Anti-Respiratory Syncytial Virus Activity of Plantago asiatica and Clerodendrum trichotomum Extracts In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Total Aqueous Extract Preparation
2.2. Reagents, Chemicals and Antibodies
2.3. Cell Culture and Virus Infection
2.4. Antiviral Assays
2.5. Determination of Effective Concentration (EC50) of Extracts and Acteoside
2.6. Determination of Cytotoxic Concentration (CC50) of Extracts and Acteoside
2.7. Quantitative RT-PCR (qRT PCR)
2.8. Immunoblot Analysis
2.9. Syncytium Formation Assay
2.10. RSV-GFP Challenge Experiment in Mouse Model
2.11. Ethical Approval
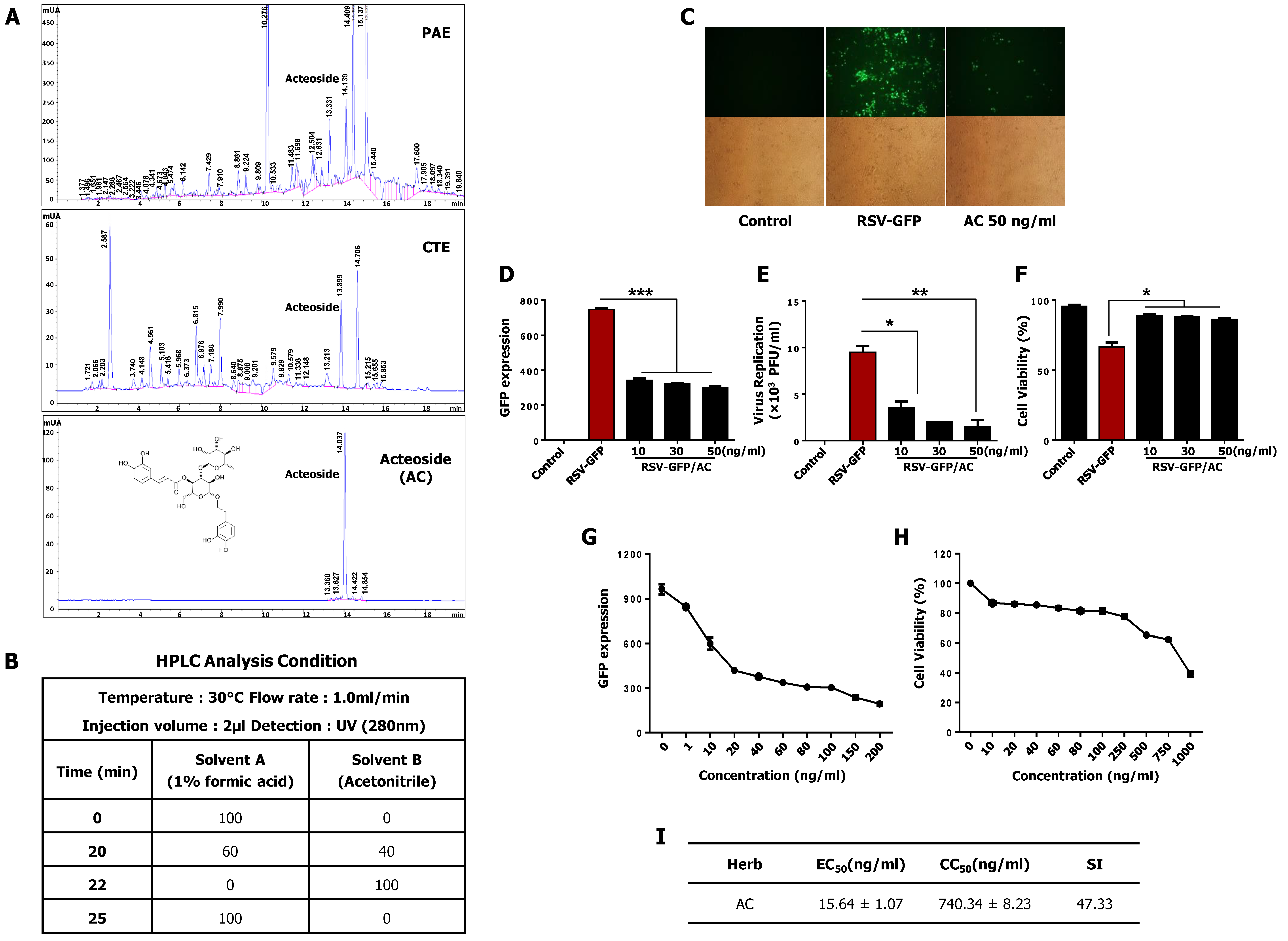
2.12. Identification of Acteoside through HPLC
2.13. Statistical Analysis
3. Results
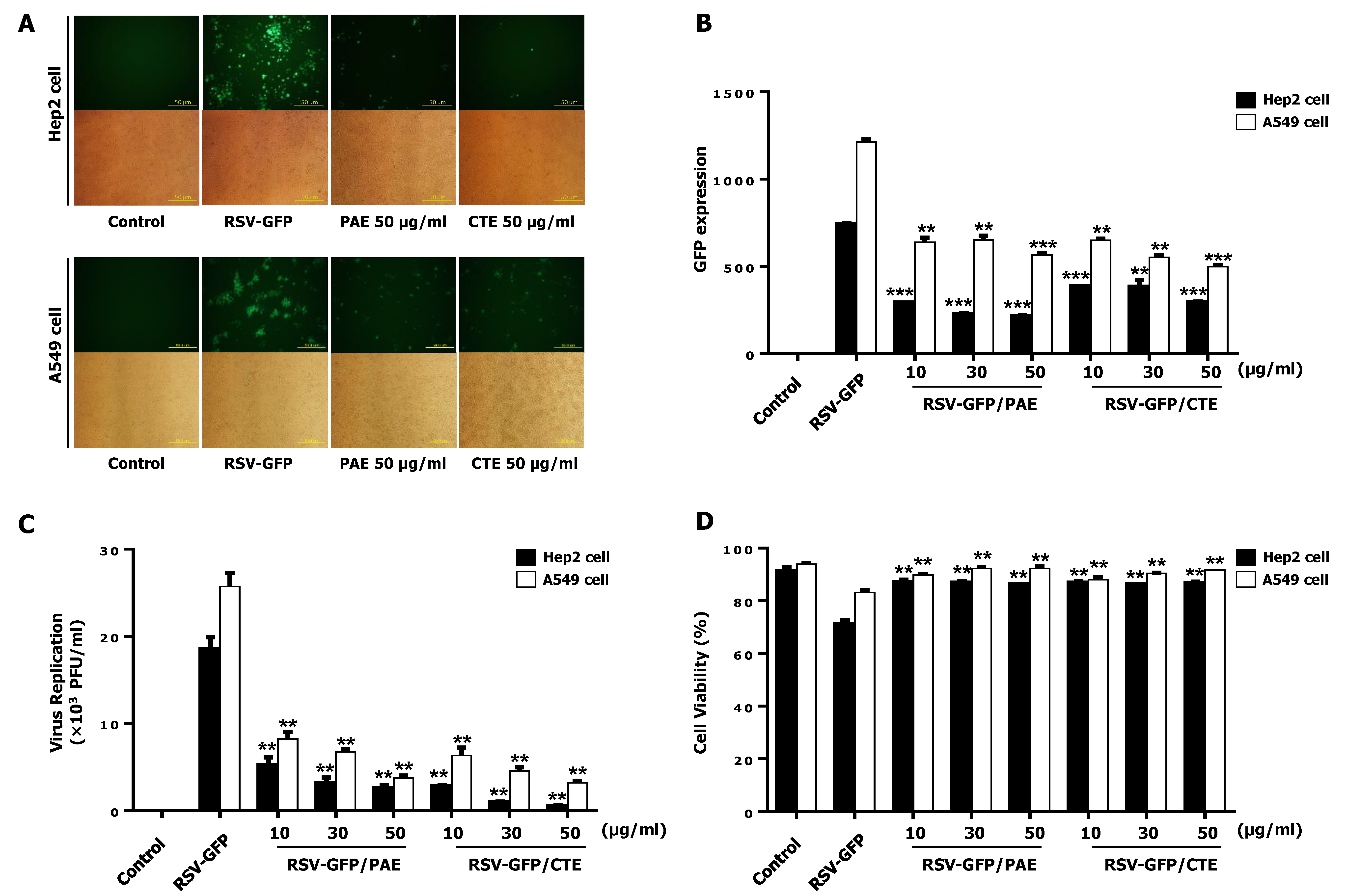
3.1. Antiviral Effects of PAE and CTE
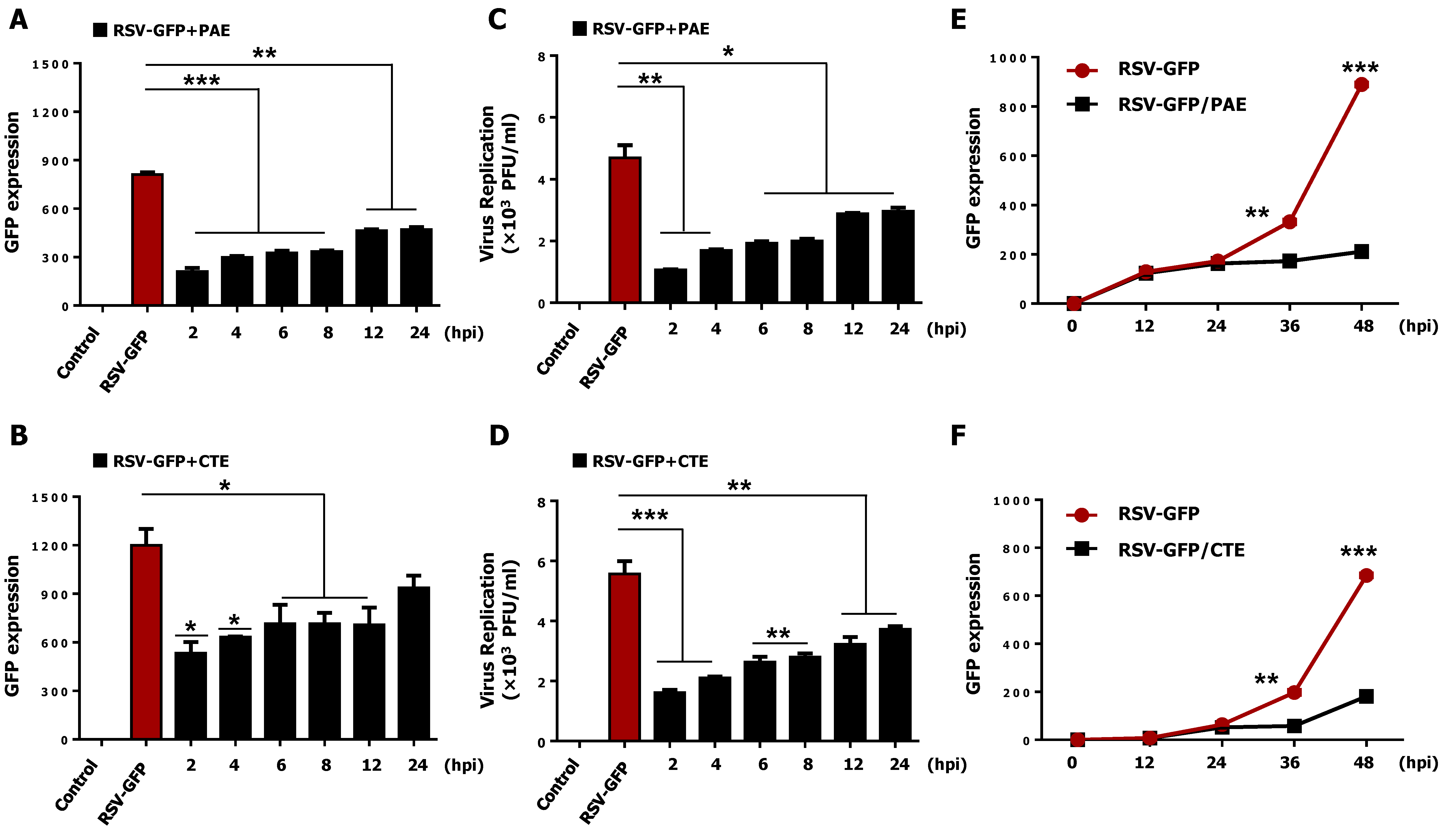
3.2. Therapeutic Effect of PAE and CTE against RSV Infection
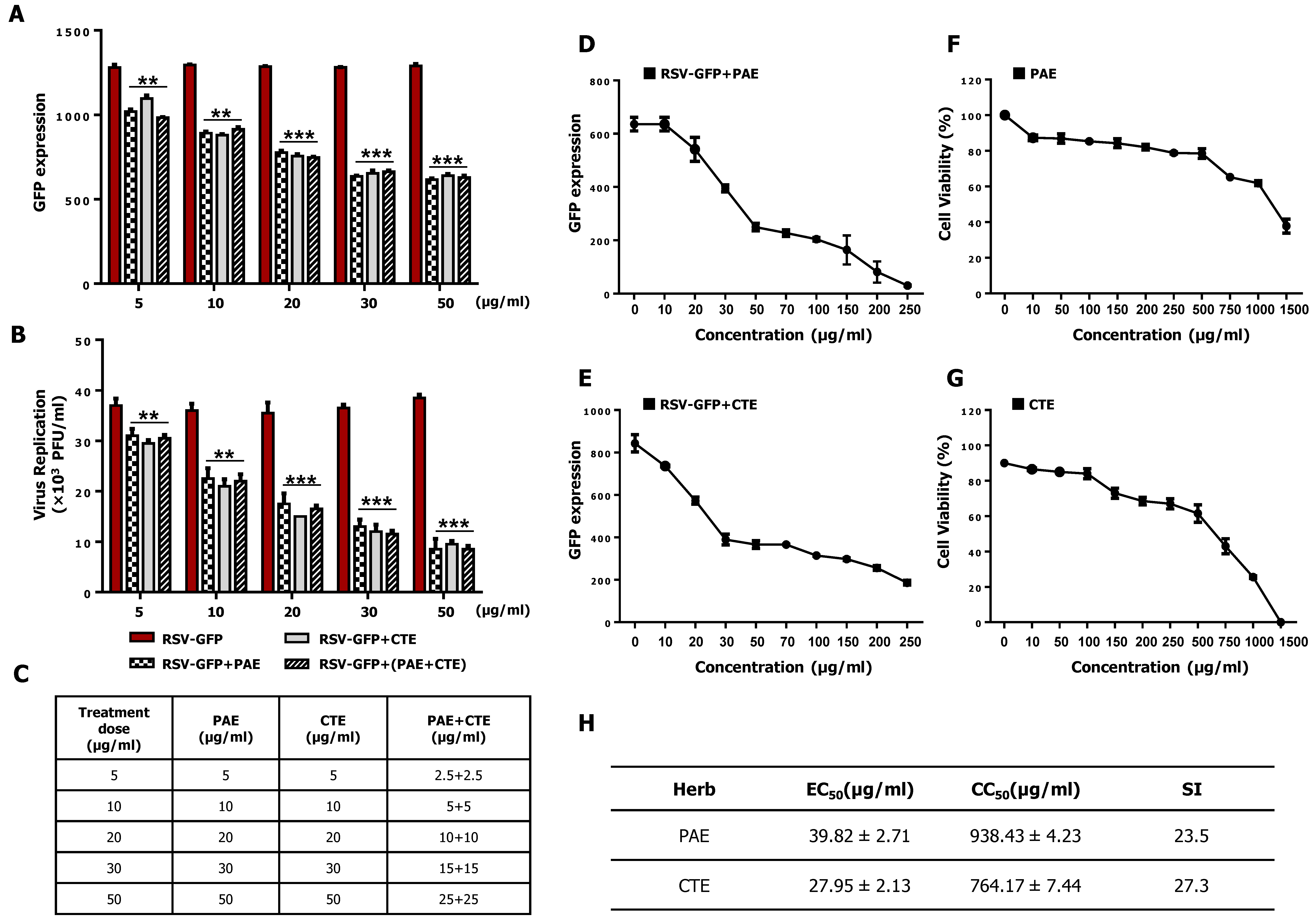
3.3. Synergistic Anti-RSV Effect of PAE and CTE in HEp2 Cells
3.4. Determination of the Effective Concentration (EC50) and Cytotoxic Concentration (CC50) of PAE and CTE
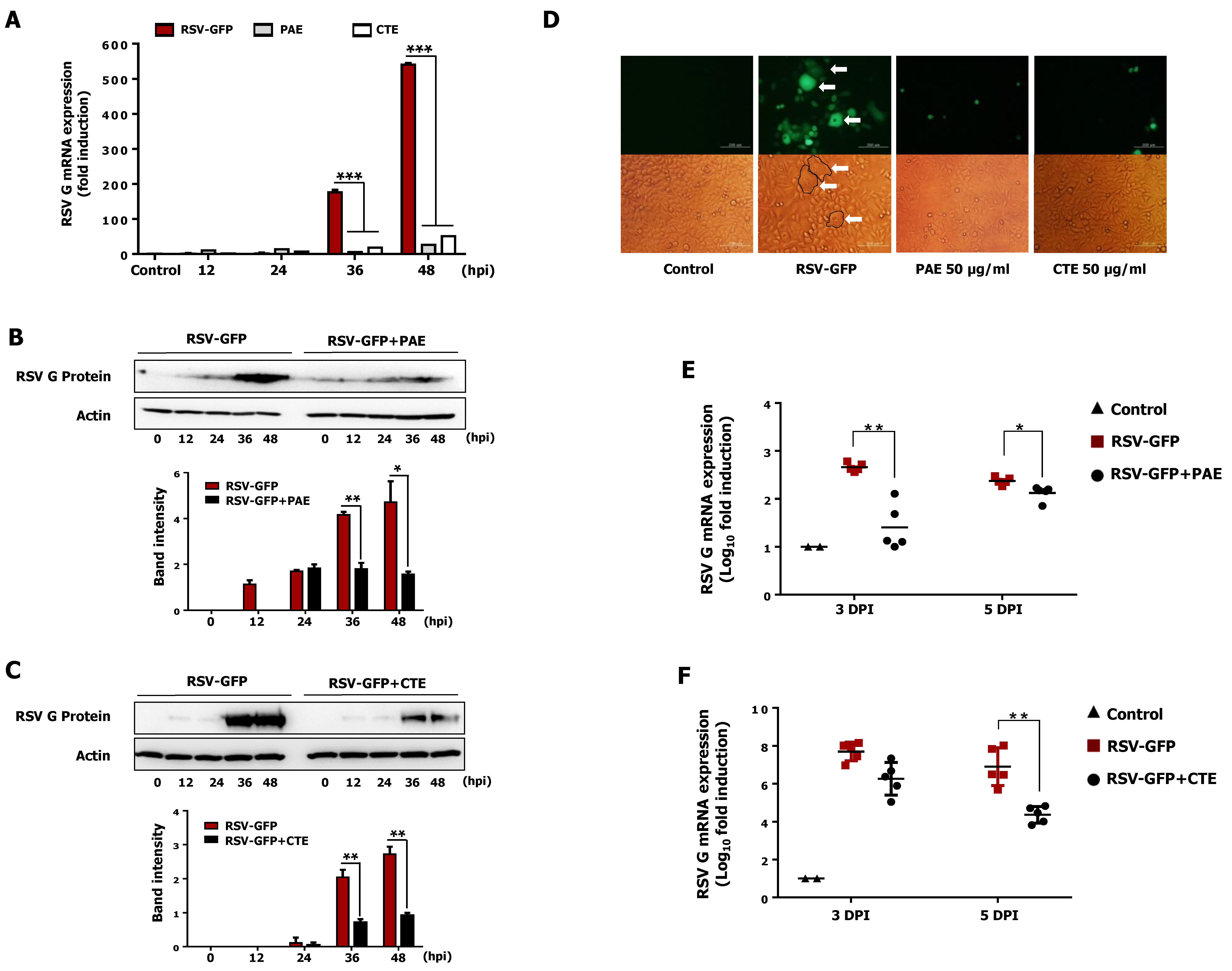
3.5. Effect of PAE and CTE on the Production of Viral RNA and Protein in HEp2 Cells
3.6. PAE and CTE Inhibits RSV Syncytium Formation
3.7. Oral Administration of PAE and CTE Enhance Protection against RSV Infection in BALB/c Mice
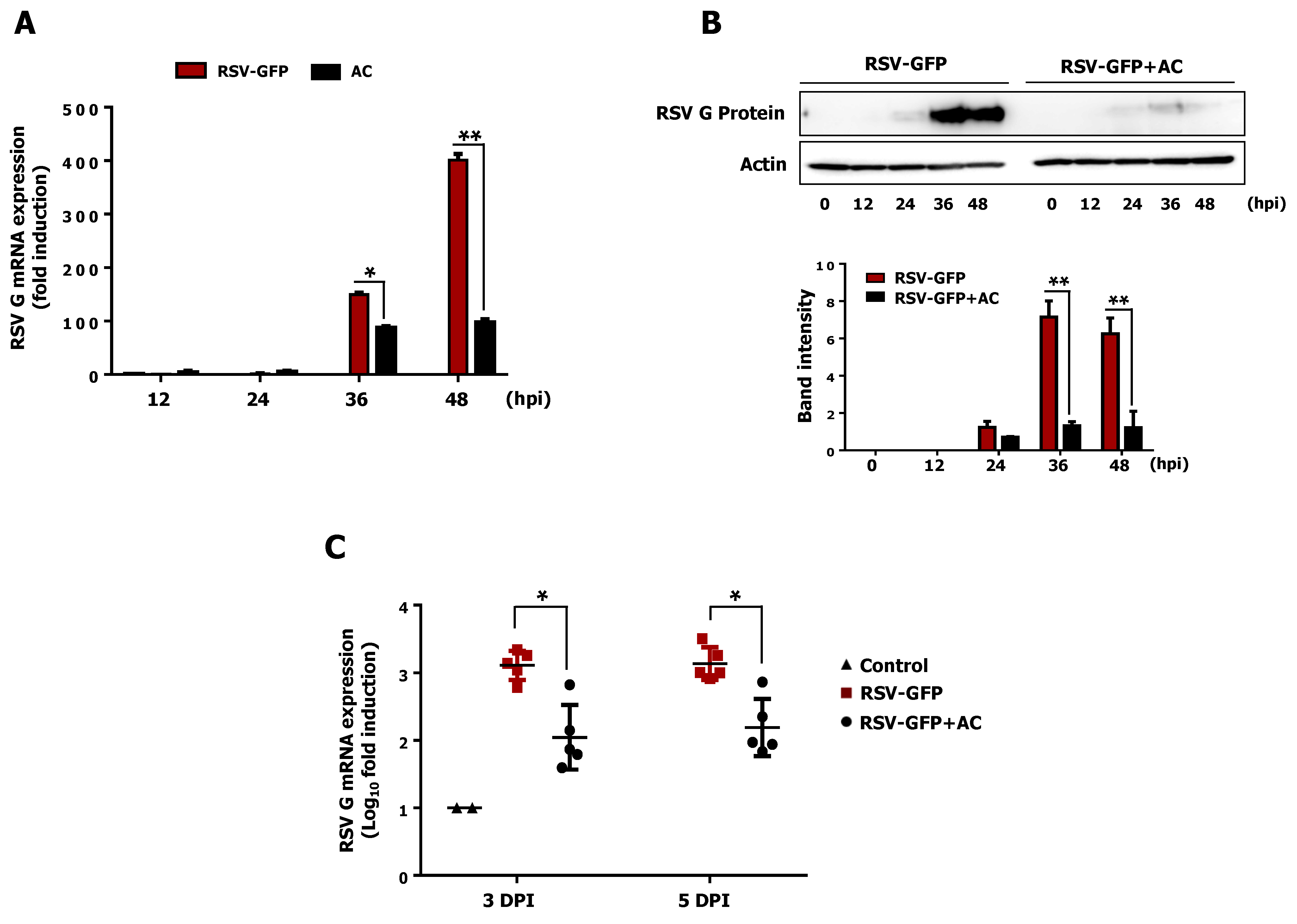
3.8. Acteoside Inhibits RSV Replication at Non-Cytotoxic Concentrations in vitro
