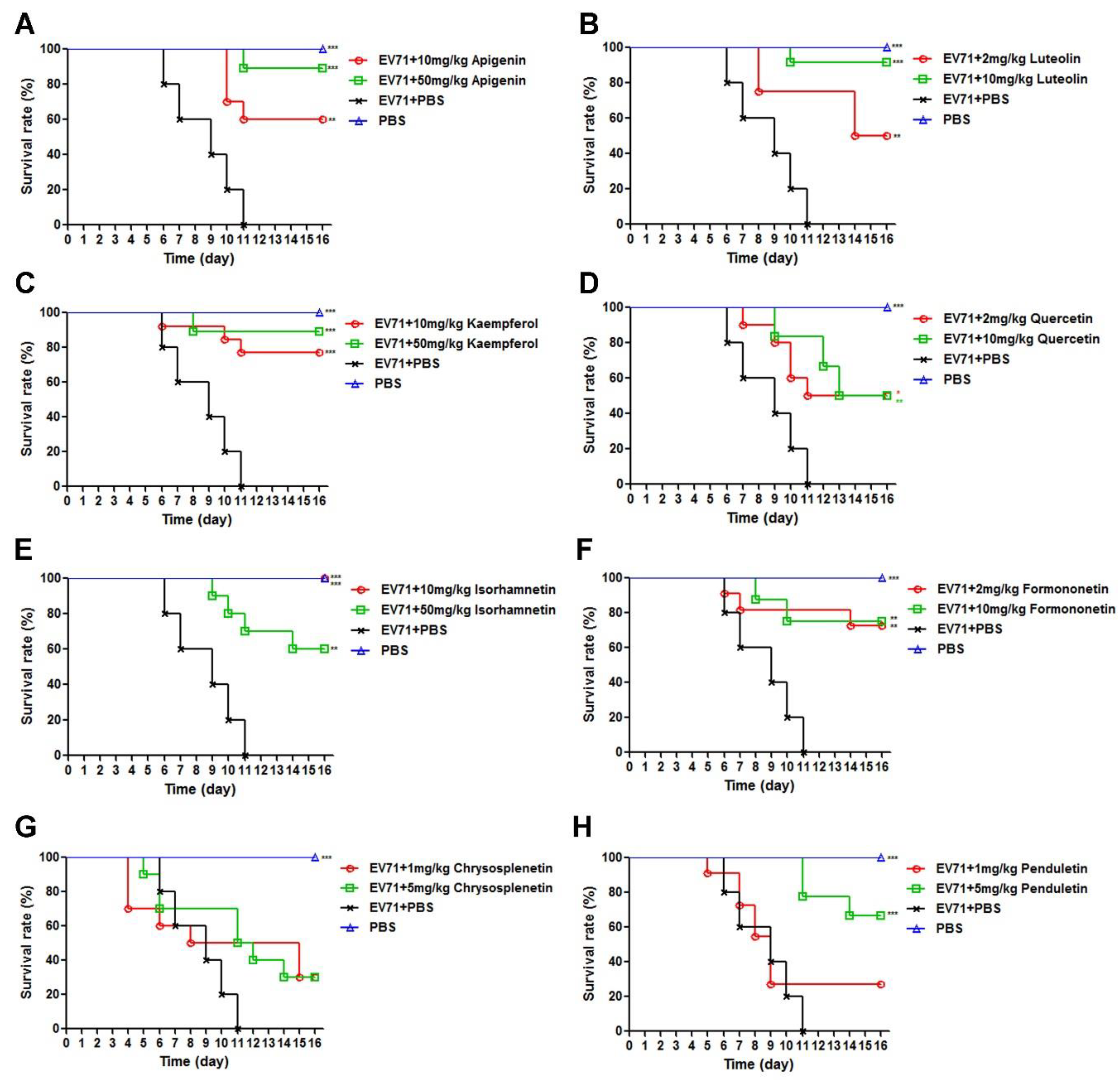
2.6. Protective Efficacy against EV71 in Newborn Mice
2.7. Statistical Analysis
3. Results
3.1. Flavonoids Protected Cells from EV71-induced CPE
3.2. Flavonoids Caused Reduced EV71 Replication in Cells
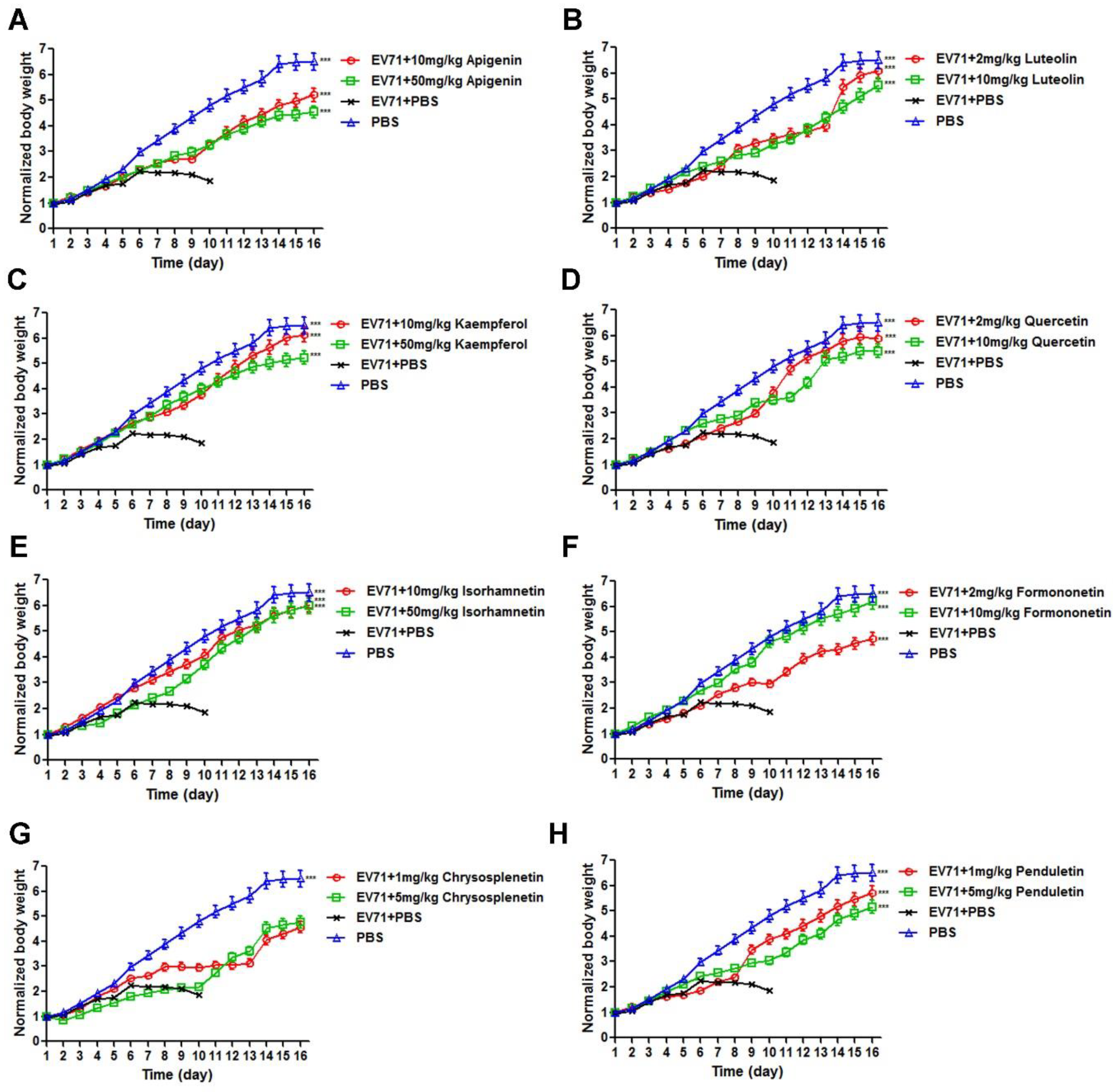
3.3. Flavonoids Protected Newborn BALB/c Mice from EV71-induced Lethality
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solomon, T.; Lewthwaite, P.; Perera, D.; Cardosa, M.J.; McMinn, P.; Ooi, M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010, 10, 778–790. [Google Scholar] [CrossRef]
- Lei, X.; Cui, S.; Zhao, Z.; Wang, J. Etiology, pathogenesis, antivirals and vaccines of hand, foot, and mouth disease. Natl. Sci. Rev. 2015, 2, 268–284. [Google Scholar] [CrossRef]
- McMinn, P.C. An overview of the evolution of enterovirus 71 and its clinical and public health significance. Fems Microbiol. Rev. 2002, 26, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Liao, Q.; Viboud, C.; Zhang, J.; Sun, J.; Wu, J.T.; Chang, Z.; Liu, F.; Fang, V.J.; Zheng, Y.; et al. Hand, foot, and mouth disease in China, 2008–2012: An epidemiological study. Lancet Infect. Dis. 2014, 14, 308–318. [Google Scholar] [CrossRef]
- Ho, M.; Chen, E.R.; Hsu, K.H.; Twu, S.J.; Chen, K.T.; Tsai, S.F.; Wang, J.R.; Shih, S.R. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N. Engl. J. Med. 1999, 341, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.; He, F.; Kwang, J. Recent Progress towards Novel EV71 Anti-Therapeutics and Vaccines. Viruses 2015, 7, 6441–6457. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Wang, Y.; Bian, L.; Xu, M.; Liang, Z. EV-A71 vaccine licensure: A first step for multivalent enterovirus vaccine to control HFMD and other severe diseases. Emerg. Microbes Infect. 2016, 5, e75. [Google Scholar] [CrossRef]
