Human-Derived A/Guangdong/Th005/2017 (H7N9) Exhibits Extremely High Replication in the Lungs of Ferrets and Is Highly Pathogenic in Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Biosafety Statements
2.2. Viruses
2.3. Cells
2.4. Growth Kinetics of H7N9 Viruses in Cells
2.5. Viral Receptor-Binding Characteristics
2.6. Ferret Experiments
2.7. Chicken Experiments
2.8. Mouse Experiments
2.9. Virus Titrations
2.10. Histopathology Evaluation
2.11. Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-qPCR)
2.12. HI Assays
2.13. Nucleotide Sequencing and Analysis
2.14. Statistical Analyses
3. Results
3.1. Pathogenicity and Transmissibility of H7N9 Avian Influenza Viruses in Ferrets
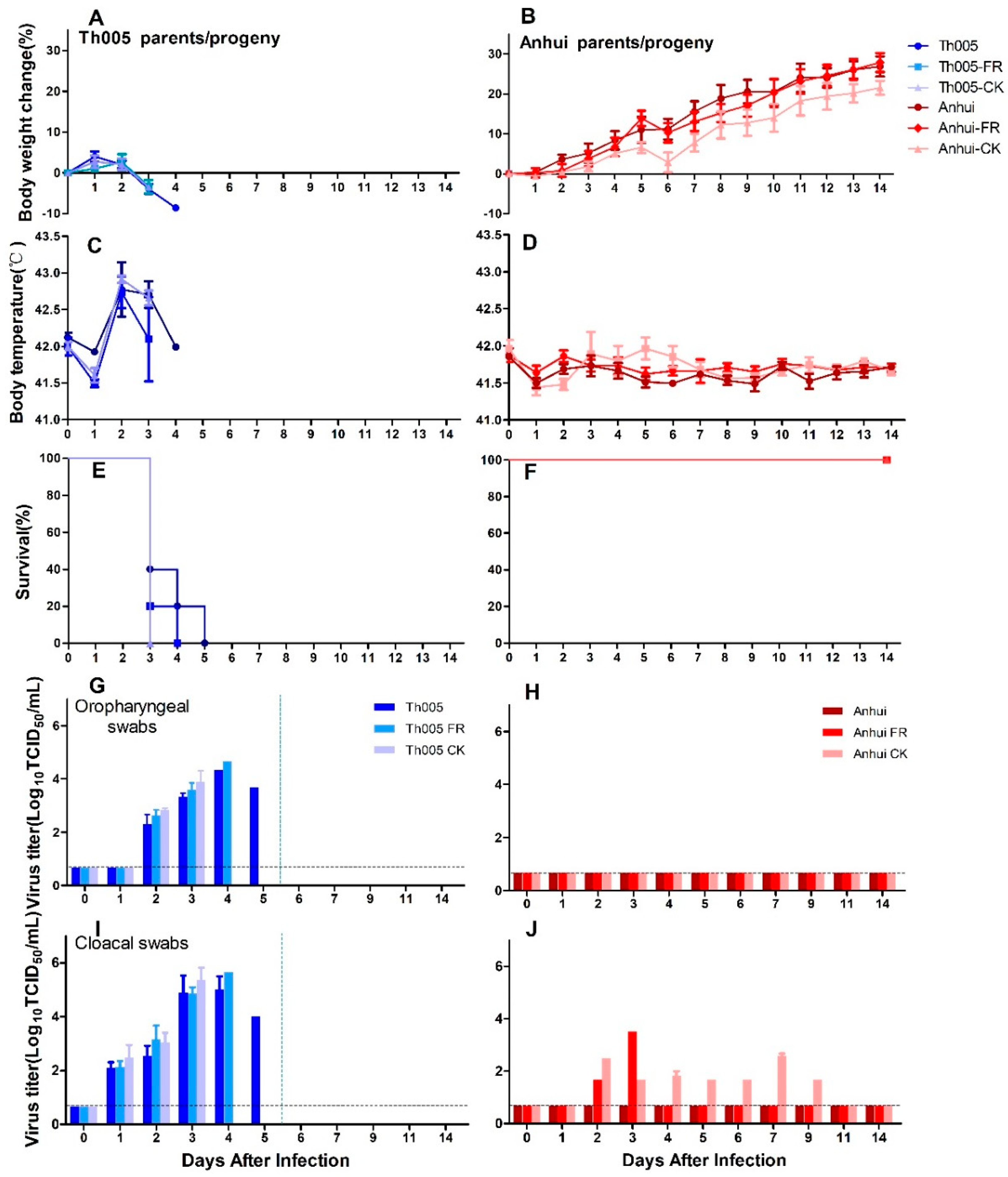
3.1.1. Infection Group
3.1.2. Transmission Group
3.2. Pathogenicity of H7N9 Avian-Passaged and Mammalian-Passaged Strains in Chickens
3.2.1. The IVPI of Chickens
3.2.2. Pathogenicity in Nasally Inoculated Chickens
3.3. Pathogenicity of H7N9 Avian-Passaged and Mammalian-Passaged Strains in Mammals
3.4. Viral Growth Replication Curves
3.5. Viral Receptor-Binding Characteristics
3.6. Analysis of Mutations in Key Pathogenic Sites (Table 1)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W.; Chen, J.; Jie, Z.; Qiu, H.; Xu, K.; et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013, 368, 1888–1897. [Google Scholar] [CrossRef]
- Agriculture and Consumer Protection Department. Animal Protection and Health. Available online: http://www.fao.org/ag/againfo/programmes/en/empres/h7n9/situation_update.html (accessed on 29 May 2019).