3.9. Intraperitoneal Administration of Acteoside Inhibits RSV Infection In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Piedimonte, G.; Perez, M.K. Alternative Mechanisms for Respiratory Syncytial Virus (RSV) Infection and Persistence: Could RSV Be Transmitted Through the Placenta And Persist Into Developing Fetal Lungs? Curr. Opin. Pharmacol. 2014, 16, 82–88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazur, N.I.; Martinón-Torres, F.; Baraldi, E.; Fauroux, B.; Greenough, A.; Heikkinen, T.; Manzoni, P.; Mejias, A.; Nair, H.; Papadopoulos, N.G.; et al. Lower Respiratory Tract Infection Caused by Respiratory Syncytial Virus: Current Management and New Therapeutics. Lancet Respir. Med. 2015, 3, 888–900. [Google Scholar] [CrossRef]
- Walsh, E.; Falsey, A.R. Respiratory Syncytial Virus Infection in Adult Populations. Infect. Disord.-Drug Targets 2012, 12, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Le Nouen, C.; Brock, L.G.; Luongo, C.; McCarty, T.; Yang, L.; Mehedi, M.; Wimmer, E.; Mueller, S.; Collins, P.L.; Buchholz, U.J.; et al. Attenuation of Human Respiratory Syncytial Virus by Genome-Scale Codon-Pair Deoptimization. Proc. Natl. Acad. Sci. USA 2014, 111, 13169–13174. [Google Scholar] [CrossRef]
- Kneyber, M.C.; Moll, H.A.; De Groot, R. Treatment and prevention of respiratory syncytial virus infection. Eur. J. Pediatr. 2000, 159, 399–411. [Google Scholar] [CrossRef]
- Solecki, R.S.; Shanidar, I.V. A Neanderthal Flower Burial in Northern Iraq. Science 1975, 190, 880–881. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Schuppan, D.; Afdhal, N.H. Liver Cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Lin, C.C.; Kan, W.S. Medicinal Plants Used for the Treatment of Hepatitis in Taiwan. Am. J. Chin. Med. 1990, 18, 35–43. [Google Scholar] [CrossRef]