- Matsubayashi, T.; Miwa, Y.; Takeda, S.; Koide, M.; Enoki, H.; Mizukami, A.; Matsubayashi, R. Percutaneous cardiopulmonary support in a child with enterovirus 71 encephalitis. Pediatrics Int. Off. J. Jpn. Pediatric Soc. 2006, 48, 327–329. [Google Scholar] [CrossRef]
- Ooi, M.H.; Wong, S.C.; Lewthwaite, P.; Cardosa, M.J.; Solomon, T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010, 9, 1097–1105. [Google Scholar] [CrossRef]
- Wang, J.N.; Yao, C.T.; Yeh, C.N.; Huang, C.C.; Wang, S.M.; Liu, C.C.; Wu, J.M. Critical management in patients with severe enterovirus 71 infection. Pediatrics Int. Off. J. Jpn. Pediatric Soc. 2006, 48, 250–256. [Google Scholar] [CrossRef]
- Yen, M.H.; Chiu, C.H.; Huang, Y.C.; Lin, T.Y. Effects of lactoferrin-containing formula in the prevention of enterovirus and rotavirus infection and impact on serum cytokine levels: A randomized trial. Chang Gung Med. J. 2011, 34, 395–402. [Google Scholar] [PubMed]
- Zhang, G.; Zhou, F.; Gu, B.; Ding, C.; Feng, D.; Xie, F.; Wang, J.; Zhang, C.; Cao, Q.; Deng, Y.; et al. In vitro and in vivo evaluation of ribavirin and pleconaril antiviral activity against enterovirus 71 infection. Arch. Virol. 2012, 157, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Song, Z.G.; Jiang, T.; Shi, B.S.; Hu, Y.W.; Yuan, Z.H. Rupintrivir is a promising candidate for treating severe cases of Enterovirus-71 infection. World J. Gastroenterol. 2010, 16, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Manthey, J.A.; Grohmann, K.; Guthrie, N. