Porcine HMGCR Inhibits Porcine Circovirus Type 2 Infection by Directly Interacting with the Viral Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Cells
2.2. Drugs and Antibodies
2.3. Plasmid Construction
2.4. Virus Infection and Treatment
2.5. Real-Time PCR
2.6. MTS Assay
2.7. Confocal Fluorescence Microscopy
2.8. Coimmunoprecipitation (Co-IP)
2.9. Western Blot (WB)
2.10. Statistical Analysis
3. Results
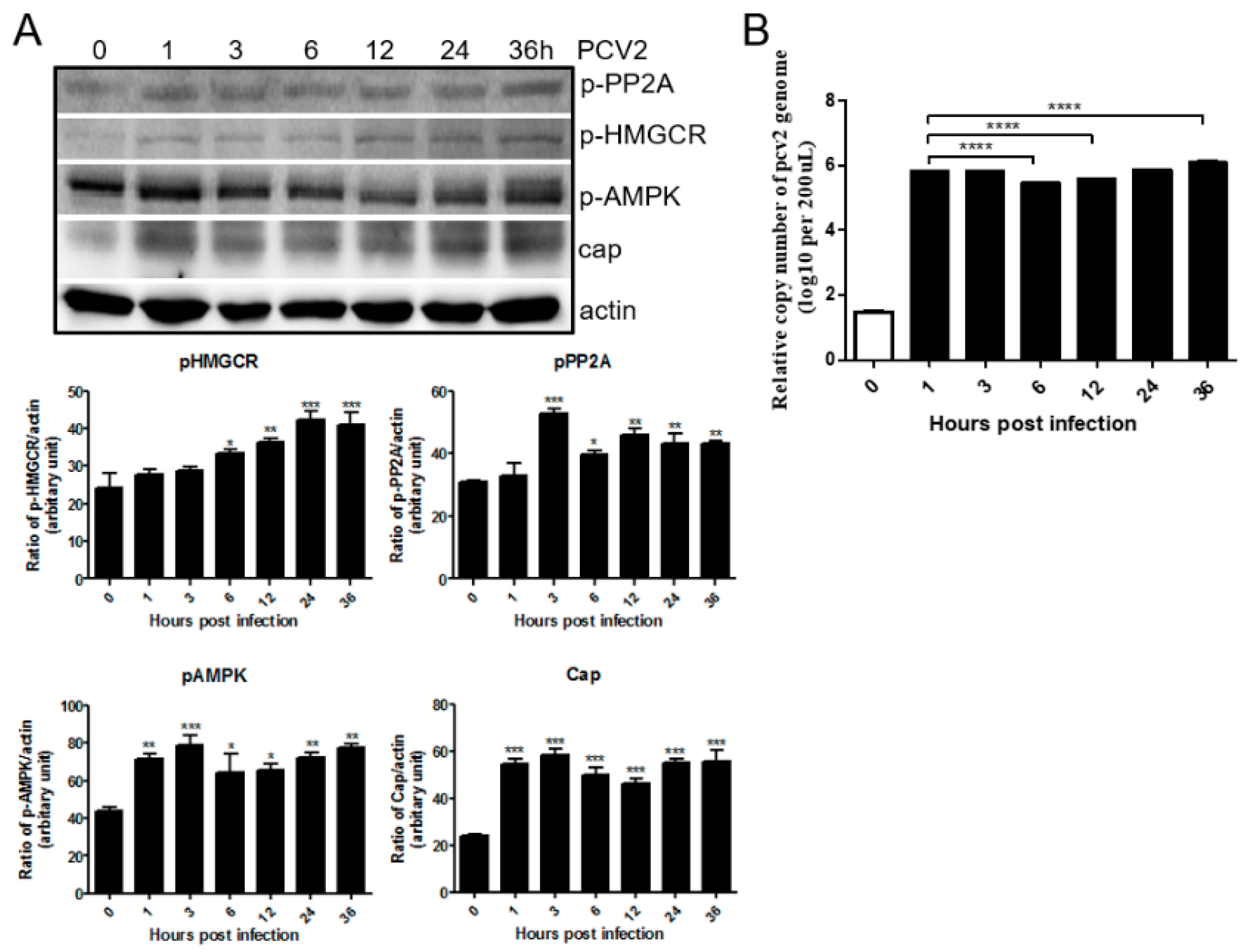
3.1. PCV2 Infection Increases Phosphorylation of PP2A, AMPK and HMGCR
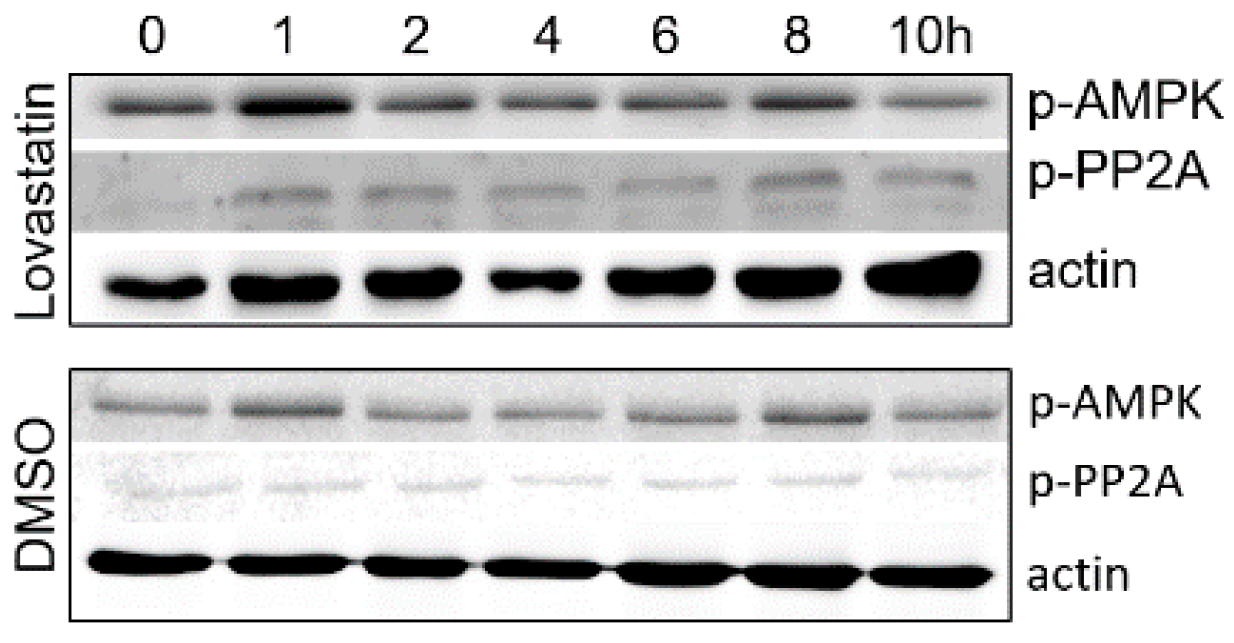
3.2. Inhibition of HMGCR by Lovastatin Has No Effect on the Activities of AMPK and PP2A during PCV2 Infection
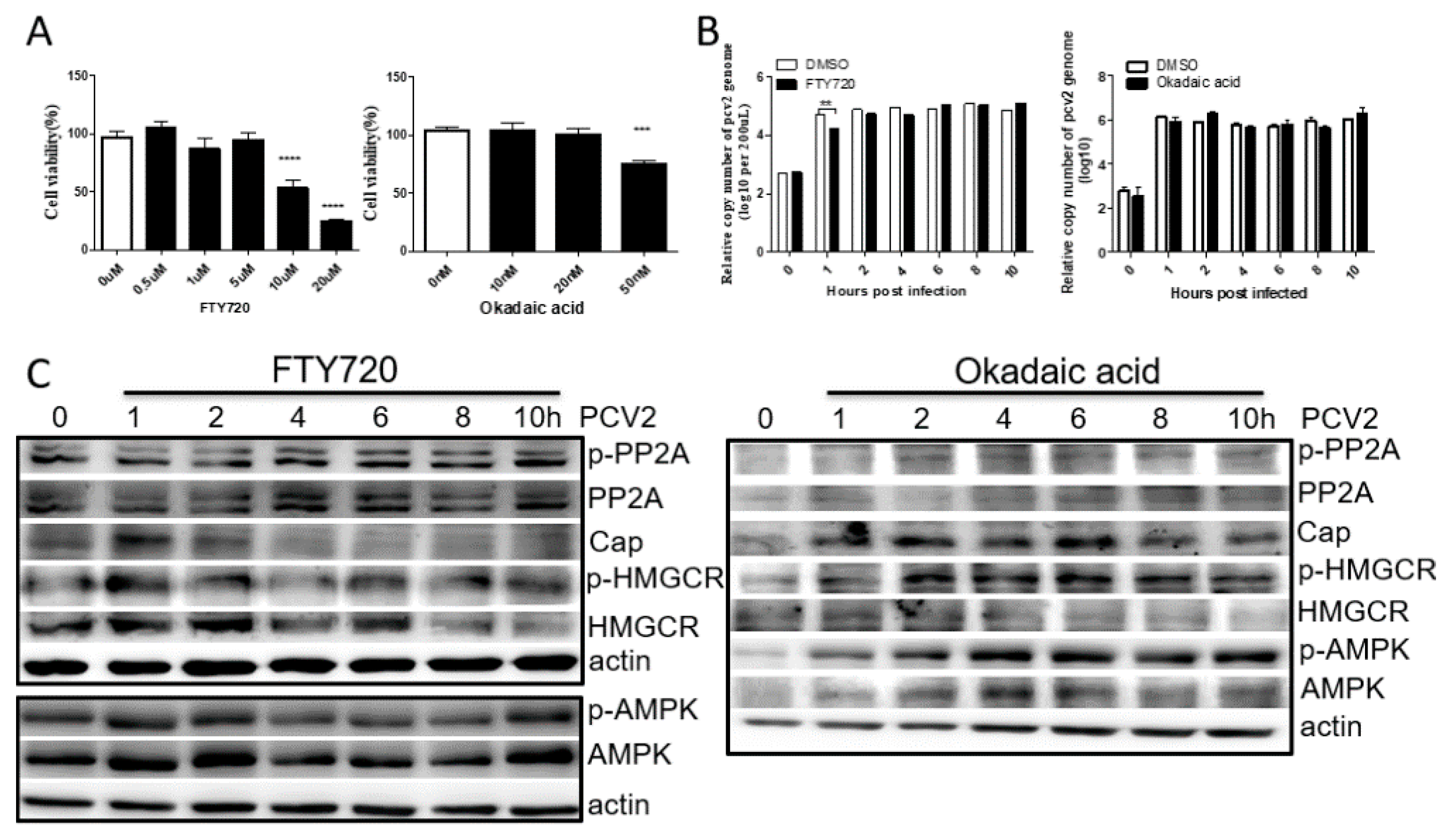
3.3. PP2A Has Little Effect on PCV2 Infection and HMGCR Activity
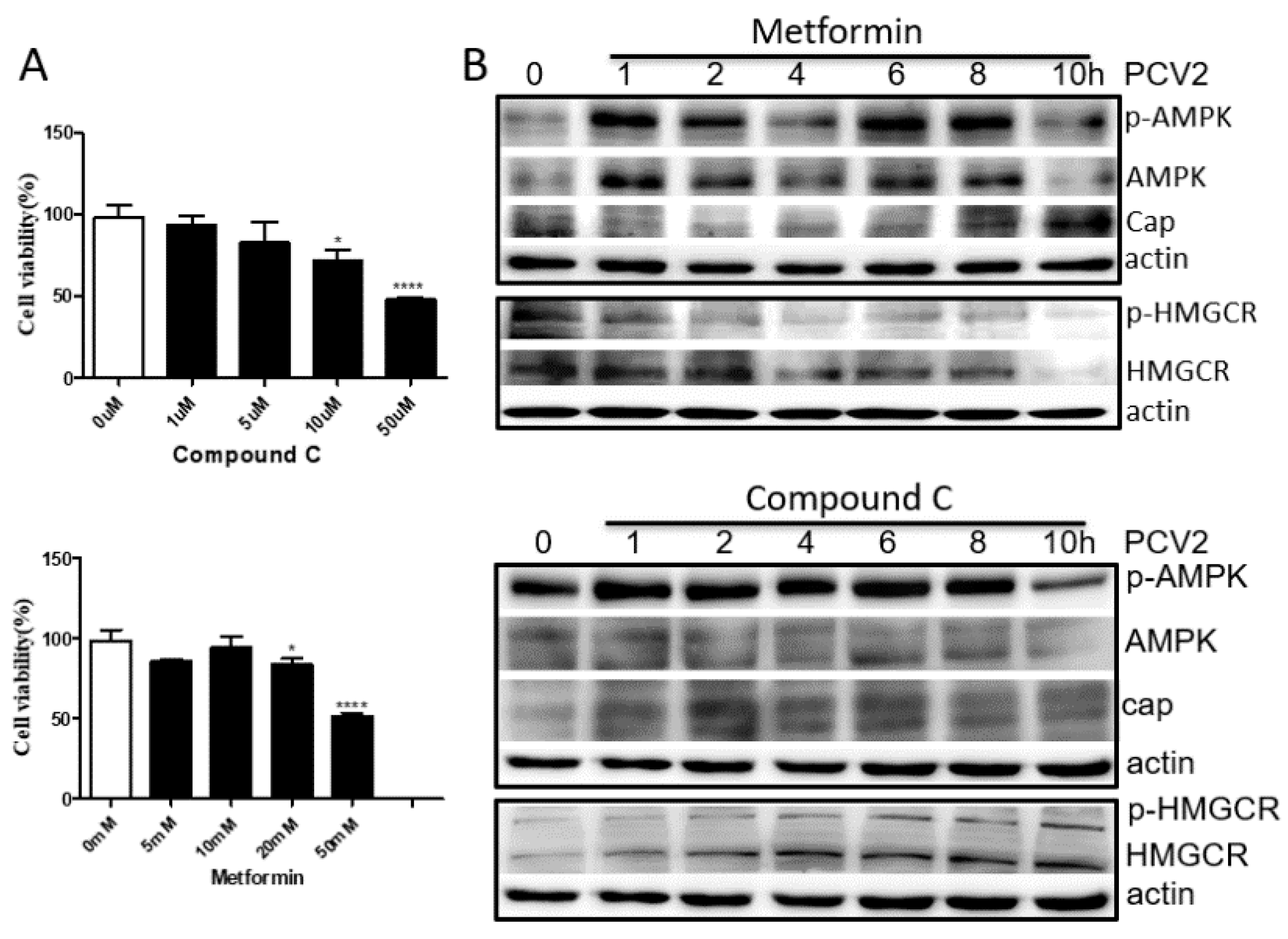
3.4. HMGCR Activity Is Mainly Regulated by AMPK during PCV2 Infection
3.5. Construction of Recombinant Expression Plasmids
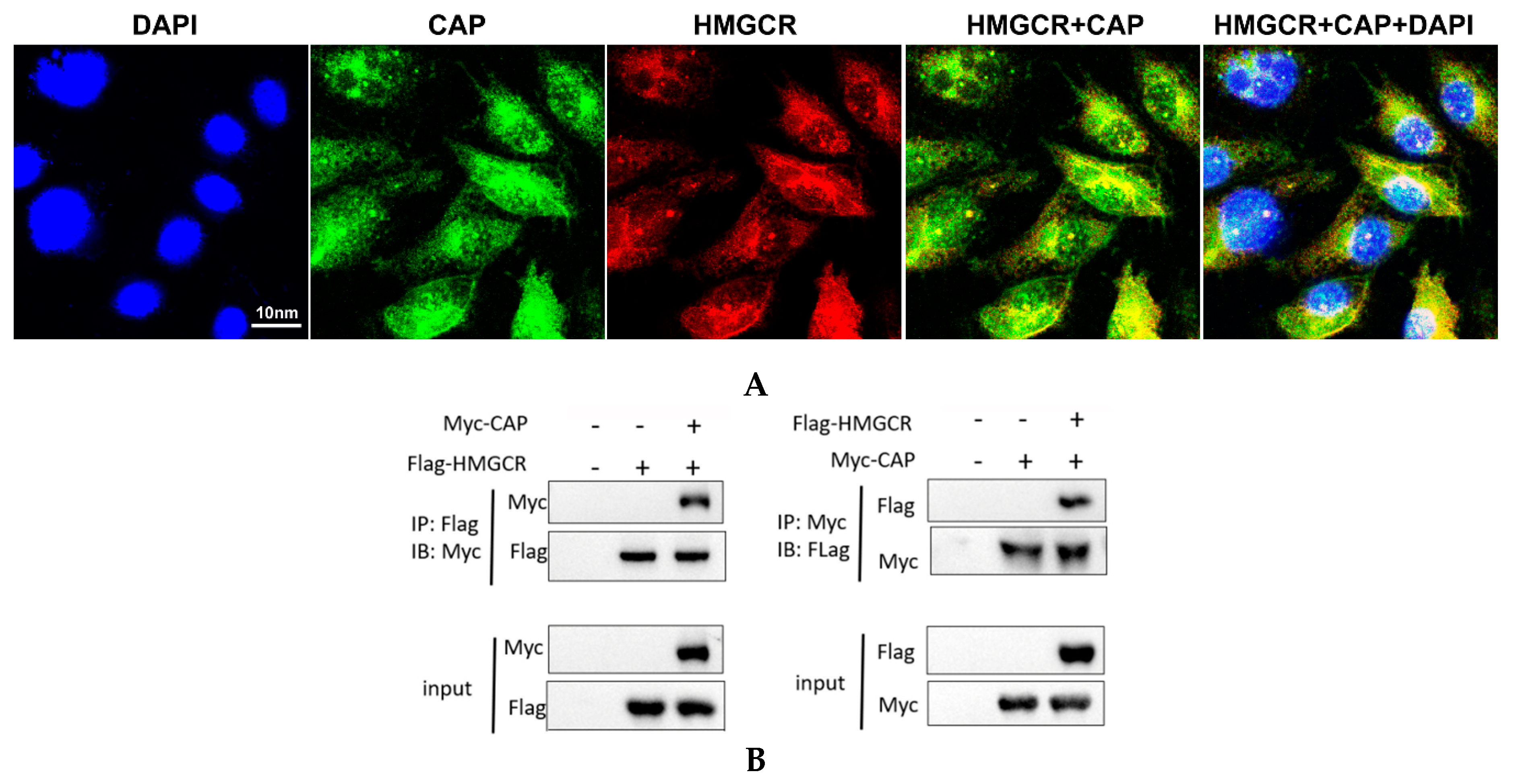
3.6. HMGCR Interacts with the Cap Protein of PCV2
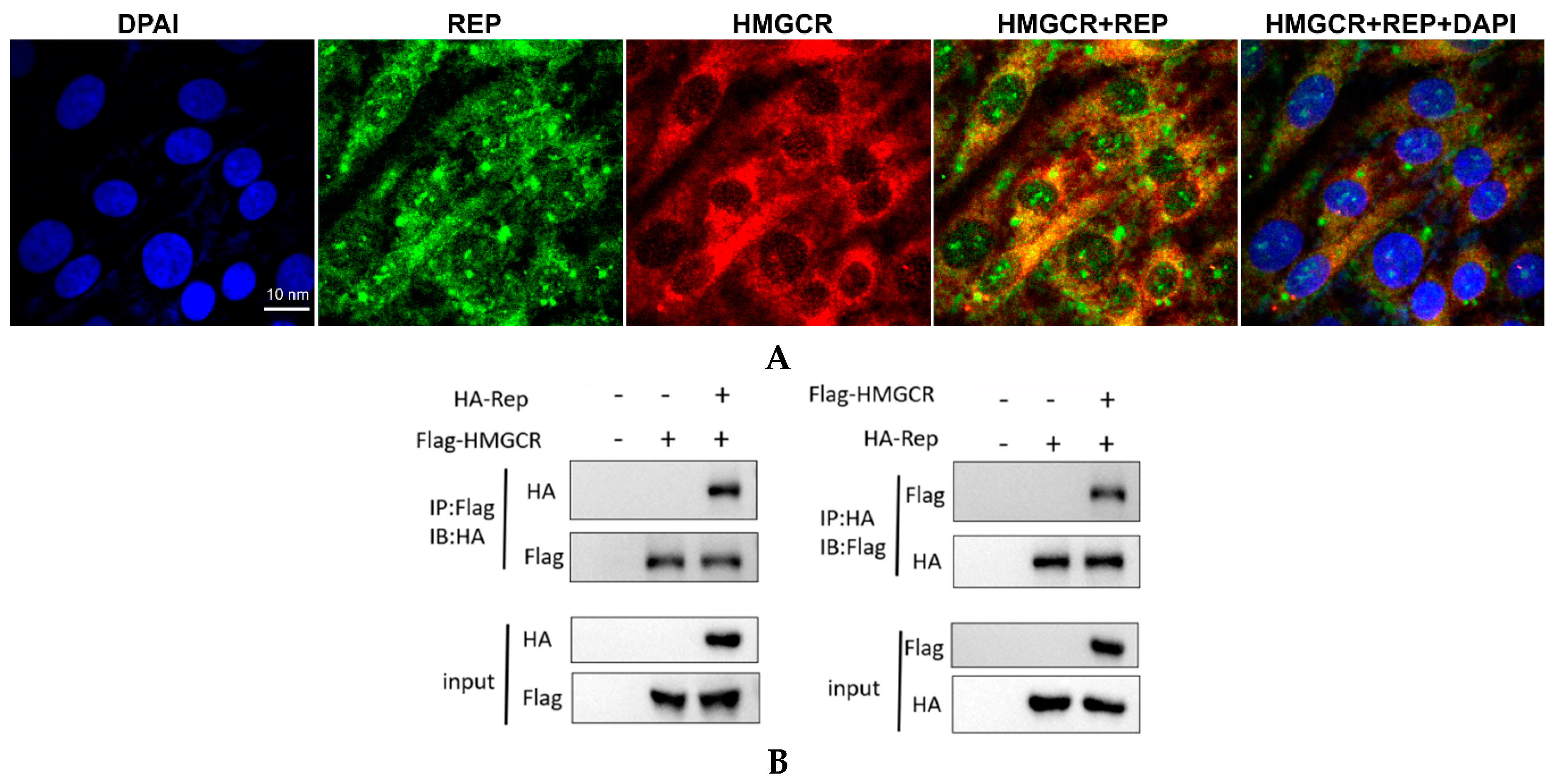
3.7. HMGCR Interacts with the Rep Protein of PCV2
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Finsterbusch, T.; Mankertz, A. Porcine circoviruses—Small but powerful. Virus Res. 2009, 143, 177–183. [Google Scholar] [CrossRef]
