Human Adenovirus Serotype 5 Is Sensitive to IgM-Independent Neutralization In Vitro and In Vivo
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethics Statement
2.2. Cells and Viral Vectors
2.3. Fluorescent Labelling of HAdV Particles
2.4. Nanoparticle Tracking Analysis (NTA)
2.5. Serum Neutralization Assay
2.6. Isolation of Proteins Bound to the Virions
2.7. Liquid Chromatography-Mass Spectrometry (LC-MS/MS)
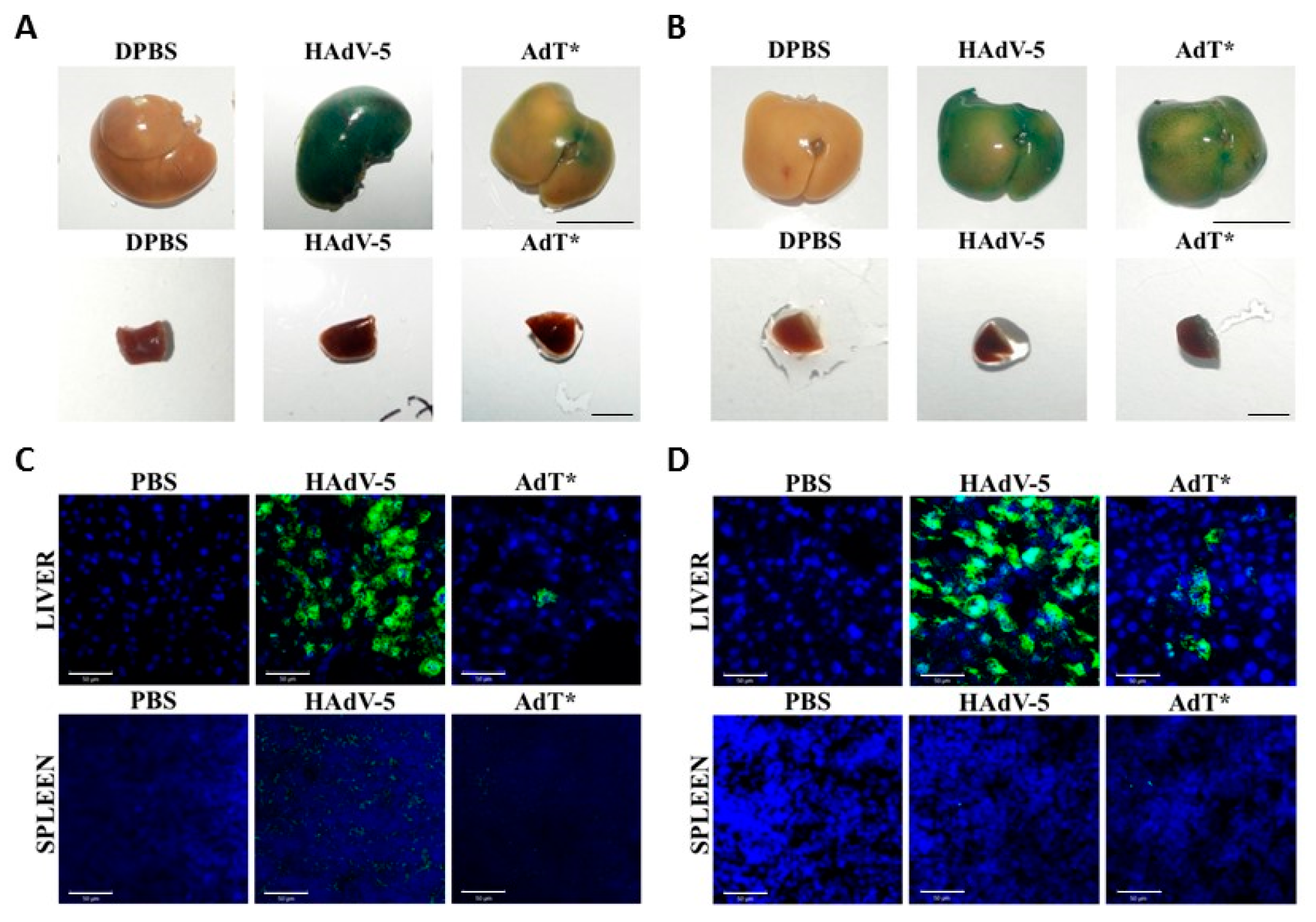
2.8. In Vivo Experiments
2.9. Detection of IgM and IgG
2.10. Statistical Analysis
3. Results
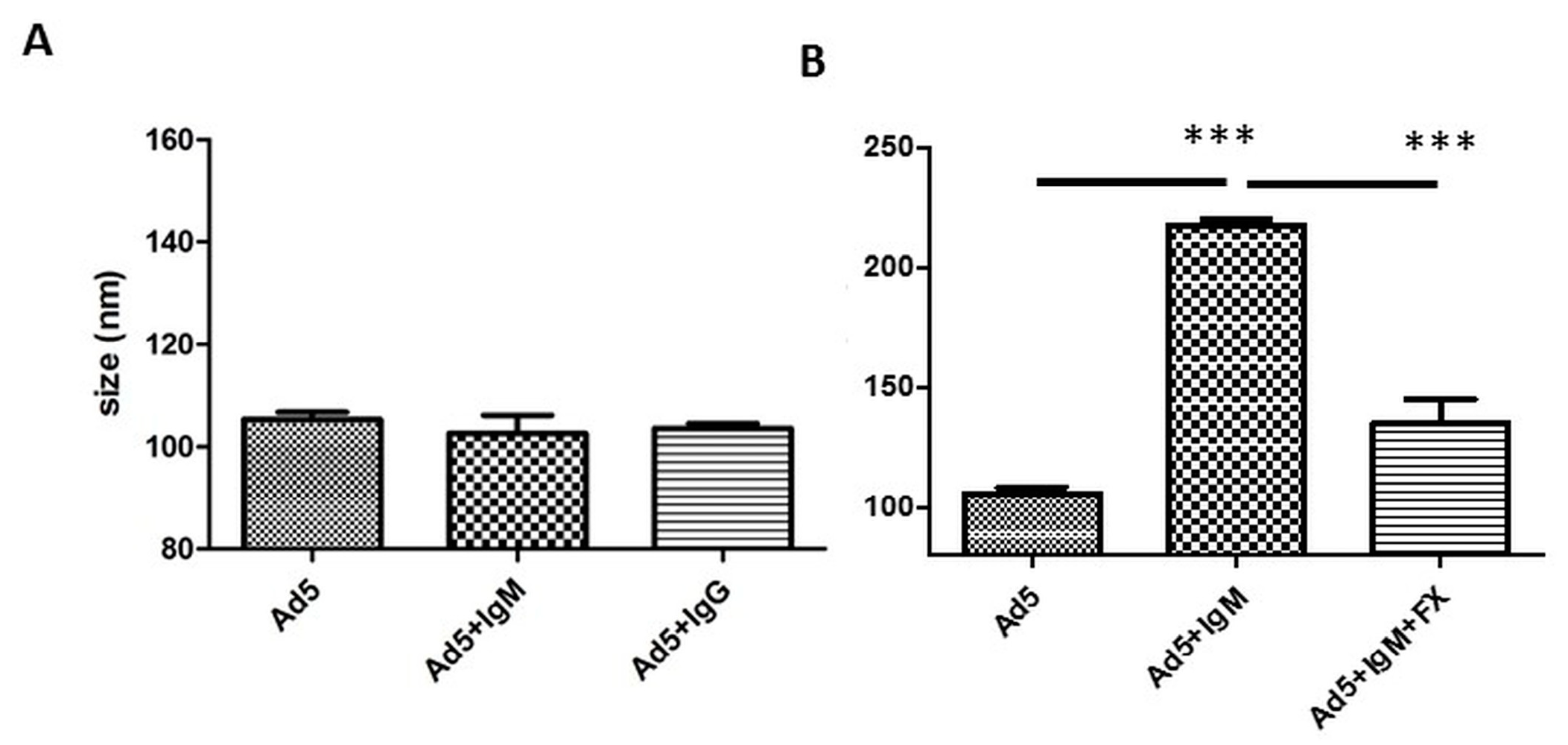
3.1. Differential Binding of Different Sources of IgM
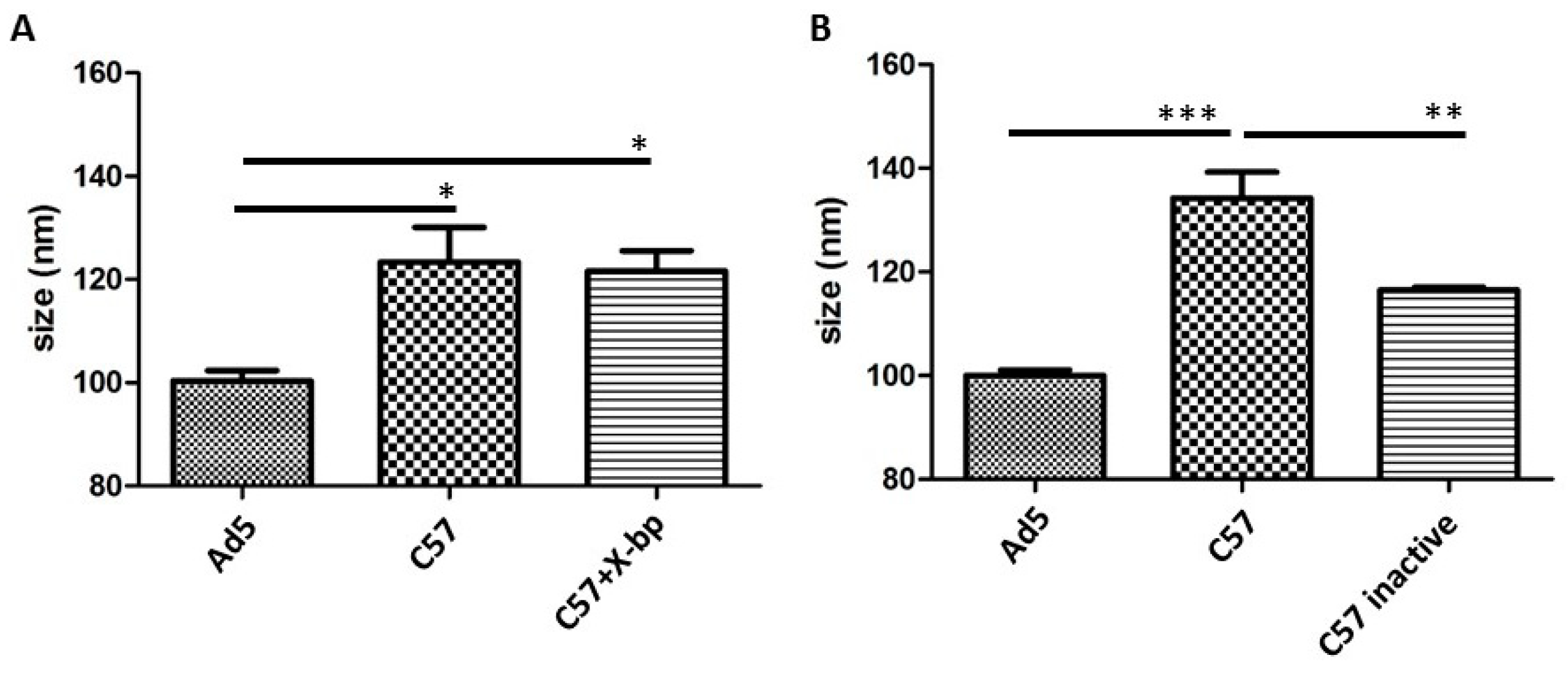
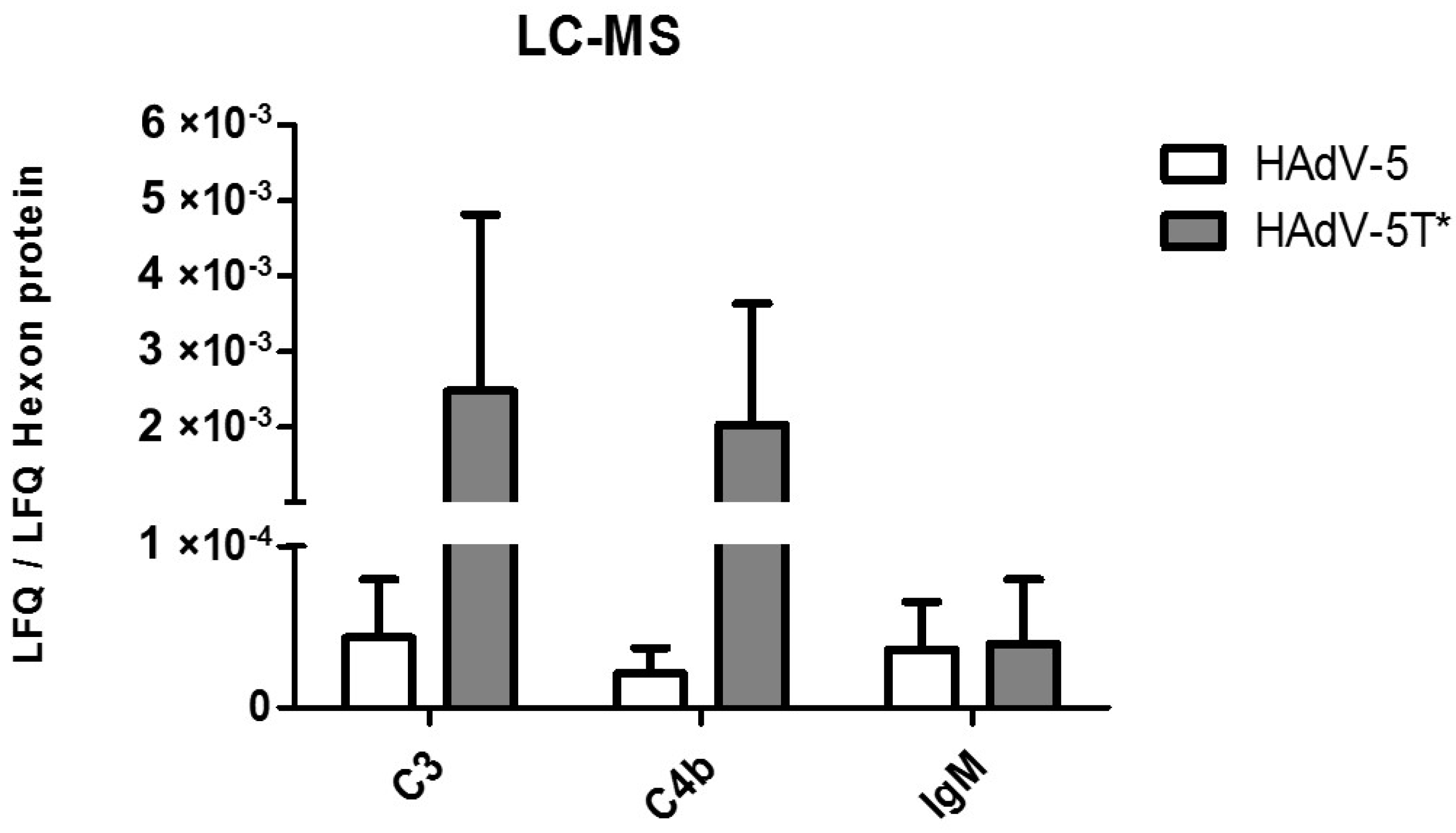
3.2. Differential Binding of Serum Components to HAdV-5 Virions
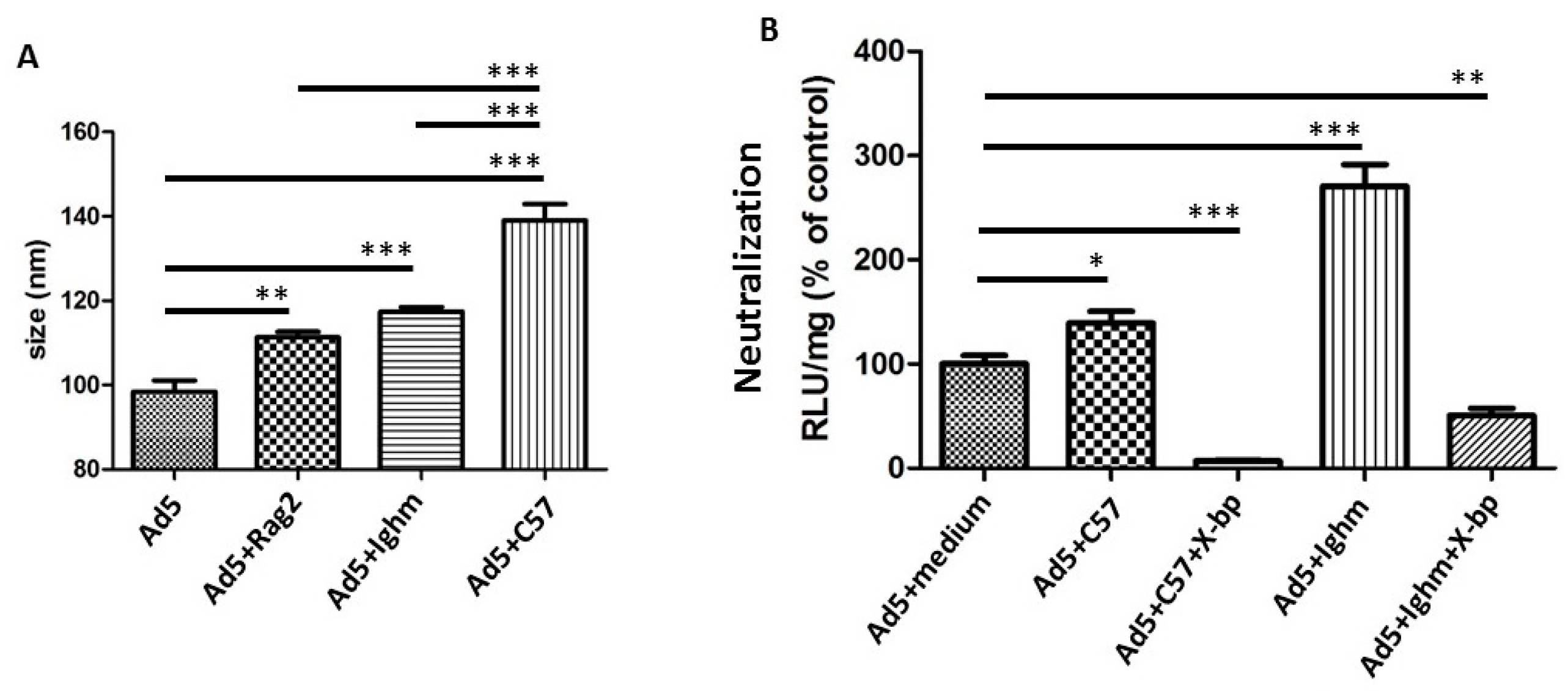
3.3. Murine IgM and IgG are not Necessary for Neutralization In Vitro
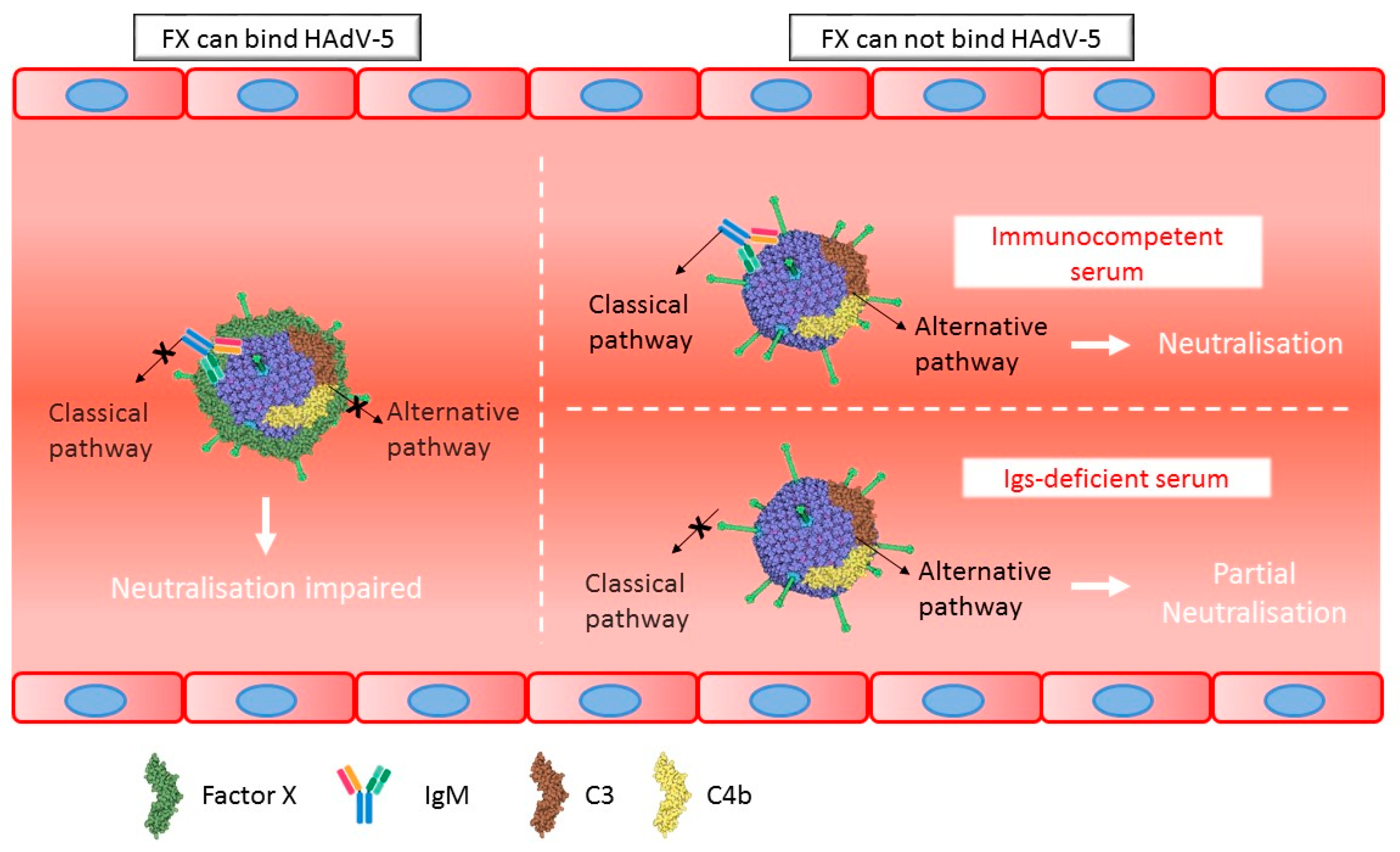
3.4. IgM, C3 and C4b Bind to HAdV-5 in the Presence of FX
3.5. HAdV-5T* Is Partially Neutralized in Immunoglobulin-Deficient Mice