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr. Med. Chem. 2001, 8, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Orhan, D.D.; Ozcelik, B.; Ozgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Qiu, M.; Chen, D.; Zheng, N.; Jin, Y.; Wu, Z. Apigenin inhibits enterovirus 71 replication through suppressing viral IRES activity and modulating cellular JNK pathway. Antivir. Res. 2014, 109, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Su, W.; Jin, J.; Chen, J.; Li, X.; Zhang, X.; Sun, M.; Sun, S.; Fan, P.; An, D.; et al. Identification of luteolin as enterovirus 71 and coxsackievirus A16 inhibitors through reporter viruses and cell viability-based screening. Viruses 2014, 6, 2778–2795. [Google Scholar] [CrossRef]
- Tsai, F.J.; Lin, C.W.; Lai, C.C.; Lan, Y.C.; Lai, C.H.; Hung, C.H.; Hsueh, K.C.; Lin, T.H.; Chang, H.C.; Wan, L.; et al. Kaempferol inhibits enterovirus 71 replication and internal ribosome entry site (IRES) activity through FUBP and HNRP proteins. Food Chem. 2011, 128, 312–322. [Google Scholar] [CrossRef]
- Wang, C.Y.; Huang, S.C.; Zhang, Y.; Lai, Z.R.; Kung, S.H.; Chang, Y.S.; Lin, C.W. Antiviral Ability ofKalanchoe gracilisLeaf Extract against Enterovirus 71 and Coxsackievirus A16. Evid. Based Complementary Altern. Med. 2012, 2012, 203165. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, D.; Ge, M.; Li, Z.; Jiang, J.; Li, Y. Formononetin inhibits enterovirus 71 replication by regulating COX- 2/PGE(2) expression. Virol. J. 2015, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.C.; Wang, Y.; Liu, Y.P.; Zhang, R.Q.; Li, X.; Su, W.H.; Long, F.; Luo, X.D.; Peng, T. Inhibition of enterovirus 71 replication by chrysosplenetin and penduletin. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2011, 44, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, P.; Jin, J.; Su, W.; An, D.; Xu, L.; Sun, S.; Zhang, Y.; Meng, X.; Gao, F.; et al. Establishment of cell lines with increased susceptibility to EV71/CA16 by stable overexpression of