- Segales, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef]
- Ren, L.; Chen, X.; Ouyang, H. Interactions of porcine circovirus 2 with its hosts. Virus Genes 2016. [Google Scholar] [CrossRef]
- Misinzo, G.; Delputte, P.L.; Meerts, P.; Lefebvre, D.J.; Nauwynck, H.J. Porcine circovirus 2 uses heparan sulfate and chondroitin sulfate B glycosaminoglycans as receptors for its attachment to host cells. J. Virol. 2006, 80, 3487–3494. [Google Scholar] [CrossRef]
- Roitelman, J.; Olender, E.H.; Bar-Nun, S.; Dunn, W.A., Jr.; Simoni, R.D. Immunological evidence for eight spans in the membrane domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase: Implications for enzyme degradation in the endoplasmic reticulum. J. Cell Biol. 1992, 117, 959–973. [Google Scholar] [CrossRef]
- Ravi, L.I.; Liang, L.; Wong, P.S.; Brown, G.; Tan, B.H.; Sugrue, R.J. Increased hydroxymethylglutaryl coenzyme A reductase activity during respiratory syncytial virus infection mediates actin dependent inter-cellular virus transmission. Antivir. Res. 2013, 100, 259–268. [Google Scholar] [CrossRef]
- Soto-Acosta, R.; Mosso, C.; Cervantes-Salazar, M.; Puerta-Guardo, H.; Medina, F.; Favari, L.; Ludert, J.E.; Angel, R.M. The increase in cholesterol levels at early stages after dengue virus infection correlates with an augment in LDL particle uptake and HMG-CoA reductase activity. Virology 2013, 442, 132–147. [Google Scholar] [CrossRef]
- Soto-Acosta, R.; Bautista-Carbajal, P.; Cervantes-Salazar, M.; Angel-Ambrocio, A.H.; Del Angel, R.M. DENV up-regulates the HMG-CoA reductase activity through the impairment of AMPK phosphorylation: A potential antiviral target. PLoS Pathog. 2017, 13, e1006257. [Google Scholar] [CrossRef]
- Serquina, A.K.P.; Kambach, D.M.; Sarker, O.; Ziegelbauer, J.M. Viral MicroRNAs Repress the Cholesterol Pathway, and 25-Hydroxycholesterol Inhibits Infection. MBio 2017, 8, e00576. [Google Scholar] [CrossRef]
- Yang, X.; Ma, T.; Ouyang, H.; Chen, F.; Peng, Z.; Li, C.; Ma, Y.; Chen, X.; Li, B.; Pang, D.; et al. Effect of atovastatin treatment on porcine circovirus 2 infection in BALB/c mice. Clin. Exp. Pharmacol. Physiol. 2015, 42, 817–821. [Google Scholar] [CrossRef]
- Ma, T.; Chen, X.; Ouyang, H.; Liu, X.; Ouyang, T.; Peng, Z.; Yang, X.; Chen, F.; Pang, D.; Bai, J.; et al. HMGCR inhibits the early stage of PCV2 infection, while PKC enhances the infection at the late stage. Virus Res. 2017, 229, 41–47. [Google Scholar] [CrossRef]