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lopez-Gordo, E.; Denby, L.; Nicklin, S.A.; Baker, A.H. The importance of coagulation factors binding to adenovirus: Historical perspectives and implications for gene delivery. Expert Opin. Drug Deliv. 2014, 11, 1795–1813. [Google Scholar] [CrossRef] [PubMed]
- Bauer, U.; Flunker, G.; Bruss, K.; Kallwellis, K.; Liebermann, H.; Luettich, T.; Motz, M.; Seidel, W. Detection of antibodies against adenovirus protein ix, fiber, and hexon in human sera by immunoblot assay. J. Clin. Microbiol. 2005, 43, 4426–4433. [Google Scholar] [CrossRef] [PubMed]
- Dakin, R.S.; Parker, A.L.; Delles, C.; Nicklin, S.A.; Baker, A.H. Efficient transduction of primary vascular cells by the rare adenovirus serotype 49 vector. Hum. Gene Ther. 2015, 26, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Muruve, D.A.; Barnes, M.J.; Stillman, I.E.; Libermann, T.A. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 1999, 10, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Lozier, J.N.; Csako, G.; Mondoro, T.H.; Krizek, D.M.; Metzger, M.E.; Costello, R.; Vostal, J.G.; Rick, M.E.; Donahue, R.E.; Morgan, R.A. Toxicity