SCARB2. Virol. J. 2013, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Xu, L.; Guo, S.; Sun, S.; Zhang, S.; Zhu, C.; Kong, W.; Jiang, C. Safe and objective assay of enterovirus 71 neutralizing antibodies via pseudovirus. Chem. Res. Chin. Univ. 2012, 28, 91–95. [Google Scholar]
- Crouch, S.P.; Kozlowski, R.; Slater, K.J.; Fletcher, J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 1993, 160, 81–88. [Google Scholar] [CrossRef]
- Lu, J. Viral kinetics of Enterovirus 71 in human abdomyosarcoma cells. World J. Gastroenterol. 2011, 17, 4135. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, M.; van Maarseveen, N.; Schuurman, R.; Verkuijlen, S.; de Vos, M.; Hendriksen, K.; van Loon, A.M. Rapid and sensitive routine detection of all members of the genus enterovirus in different clinical specimens by real-time PCR. J. Clin. Microbiol. 2002, 40, 3666–3670. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wu, Y.; Bi, J.; Lu, X.; Hou, A.; Zhou, Y.; Sun, B.; Kong, W.; Barbier, J.; Cintrat, J.C.; et al. Antiviral effects of Retro-2(cycl) and Retro-2.1 against Enterovirus 71 in vitro and in vivo. Antivir. Res. 2017, 144, 311–321. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.; Qin, B.; Zhang, X.; Chen, L.; Hu, Y.; Yuan, Z. Rupintrivir is a promising candidate for treating severe cases of enterovirus-71 infection: Evaluation of antiviral efficacy in a murine infection model. Antivir. Res. 2013, 97, 264–269. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Shimizu, M.; Hiyama, Y.; Itoh, K.; Hashimoto, Y.; Nakayama, M.; Horie, T.; Morita, N. Antiviral activity of natural occurring flavonoids in vitro. Chem. Pharm. Bull. 1985, 33, 3881–3886. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, J.L.; Vanden Berghe, D.; Carrasco, L. 3-Methylquercetin is a potent and selective inhibitor of poliovirus RNA synthesis. Virology 1986, 152, 219–227. [Google Scholar] [CrossRef]