- Yang, X.; Ouyang, H.; Chen, F.; Pang, D.; Dong, M.; Yang, S.; Liu, X.; Peng, Z.; Wang, F.; Zhang, X.; et al. HMG-CoA reductase is negatively associated with PCV2 infection and PCV2-induced apoptotic cell death. J. Gen. Virol. 2014, 95, 1330–1337. [Google Scholar] [CrossRef][Green Version]
- Goldstein, J.L.; Brown, M.S. Regulation of the mevalonate pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef]
- Yang, X.; Chen, F.; Cao, Y.; Pang, D.; Ouyang, H.; Ren, L. Complete genome sequence of porcine circovirus 2b strain CC1. J. Virol. 2012, 86, 9536. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, T.; Ouyang, H.; Liu, X.; Niu, G.; Huo, W.; Yin, W.; Pang, D.; Ren, L. Human cells are permissive for the productive infection of porcine circovirus type 2 in vitro. Sci. Rep. 2019, 9, 5638. [Google Scholar] [CrossRef]
- Grierson, S.S.; King, D.P.; Sandvik, T.; Hicks, D.; Spencer, Y.; Drew, T.W.; Banks, M. Detection and genetic typing of type 2 porcine circoviruses in archived pig tissues from the UK. Arch. Virol. 2004, 149, 1171–1183. [Google Scholar] [CrossRef]
- Shen, H.G.; Zhou, J.Y.; Huang, Z.Y.; Guo, J.Q.; Xing, G.; He, J.L.; Yan, Y.; Gong, L.Y. Protective immunity against porcine circovirus 2 by vaccination with ORF2-based DNA and subunit vaccines in mice. J. Gen. Virol. 2008, 89, 1857–1865. [Google Scholar] [CrossRef]