- Marlett, J.A.; Fischer, M.H. The Active Fraction of Psyllium Seed Husk. Proc. Nutr. Soc. 2003, 62, 207–209. [Google Scholar] [CrossRef]
- Ramkumar, D.; Rao, S.S. Efficacy and Safety of Traditional Medical Therapies for Chronic Constipation: Systematic Review. Am. J. Gastroenterol. 2005, 100, 936–971. [Google Scholar] [CrossRef] [PubMed]
- Singh, B. Psyllium as Therapeutic and Drug Delivery Agent. Int. J. Pharm. 2007, 334, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Lin, C.C. In Vitro Cytotoxic, Antiviral and Immunomodulatory Effects of Plantago Major and Plantago Asiatica. Am. J. Chin. Med. 2003, 31, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Whang, W.K.; Kim, H.J. Studies on the Anti-Inflammatory Effects Ofclerodendron Trichotomum Thunberg Leaves. Arch. Pharmacal Res. 2004, 27, 189–193. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, S.; Jung, M.Y.; Ham, I.H.; Whang, W.K. Anti-Inflammatory Phenylpropanoid Glycosides from Clerodendron Trichotomum Leaves. Arch. Pharmacal Res. 2009, 32, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.G.; Lee, Y.S.; Kim, H.J.; Lee, Y.M.; Lee, H.S. Angiotensin Converting Enzyme Inhibitory Phenylpropanoid Glycosides from Clerodendron Trichotomum. J. Ethnopharmacol. 2003, 89, 151–154. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.G.; Sim, S.S.; Whang, W.K.; Kim, C.J. Anti-Asthmatic Effects of Phenylpropanoid Glycosides from Clerodendron Trichotomum Leaves and Rumex Gmelini Herbes in Conscious Guinea-Pigs Challenged with Aerosolized Ovalbumin. Phytomedicine 2011, 18, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.W.; Kang, K.A.; Kim, J.S.; Kim, H.K.; Lee, E.J.; Hyun, J.W.; Kang, S.S. Antioxidant activities of acetylmartynosides from Clerodendron trichotomum. J. Appl. Biol. Chem. 2007, 50, 270–274. [Google Scholar]