of a first-generation adenoviral vector in rhesus macaques. Hum. Gene Ther. 2002, 13, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Morral, N.; O’Neal, W.K.; Rice, K.; Leland, M.M.; Piedra, P.A.; Aguilar-Córdova, E.; Carey, K.D.; Beaudet, A.L.; Langston, C. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum. Gene Ther. 2002, 13, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Atencio, I.A.; Grace, M.; Bordens, R.; Fritz, M.; Horowitz, J.A.; Hutchins, B.; Indelicato, S.; Jacobs, S.; Kolz, K.; Maneval, D.; et al. Biological activities of a recombinant adenovirus p53 (sch 58500) administered by hepatic arterial infusion in a phase 1 colorectal cancer trial. Cancer Gene Ther. 2006, 13, 169–181. [Google Scholar] [CrossRef]
- Alba, R.; Bradshaw, A.C.; Parker, A.L.; Bhella, D.; Waddington, S.N.; Nicklin, S.A.; van Rooijen, N.; Custers, J.; Goudsmit, J.; Barouch, D.H.; et al. Identification of coagulation factor (f)x binding sites on the adenovirus serotype 5 hexon: Effect of mutagenesis on fx interactions and gene transfer. Blood 2009, 114, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Shayakhmetov, D.M.; Gaggar, A.; Ni, S.; Li, Z.Y.; Lieber, A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 2005, 79, 7478–7491. [Google Scholar] [CrossRef]
- Bradshaw, A.C.; Parker, A.L.; Duffy, M.R.; Coughlan, L.; van Rooijen, N.; Kähäri, V.M.; Nicklin, S.A.; Baker, A.H. Requirements for receptor engagement during infection by adenovirus complexed with blood coagulation factor x. PLoS Pathog. 2010, 6, e1001142. [Google Scholar] [CrossRef]
- Xu, Z.; Qiu, Q.; Tian, J.; Smith, J.S.; Conenello, G.M.; Morita, T.; Byrnes, A.P. Coagulation factor x shields adenovirus type 5 from attack by natural antibodies and complement. Nat. Med. 2013, 19, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Duffy, M.R.; Deng, L.; Dakin, R.S.; Uil, T.; Custers, J.; Kelly, S.M.; McVey, J.H.; Nicklin, S.A.; Baker, A.H. Manipulating adenovirus hexon hypervariable loops dictates immune neutralisation and coagulation factor x-dependent cell interaction in vitro and in vivo. PLoS Pathog. 2015, 11, e1004673. [Google Scholar] [CrossRef] [PubMed]