- Chen, F.; Yang, X.; Pang, D.; Peng, Z.; Dong, M.; Liu, X.; Ouyang, H.; Ren, L. Expression, purification and antibody preparation using different constructs of PCV2 capsid protein. Int. J. Biol. Macromol. 2014, 67, 289–294. [Google Scholar] [CrossRef]
- Peng, Z.; Ma, T.; Pang, D.; Su, D.; Chen, F.; Chen, X.; Guo, N.; Ouyang, T.; Ouyang, H.; Ren, L. Expression, purification and antibody preparation of PCV2 Rep and ORF3 proteins. Int. J. Biol. Macromol. 2016, 86, 277–281. [Google Scholar] [CrossRef]
- Wu, Y.; Song, P.; Xu, J.; Zhang, M.; Zou, M.H. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J. Biol. Chem. 2007, 282, 9777–9788. [Google Scholar] [CrossRef]
- Joseph, B.K.; Liu, H.Y.; Francisco, J.; Pandya, D.; Donigan, M.; Gallo-Ebert, C.; Giordano, C.; Bata, A.; Nickels, J.T., Jr. Inhibition of AMP Kinase by the Protein Phosphatase 2A Heterotrimer, PP2APpp2r2d. J. Biol. Chem. 2015, 290, 10588–10598. [Google Scholar] [CrossRef]
- Park, S.; Scheffler, T.L.; Rossie, S.S.; Gerrard, D.E. AMPK activity is regulated by calcium-mediated protein phosphatase 2A activity. Cell Calcium 2013, 53, 217–223. [Google Scholar] [CrossRef]