- Tan, C.; Lai, J.K.; Sam, I.C.; Chan, Y. Recent developments in antiviral agents against enterovirus 71 infection. J. Biomed. Sci. 2014, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.P.; Peng, Z.Y.; Peng, M.J.; Yan, W.B.; Ouyang, Y.Z.; Zhu, H.L. Flavonoids health benefits and their molecular mechanism. Mini Rev. Med. Chem. 2011, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Vrijsen, R.; Everaert, L.; Boeye, A. Antiviral activity of flavones and potentiation by ascorbate. J. Gen. Virol. 1988, 69, 1749–1751. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Wang, Q.; Nanua, S.; Hershenson, M.B.; Sajjan, U.S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 2012, 94, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Fan, W.; Qian, P.; Zhang, D.; Wei, Y.; Chen, H.; Li, X. Apigenin restricts FMDV infection and inhibits viral IRES driven translational activity. Viruses 2015, 7, 1613–1626. [Google Scholar] [CrossRef]
- Murali, K.S.; Sivasubramanian, S.; Vincent, S.; Murugan, S.B.; Giridaran, B.; Dinesh, S.; Gunasekaran, P.; Krishnasamy, K.; Sathishkumar, R. Anti—chikungunya activity of luteolin and apigenin rich fraction from Cynodon dactylon. Asian Pac. J. Trop. Med. 2015, 8, 352–358. [Google Scholar] [CrossRef]
- Keramagi, A.R.; Skariyachan, S. Prediction of binding potential of natural leads against the prioritized drug targets of chikungunya and dengue viruses by computational screening. 3 Biotech 2018, 8, 274. [Google Scholar] [CrossRef]
- Ghosh, A.; Desai, A.; Ravi, V.; Narayanappa, G.; Tyagi, B.K. Chikungunya Virus Interacts with Heat Shock Cognate 70 Protein to Facilitate Its Entry into Mosquito Cell Line. Intervirology 2017, 60, 247–262. [Google Scholar] [CrossRef]