- Lee, S.J.; Moon, H.I. Immunotoxicity activity of 2,6,10,15-tetrameheptadecane from the essential oils of Clerodendron trichotomum Thunb. against Aedes aegypti L. Immunopharmacol. Immunotoxicol. 2010, 32, 705–707. [Google Scholar] [CrossRef]
- Moon, H.J.; Lee, J.S.; Choi, Y.K.; Park, J.Y.; Talactac, M.R.; Chowdhury, M.Y.; Poo, H.; Sung, M.H.; Lee, J.H.; Jung, J.H.; et al. Induction of Type I Interferon by High-Molecular Poly-Γ-Glutamate Protects B6.A2G-Mx1 Mice Against Influenza a Virus. Antiviral Res. 2012, 94, 98–102. [Google Scholar] [CrossRef]
- Nguyen, D.T.; De Witte, L.; Ludlow, M.; Yüksel, S.; Wiesmüller, K.H.; Geijtenbeek, T.B.H.; Osterhaus, A.D.M.E.; De Swart, R.L. The Synthetic Bacterial Lipopeptide Pam3csk4 Modulates Respiratory Syncytial Virus Infection Independent of TLR Activation. PLoS Pathog. 2010, 6, e1001049. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001. [Google Scholar] [CrossRef]
- Kuhn, D.M.; Balkis, M.; Chandra, J.; Mukherjee, P.; Ghannoum, M. Uses and Limitations of the XTT Assay in Studies of Candida Growth and Metabolism. J. Clin. Microbiol. 2003, 41, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Magadula, J.; Suleimani, H. Cytotoxic and Anti-HIV Activities of Some Tanzanian Garcinia Species. Tanzan. J. Health Res. 2010, 12, 144–149. [Google Scholar] [CrossRef]
- Lee, B.H.; Chathuranga, K.; Uddin, M.B.; Weeratunga, P.; Kim, M.S.; Cho, W.K.; Kim, H.I.; Ma, J.Y.; Lee, J.S. Coptidis rhizoma extract inhibits replication of respiratory syncytial virus in vitro and in vivo by inducing antiviral state. J. Microbiol. 