- Huang, L.; Van Renne, N.; Liu, C.; Nauwynck, H.J. A sequence of basic residues in the porcine circovirus type 2 capsid protein is crucial for its co-expression and co-localization with the replication protein. J. Gen. Virol. 2015, 96, 3566–3576. [Google Scholar] [CrossRef][Green Version]
- Timmusk, S.; Fossum, C.; Berg, M. Porcine circovirus type 2 replicase binds the capsid protein and an intermediate filament-like protein. J. Gen. Virol. 2006, 87, 3215–3223. [Google Scholar] [CrossRef]
- Rodriguez-Carino, C.; Duffy, C.; Sanchez-Chardi, A.; McNeilly, F.; Allan, G.M.; Segales, J. Porcine circovirus type 2 morphogenesis in a clone derived from the l35 lymphoblastoid cell line. J. Comp. Pathol. 2011, 144, 91–102. [Google Scholar] [CrossRef]
- Sarker, S.; Terron, M.C.; Khandokar, Y.; Aragao, D.; Hardy, J.M.; Radjainia, M.; Jimenez-Zaragoza, M.; de Pablo, P.J.; Coulibaly, F.; Luque, D.; et al. Structural insights into the assembly and regulation of distinct viral capsid complexes. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Hou, Q.; Hou, S.H.; Chen, Q.; Jia, H.; Xin, T.; Jiang, Y.T.; Guo, X.Y.; Zhu, H.F. Nuclear localization signal regulates porcine circovirus type 2 capsid protein nuclear export through phosphorylation. Virus Res. 2018, 246, 12–22. [Google Scholar] [CrossRef]