- Senne, D.A.; Panigrahy, B.; Kawaoka, Y.; Pearson, J.E.; Suss, J.; Lipkind, M.; Kida, H.; Webster, R.G. Survey of the hemagglutinin (HA) cleavage site sequence of H5 and H7 avian influenza viruses: amino acid sequence at the HA cleavage site as a marker of pathogenicity potential. Avian Dis. 1996, 40, 425–437. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Weekly Epidemiological Record Relevé Épidémiologique Hebdomadaire 2017; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Wang, X.; Jiang, H.; Wu, P.; Uyeki, T.M.; Feng, L.; Lai, S.; Wang, L.; Huo, X.; Xu, K.; Chen, E.; et al. Evolving epidemiology of human infections with avian influenza A(H7N9) virus across five epidemic waves in mainland China, 2013–17: An epidemiological study of laboratory-confirmed case series. Lancet Infect. Dis. 2017, 17, 822–832. [Google Scholar] [CrossRef]
- Yang, J.R.; Liu, M.T. Human infection caused by an avian influenza A (H7N9) virus with a polybasic cleavage site in Taiwan, 2017. J. Formos. Med. Assoc. 2017, 116, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Bi, Y.; Wang, J.; Wong, G.; Shi, W.; Hu, F.; Yang, Y.; Yang, L.; Deng, X.; Jiang, S.; et al. Human infections with recently-emerging highly pathogenic H7N9 avian influenza virus in China. J. Infect. 2017, 75, 71–75. [Google Scholar] [CrossRef]
- Ke, C.; Mok, C.K.P.; Zhu, W.; Zhou, H.; He, J.; Guan, W.; Wu, J.; Song, W.; Wang, D.; Liu, J. Human infection with highly pathogenic avian influenza A (H7N9) virus, China. Emerg. Infect. Dis. 2017, 23, 1332–1340. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, Y.; Kang, M.; Liu, F.; Ren, R.; Wang, Y.; Chen, T.; Yang, Y.; Li, C.; Wu, J. Preliminary epidemiology of human infections with highly pathogenic avian influenza A (H7N9) virus, China, 2017. Emerg. Infect. Dis. 2017, 23, 1355–1359. [Google Scholar] [CrossRef]
- Xu, L.; Bao, L.; Deng, W.; Dong, L.; Zhu, H.; Chen, T.; Lv, Q.; Li, F.; Yuan, J.; Xiang, Z.; et al. Novel avian-origin human influenza A(H7N9) can be transmitted between ferrets via respiratory droplets. J. Infect. Dis. 2014, 209, 551–556. [Google Scholar] [CrossRef]
- Reuman, P.D.; Keely, S.; Schiff, G.M. Assessment of signs of influenza illness in the ferret model. J. Virol. Methods 1989, 24, 27–34. [Google Scholar] [CrossRef]
- World Organization for Animal Health (OIE). Avian influenza (infection with avian influenza viruses). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE: Paris, France, 2016; pp. 1–23. [Google Scholar]
- Shi, J.; Deng, G.; Kong, H.; Gu, C.; Ma, S.; Yin, X.; Zeng, X.; Cui, P.; Chen, Y.; Yang, H.; et al. H7N9 virulent mutants detected in chickens in China pose an increased threat to humans. Cell Res. 2017, 27, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.; Schrauwen, E.J.A.; de Graaf, M.; Bestebroer, T.M.; Spronken, M.I.J.; van Boheemen, S.; de Meulder, D.; Lexmond, P.; Linster, M.; Herfst, S.; et al. Limited airborne transmission of H7N9 influenza A virus between ferrets. Nature 2013, 501, 560–563. [Google Scholar] [CrossRef]
- Matrosovich, M.; Tuzikov, A.; Bovin, N.; Gambaryan, A.; Klimov, A.; Castrucci, M.R.; Donatelli, I.; Kawaoka, Y. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J. Virol. 2000, 74, 8502–8512. [Google Scholar] [CrossRef]