- Shibata, C.; Ohno, M.; Otsuka, M.; Kishikawa, T.; Goto, K.; Muroyama, R.; Kato, N.; Yoshikawa, T.; Takata, A.; Koike, K. The flavonoid apigenin inhibits hepatitis C virus replication by decreasing mature microRNA122 levels. Virology 2014, 462, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Del Campo, J.A.; Clement, S.; Lemasson, M.; Garcia-Valdecasas, M.; Gil-Gomez, A.; Ranchal, I.; Bartosch, B.; Bautista, J.D.; Rosenberg, A.R.; et al. Effect of Quercetin on Hepatitis C Virus Life Cycle: From Viral to Host Targets. Sci. Rep. 2016, 6, 31777. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.W.; Abou El-Kassem, L.T.; Shaher, F.; Al-Farawati, R. In vitro inhibition of Hepatitis C virus protease and antioxidant by flavonoid glycosides from the Saudi costal plant Sarcocornia fruticosa. Nat. Prod. Res. 2018, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Watanabe, S.; Chan, K.W.K.; He, Q.; Zhao, Y.; Zhang, Z.; Lai, X.; Luo, D.; Vasudevan, S.G.; Li, G. Luteolin restricts dengue virus replication through inhibition of the proprotein convertase furin. Antivir. Res. 2017, 143, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Qian, S.; Qian, P.; Li, X. Antiviral activity of luteolin against Japanese encephalitis virus. Virus Res. 2016, 220, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, Z.; Du, J.; Hu, Y.; Liu, L.; Yang, F.; Jin, Q. Anti-Japanese-encephalitis-viral effects of kaempferol and daidzin and their RNA-binding characteristics. PLoS ONE 2012, 7, e30259. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; Patel, T.; Gaikwad, R.; Patil, U.K.; Gayen, S. Identification of structural requirements and prediction of inhibitory activity of natural flavonoids against Zika virus through molecular docking and Monte Carlo based QSAR Simulation. Nat. Prod. Res. 2017, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]