- Kovacs, W.J.; Tape, K.N.; Shackelford, J.E.; Duan, X.Y.; Kasumov, T.; Kelleher, J.K.; Brunengraber, H.; Krisans, S.K. Localization of the pre-squalene segment of the isoprenoid biosynthetic pathway in mammalian peroxisomes. Histochem. Cell Biol. 2007, 127, 273–290. [Google Scholar] [CrossRef]
- DeBose-Boyd, R.A. Feedback regulation of cholesterol synthesis: Sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell Res. 2008, 18, 609–621. [Google Scholar] [CrossRef]
- Wang, Z.J.; Xu, C.M.; Song, Z.B.; Wang, M.; Liu, Q.Y.; Jiang, P.; Li, Y.F.; Bai, J.; Wang, X.W. Vimentin modulates infectious porcine circovirus type 2 in PK-15 cells. Virus Res. 2018, 243, 110–118. [Google Scholar] [CrossRef]
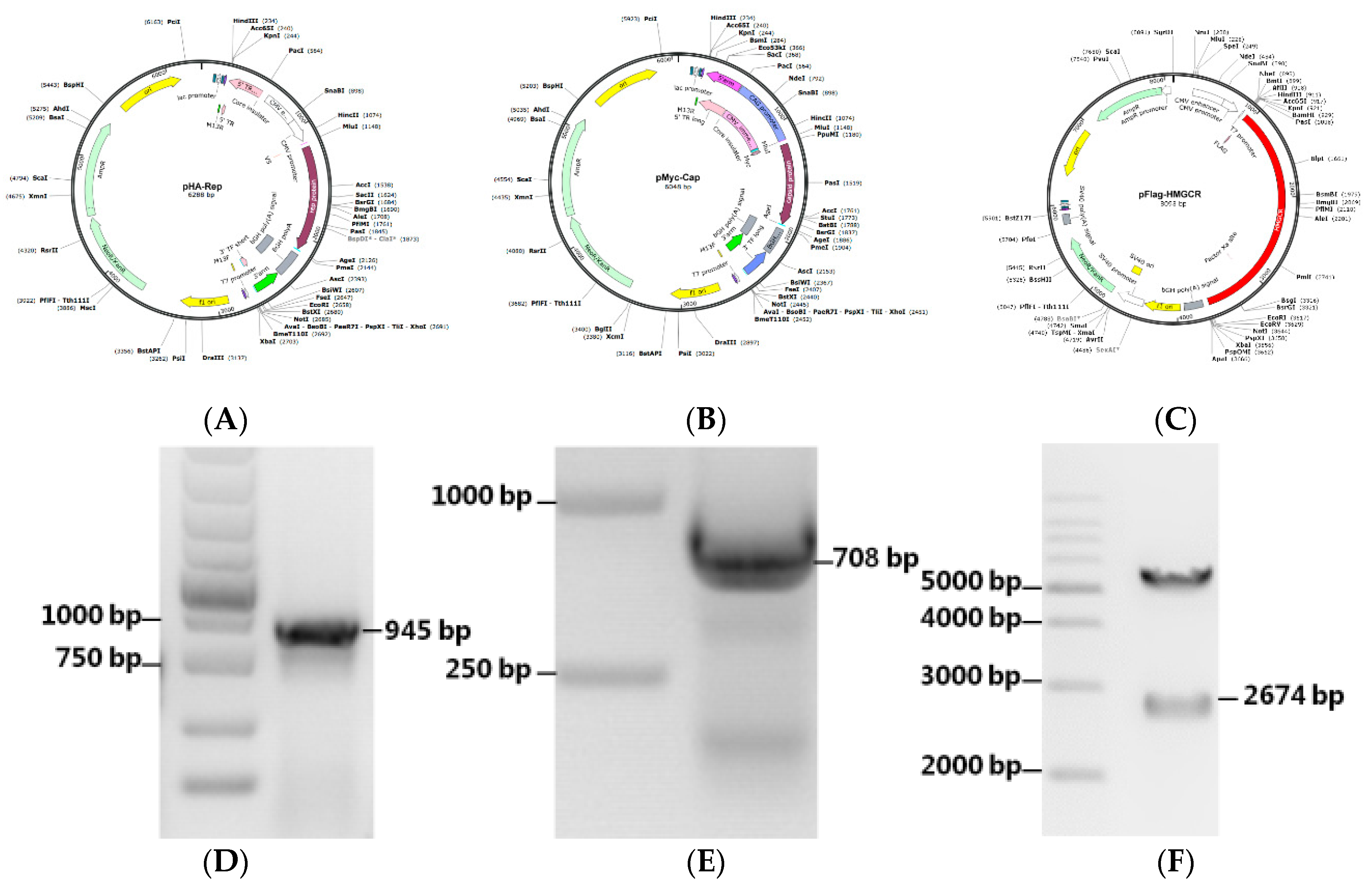
| Primer | Oligonucleotide Sequences (5′–3′) | Cloning Site | Size (bp) |
|---|---|---|---|
| Rep-F | acgcgtatgtacccatacgacgtaccagattacgctcccagcaaaaagaatggaagaag | Mlu I Age I | 945 |
| Rep-R | cgaccggttcagtaatttatttcatatggaaattcag | ||
| Cap-F | acgcgtatggagcagaagctgatctcagaggaggacctgacgtatccaaggaggcgtta | Mlu I Age I | 708 |
| Cap-R | cgaccggtttaagggttaagtggggggtctt | ||
| HMGCR-F | ggatccatgttgtcaagactcttccgaatgc | BamH I EcoR I | 2674 |