- Alba, R.; Bradshaw, A.C.; Coughlan, L.; Denby, L.; McDonald, R.A.; Waddington, S.N.; Buckley, S.M.; Greig, J.A.; Parker, A.L.; Miller, A.M.; et al. Biodistribution and retargeting of fx-binding ablated adenovirus serotype 5 vectors. Blood 2010, 116, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Doszpoly, A.; Turner, G.; Nicklin, S.A.; Baker, A.H. The relevance of coagulation factor x protection of adenoviruses in human sera. Gene Ther. 2016, 23, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, S.A.; Baker, A.H. Simple methods for preparing recombinant adenoviruses for high-efficiency transduction of vascular cells. Methods Mol. Med. 1999, 30, 271–283. [Google Scholar] [PubMed]
- Parker, A.L.; Waddington, S.N.; Nicol, C.G.; Shayakhmetov, D.M.; Buckley, S.M.; Denby, L.; Kemball-Cook, G.; Ni, S.; Lieber, A.; McVey, J.H.; et al. Multiple vitamin k-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 2006, 108, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- De la Cuesta, F.; Baldan-Martin, M.; Moreno-Luna, R.; Alvarez-Llamas, G.; Gonzalez-Calero, L.; Mourino-Alvarez, L.; Sastre-Oliva, T.; López, J.A.; Vázquez, J.; Ruiz-Hurtado, G.; et al. Kalirin and chd7: Novel endothelial dysfunction indicators in circulating extracellular vesicles from hypertensive patients with albuminuria. Oncotarget 2017, 8, 15553–15562. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed maxlfq. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef]
- Atoda, H.; Ishikawa, M.; Mizuno, H.; Morita, T. Coagulation factor x-binding protein from deinagkistrodon acutus venom is a gla domain-binding protein. Biochemistry 1998, 37, 17361–17370. [Google Scholar] [CrossRef]
- Boes, M.; Esau, C.; Fischer, M.B.; Schmidt, T.; Carroll, M.; Chen, J. Enhanced b-1 cell development, but impaired igg antibody responses in mice deficient in secreted igm. J. Immunol. 1998, 160, 4776–4787. [Google Scholar]
- Lopez-Gordo, E.; Doszpoly, A.; Duffy, M.R.; Coughlan, L.; Bradshaw, A.C.; White, K.M.; Denby, L.; Nicklin, S.A.; Baker, A.H. Defining a novel role for the coxsackievirus and adenovirus receptor in human adenovirus serotype 5 transduction. J. Virol. 2017, 91, e02487-16. [Google Scholar] [CrossRef] [PubMed]