2017, 55, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.F.; Tang, Y.F.; Nie, S.P.; Wan, Y.; Xie, M.Y.; Xie, X.M. Effect of Phenylethanoid Glycosides and Polysaccharides from The Seed of Plantago Asiatica L. On the Maturation of Murine Bone Marrow-Derived Dendritic Cells. Eur. J. Pharmacol. 2009, 620, 105–111. [Google Scholar] [CrossRef]
- Song, X.; He, J.; Xu, H.; Hu, X.P.; Wu, X.L.; Wu, H.Q.; Liu, L.Z.; Liao, C.H.; Zeng, Y.; Li, Y. The Antiviral Effects of Acteoside and The Underlying IFN-γ-Inducing Action. Food Funct. 2016, 7, 3017–3030. [Google Scholar] [CrossRef]
- Ma, S.C.; Du, J.; But, P.P.H.; Deng, X.L.; Zhang, Y.W.; Ooi, V.E.C.; Xu, H.X.; Lee, S.H.S.; Lee, S.F. Antiviral Chinese medicinal herbs against respiratory syncytial virus. J. Ethnopharmacol. 2002, 79, 205–211. [Google Scholar] [CrossRef]
- Li, L.; Yu, C.H.; Ying, H.Z.; Yu, J.M. Antiviral effects of modified Dingchuan decoction against respiratory syncytial virus infection in vitro and in an immunosuppressive mouse model. J. Ethnopharmacol. 2013, 147, 238–244. [Google Scholar] [CrossRef]
- Pan, S.Y.; Zhou, S.F.; Gao, S.H.; Yu, Z.L.; Zhang, S.; Tang, M.; Sun, J.; Ma, D.; Han, Y.; Fong, W.; et al. New Perspectives on How to Discover Drugs from Herbal Medicines: CAM’s Outstanding Contribution to Modern Therapeutics. Evid.-Based Complement. Altern. Med. 2013, 2013, 627375. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Peng, T. Traditional Chinese Herbal Medicine as A Source of Molecules with Antiviral Activity. Antivir. Res. 2013, 97, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Tsai, F.J.; Tsai, C.H.; Lai, C.C.; Wan, L.; Ho, T.Y.; Hsieh, C.; Chao, P. Anti-SARS Coronavirus 3C-Like