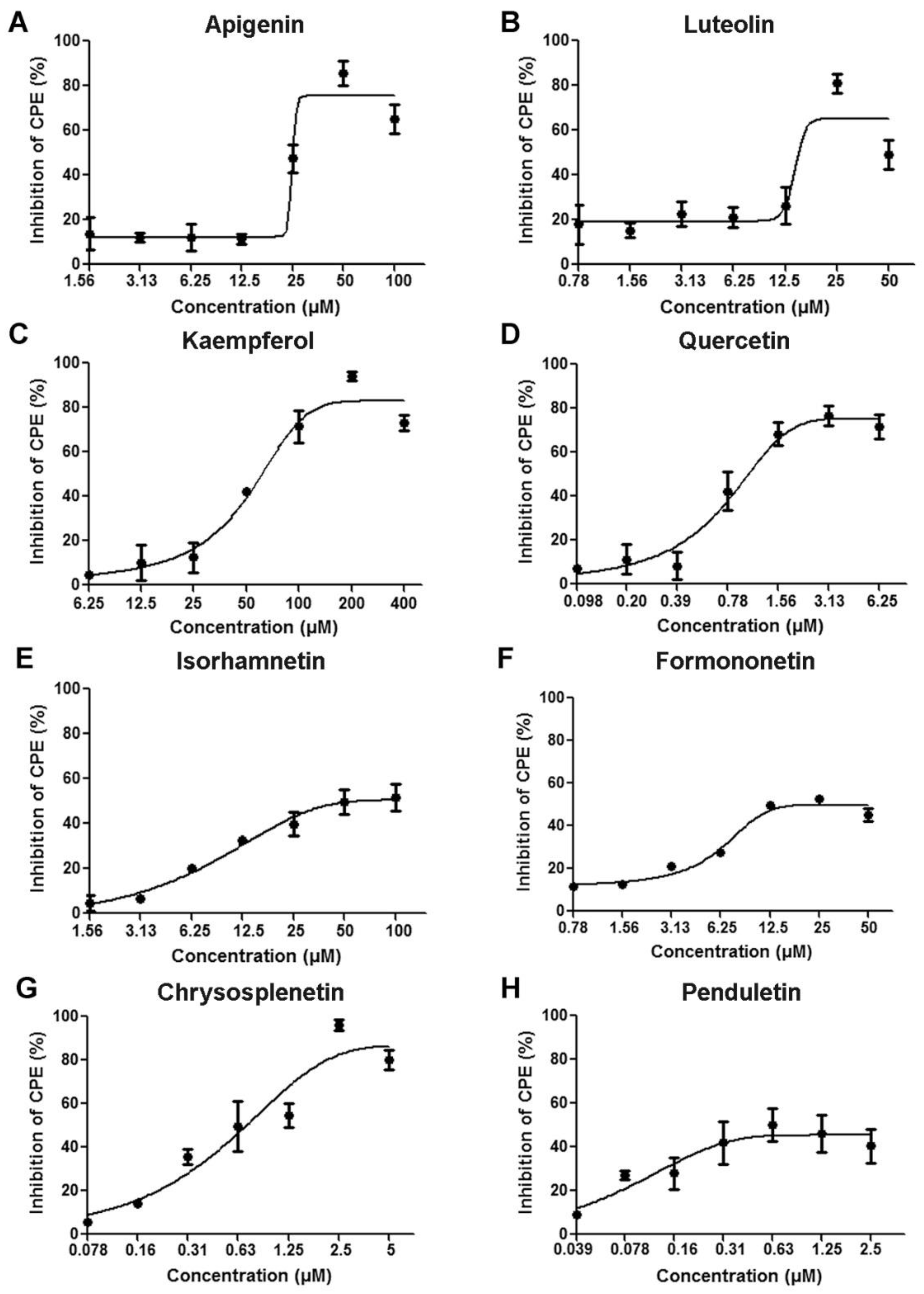
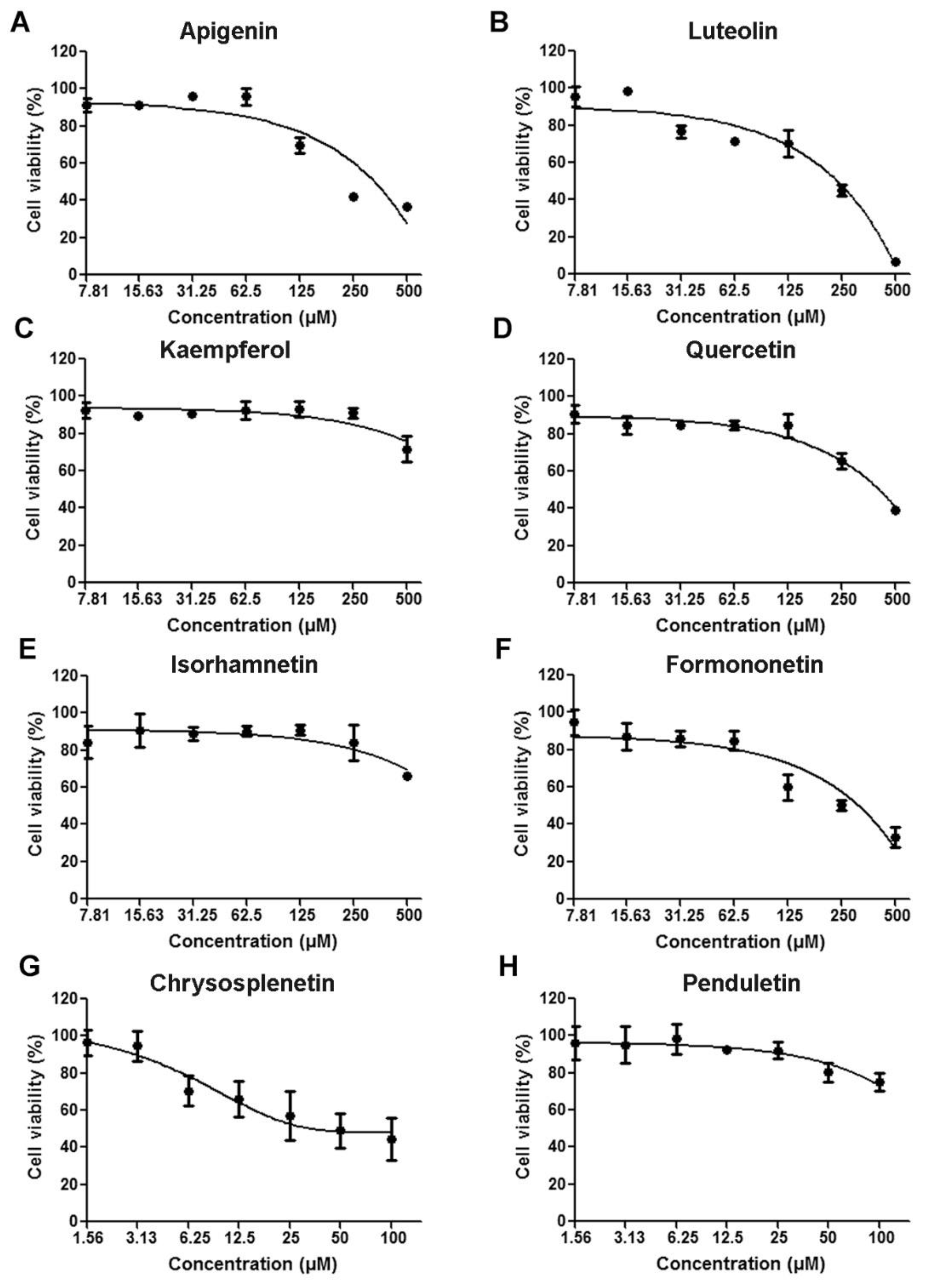

| Cytotoxicity | Antiviral Activity | ||
|---|---|---|---|
| Compounds | CC50 a (μM) | EC50 b (μM) | SI c |
| Apigenin | 256.1 | 24.74 | 10.35 |
| Luteolin | 168.2 | 13.5 | 12.46 |
| Kaempferol | >500 | 52.75 | >9.48 |
| Quercetin | 444.2 | 1.2 | 370.17 |
| Isorhamnetin | >500 | 60.7 | 8.24 |
| Formononetin | 238.9 | 12.5 | 19.11 |
| Chrysosplenetin | 48.97 | 0.68 | 72.01 |
| Penduletin | >100 | 0.63 | 158.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, W.; Bi, J.; Li, F.; Wang, S.; Huang, X.; Meng, X.; Sun, B.; Wang, D.; Kong, W.; Jiang, C.; et al. Antiviral Efficacy of Flavonoids against Enterovirus 71 Infection in Vitro and in Newborn Mice. Viruses 2019, 11, 625. https://doi.org/10.3390/v11070625
Dai W, Bi J, Li F, Wang S, Huang X, Meng X, Sun B, Wang D, Kong W, Jiang C, et al. Antiviral Efficacy of Flavonoids against Enterovirus 71 Infection in Vitro and in Newborn Mice. Viruses. 2019; 11(7):625. https://doi.org/10.3390/v11070625
Chicago/Turabian StyleDai, Wenwen, Jinpeng Bi, Fang Li, Shuai Wang, Xinyu Huang, Xiangyu Meng, Bo Sun, Deli Wang, Wei Kong, Chunlai Jiang, and et al. 2019. "Antiviral Efficacy of Flavonoids against Enterovirus 71 Infection in Vitro and in Newborn Mice" Viruses 11, no. 7: 625. https://doi.org/10.3390/v11070625
APA StyleDai, W., Bi, J., Li, F., Wang, S., Huang, X., Meng, X., Sun, B., Wang, D., Kong, W., Jiang, C., & Su, W. (2019). Antiviral Efficacy of Flavonoids against Enterovirus 71 Infection in Vitro and in Newborn Mice. Viruses, 11(7), 625. https://doi.org/10.3390/v11070625