| HMGCR-R | gaattctcaagctgccttcttagtgcaag |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, T.; Niu, G.; Zhang, Y.; Liu, X.; Zhang, X.; Zhang, S.; Geng, Y.; Pang, D.; Ouyang, H.; Ren, L. Porcine HMGCR Inhibits Porcine Circovirus Type 2 Infection by Directly Interacting with the Viral Proteins. Viruses 2019, 11, 544. https://doi.org/10.3390/v11060544
Ouyang T, Niu G, Zhang Y, Liu X, Zhang X, Zhang S, Geng Y, Pang D, Ouyang H, Ren L. Porcine HMGCR Inhibits Porcine Circovirus Type 2 Infection by Directly Interacting with the Viral Proteins. Viruses. 2019; 11(6):544. https://doi.org/10.3390/v11060544
Chicago/Turabian StyleOuyang, Ting, Guyu Niu, Yifang Zhang, Xiaohua Liu, Xinwei Zhang, Shiqi Zhang, Yulu Geng, Daxin Pang, Hongsheng Ouyang, and Linzhu Ren. 2019. "Porcine HMGCR Inhibits Porcine Circovirus Type 2 Infection by Directly Interacting with the Viral Proteins" Viruses 11, no. 6: 544. https://doi.org/10.3390/v11060544
APA StyleOuyang, T., Niu, G., Zhang, Y., Liu, X., Zhang, X., Zhang, S., Geng, Y., Pang, D., Ouyang, H., & Ren, L. (2019). Porcine HMGCR Inhibits Porcine Circovirus Type 2 Infection by Directly Interacting with the Viral Proteins. Viruses, 11(6), 544. https://doi.org/10.3390/v11060544