Protease Effects of Isatis Indigotica Root and Plant-Derived Phenolic Compounds. Antivir. Res. 2005, 68, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Michaelis, M.; Hsu, H.K.; Tsai, C.C.; Yang, K.D.; Wu, Y.C.; Cinatl, J.; Doerr, H. Toona Sinensis Roem Tender Leaf Extract Inhibits SARS Coronavirus Replication. J. Ethnopharmacol. 2008, 120, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chan, K.H.; Jiang, Y.; Kao, R.Y.; Lu, H.T.; Fan, K.W.; Cheng, V.; Tsui, W.; Hung, I.; Lee, T. In Vitro Susceptibility of 10 Clinical Isolates of SARS Coronavirus to Selected Antiviral Compounds. J. Clin. Virol. 2004, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Haruyama, T.; Nagata, K. Anti-Influenza Virus Activity of Ginkgo Biloba Leaf Extracts. J. Nat. Med. 2012, 67, 636–642. [Google Scholar] [CrossRef]
- Makau, J.N.; Watanabe, K.; Kobayashi, N. Anti-influenza activity of Alchemilla mollis extract: Possible virucidal activity against influenza virus particles. Drug Discov. Ther. 2013, 7, 18–195. [Google Scholar] [CrossRef][Green Version]
- Reinke, D.; Kritas, S.; Polychronopoulos, P.; Skaltsounis, A.L.; Aligiannis, N.; Tran, C.D. Herbal substance, acteoside, alleviates intestinal mucositis in mice. Gastroenterol. Res. Pract. 2015, 2015, 327872. [Google Scholar] [CrossRef]
- Boukhvalova, M.S.; Yim, K.C.; Prince, G.A.; Blanco, J.C. Methods for monitoring dynamics of pulmonary RSV replication by viral culture and by real-time reverse transcription–PCR in vivo: Detection of abortive viral replication. Curr. Protoc. Cell Biol. 2010, 46, 26.6.1–26.6.19. [Google Scholar]