- Meri, S.; Pangburn, M.K. A mechanism of activation of the alternative complement pathway by the classical pathway: Protection of c3b from inactivation by covalent attachment to c4b. Eur. J. Immunol. 1990, 20, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, P.J. The amplification loop of the complement pathways. Adv. Immunol. 2009, 104, 115–149. [Google Scholar] [PubMed]
- Bottermann, M.; Foss, S.; Caddy, S.L.; Clift, D.; van Tienen, L.M.; Vaysburd, M.; Cruickshank, J.; O’Connell, K.; Clark, J.; Mayes, K.; et al. Complement c4 prevents viral infection through capsid inactivation. Cell Host Microbe 2019, 25, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Wright, M. Nanoparticle tracking analysis. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Harmon, A.W.; Moitra, R.; Xu, Z.; Byrnes, A.P. Hexons from adenovirus serotypes 5 and 48 differentially protect adenovirus vectors from neutralization by mouse and human serum. PLoS ONE 2018, 13, e0192353. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doszpoly, A.; de la Cuesta, F.; Lopez-Gordo, E.; Bénézech, C.; Nicklin, S.A.; Baker, A.H. Human Adenovirus Serotype 5 Is Sensitive to IgM-Independent Neutralization In Vitro and In Vivo. Viruses 2019, 11, 616. https://doi.org/10.3390/v11070616
Doszpoly A, de la Cuesta F, Lopez-Gordo E, Bénézech C, Nicklin SA, Baker AH. Human Adenovirus Serotype 5 Is Sensitive to IgM-Independent Neutralization In Vitro and In Vivo. Viruses. 2019; 11(7):616. https://doi.org/10.3390/v11070616
Chicago/Turabian StyleDoszpoly, Andor, Fernando de la Cuesta, Estrella Lopez-Gordo, Cécile Bénézech, Stuart A. Nicklin, and Andrew H. Baker. 2019. "Human Adenovirus Serotype 5 Is Sensitive to IgM-Independent Neutralization In Vitro and In Vivo" Viruses 11, no. 7: 616. https://doi.org/10.3390/v11070616
APA StyleDoszpoly, A., de la Cuesta, F., Lopez-Gordo, E., Bénézech, C., Nicklin, S. A., & Baker, A. H. (2019). Human Adenovirus Serotype 5 Is Sensitive to IgM-Independent Neutralization In Vitro and In Vivo. Viruses, 11(7), 616. https://doi.org/10.3390/v11070616