- Li, L.; Tsao, R.; Liu, Z.; Liu, S.; Yang, R.; Young, J.C.; Zhu, H.; Deng, Z.; Xie, M.; Fu, Z. Isolation and purification of acteoside and isoacteoside from Plantago psyllium L. by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1063, 161–169. [Google Scholar] [CrossRef]
- Miyase, T.; Ishino, M.; Akahori, C.; Ueno, A.; Ohkawa, Y.; Tanizawa, H. Phenylethanoid Glycosides from Plantago Asiatica. Phytochemistry 1991, 30, 2015–2018. [Google Scholar] [CrossRef]
- Xiong, Q.; Hase, K.; Tezuka, Y.; Tani, T.; Namba, T.; Kadota, S. Hepatoprotective Activity of Phenylethanoids Fromcistanche Deserticola. Planta Medica 1998, 64, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Schapoval, E.E.; Winter de Vargas, M.R.; Chaves, C.G.; Bridi, R.; Zuanazzi, J.A.; Henriques, A.T. Antiinflammatory and Antinociceptive Activities of Extracts and Isolated Compounds from Stachytarpheta Cayennensis. J. Ethnopharmacol. 1998, 60, 53–59. [Google Scholar] [CrossRef]
- He, Z.D.; Lau, K.M.; Xu, H.X.; Li, P.C.; Pui-Hay, B. Antioxidant Activity of Phenylethanoid Glycosides from Brandisia Hancei. J. Ethnopharmacol. 2000, 71, 483–486. [Google Scholar] [CrossRef]
- Sahpaz, S.; Garbacki, N.; Tits, M.; Bailleul, F. Isolation and Pharmacological Activity of Phenylpropanoid Esters from Marrubium Vulgare. J. Ethnopharmacol. 2002, 79, 389–392. [Google Scholar] [CrossRef]
- Kim, H.J.; Woo, E.R.; Shin, C.G.; Hwang, D.J.; Park, H.; Lee, Y.S. HIV-1 Integrase Inhibitory Phenylpropanoid Glycosides Fromclerodendron Trichotomum. Arch. Pharmacal Res. 2001, 24, 286–291. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A Review of its Occurrence, (Bio)Synthesis and Pharmacological Significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Aligiannis, N.; Mitaku, S.; Tsitsa-Tsardis, E.; Harvala, C.; Tsaknis, I.; Lalas, S.; Haroutounian, S. Methanolic Extract Ofverbascum Macrurumas a Source of Natural Preservatives Against Oxidative Rancidity. J. Agric. Food Chem. 2003, 51, 7308–7312. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chathuranga, K.; Kim, M.S.; Lee, H.-C.; Kim, T.-H.; Kim, J.-H.; Gayan Chathuranga, W.A.; Ekanayaka, P.; Wijerathne, H.M.S.M.; Cho, W.-K.; Kim, H.I.; et al. Anti-Respiratory Syncytial Virus Activity of Plantago asiatica and Clerodendrum trichotomum Extracts In Vitro and In Vivo. Viruses 2019, 11, 604. https://doi.org/10.3390/v11070604
Chathuranga K, Kim MS, Lee H-C, Kim T-H, Kim J-H, Gayan Chathuranga WA, Ekanayaka P, Wijerathne HMSM, Cho W-K, Kim HI, et al. Anti-Respiratory Syncytial Virus Activity of Plantago asiatica and Clerodendrum trichotomum Extracts In Vitro and In Vivo. Viruses. 2019; 11(7):604. https://doi.org/10.3390/v11070604
Chicago/Turabian StyleChathuranga, Kiramage, Myun Soo Kim, Hyun-Cheol Lee, Tae-Hwan Kim, Jae-Hoon Kim, W. A. Gayan Chathuranga, Pathum Ekanayaka, H. M. S. M. Wijerathne, Won-Kyung Cho, Hong Ik Kim, and et al. 2019. "Anti-Respiratory Syncytial Virus Activity of Plantago asiatica and Clerodendrum trichotomum Extracts In Vitro and In Vivo" Viruses 11, no. 7: 604. https://doi.org/10.3390/v11070604
APA StyleChathuranga, K., Kim, M. S., Lee, H.-C., Kim, T.-H., Kim, J.-H., Gayan Chathuranga, W. A., Ekanayaka, P., Wijerathne, H. M. S. M., Cho, W.-K., Kim, H. I., Ma, J. Y., & Lee, J.-S. (2019). Anti-Respiratory Syncytial Virus Activity of Plantago asiatica and Clerodendrum trichotomum Extracts In Vitro and In Vivo. Viruses, 11(7), 604. https://doi.org/10.3390/v11070604