- Wang, W.; Lu, B.; Zhou, H.; Suguitan, A.L., Jr.; Cheng, X.; Subbarao, K.; Kemble, G.; Jin, H. Glycosylation at 158N of the hemagglutinin protein and receptor binding specificity synergistically affect the antigenicity and immunogenicity of a live attenuated H5N1 A/Vietnam/1203/2004 vaccine virus in ferrets. J. Virol. 2010, 84, 6570–6577. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.K.; Lee, H.H.; Chan, M.C.; Sia, S.F.; Lestra, M.; Nicholls, J.M.; Zhu, H.; Guan, Y.; Peiris, J.M. Pathogenicity of the novel A/H7N9 influenza virus in mice. MBio 2013, 4, 16. [Google Scholar] [CrossRef]
- Wiley, D.C.; Wilson, I.A.; Skehel, J.J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature 1981, 289, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Pantin-Jackwood, M.J.; Miller, P.J.; Spackman, E.; Swayne, D.E.; Susta, L.; Costa-Hurtado, M.; Suarez, D.L. Role of poultry in the spread of novel H7N9 influenza virus in China. J. Virol. 2014, 88, 5381–5390. [Google Scholar] [CrossRef] [PubMed]
- Tharakaraman, K.; Jayaraman, A.; Raman, R.; Viswanathan, K.; Stebbins, N.W.; Johnson, D.; Shriver, Z.; Sasisekharan, V.; Sasisekharan, R. Glycan receptor binding of the influenza A virus H7N9 hemagglutinin. Cell 2013, 153, 1486–1493. [Google Scholar] [CrossRef]
- Bossart-Whitaker, P.; Carson, M.; Babu, Y.S.; Smith, C.D.; Laver, W.G.; Air, G.M. Three-dimensional structure of influenza A N9 neuraminidase and its complex with the inhibitor 2-deoxy 2,3-dehydro-N-acetyl neuraminic acid. J. Mol. Biol. 1993, 232, 1069–1083. [Google Scholar] [CrossRef]
- Neumann, G.; Macken, C.A.; Kawaoka, Y. Identification of amino acid changes that may have been critical for the genesis of A(H7N9) influenza viruses. J. Virol. 2014, 88, 4877–4896. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Kiso, M.; Yasuhara, A.; Ito, M.; Shu, Y.; Kawaoka, Y. Enhanced replication of highly pathogenic influenza A(H7N9) virus in humans. Emerg. Infect. Dis. 2018, 24, 746–750. [Google Scholar] [CrossRef]
- Yao, Y.; Mingay, L.J.; McCauley, J.W.; Barclay, W.S. Sequences in influenza A virus PB2 protein that determine productive infection for an avian influenza virus in mouse and human cell lines. J. Virol. 2001, 75, 5410–5415. [Google Scholar] [CrossRef]
- Song, W.; Wang, P.; Mok, B.W.; Lau, S.Y.; Huang, X.; Wu, W.L.; Zheng, M.; Wen, X.; Yang, S.; Chen, Y.; et al. The K526R substitution in viral protein PB2 enhances the effects of E627K on influenza virus replication. Nat. Commun. 2014, 5, 5509. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Dong, J.; Zhang, Y.; Yang, L.; Li, X.; Chen, T.; Zhao, X.; Wei, H.; Bo, H.; Zeng, X.; et al. A gene constellation in avian influenza A (H7N9) viruses may have facilitated the fifth wave outbreak in China. Cell Rep. 2018, 23, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Bao, L.; Deng, W.; Zhu, H.; Chen, T.; Lv, Q.; Li, F.; Yuan, J.; Xiang, Z.; Gao, K.; et al. The mouse and ferret models for studying the novel avian-origin human influenza A (H7N9) virus. Virol. J. 2013, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Bao, L.; Deng, W.; Zhu, H.; Li, F.; Chen, T.; Lv, Q.; Yuan, J.; Xu, Y.; Li, Y.; et al. Rapid adaptation of avian H7N9 virus in pigs. Virology 2014, 452–453, 231–236. [Google Scholar] [CrossRef]
- Xiong, X.; Martin, S.R.; Haire, L.F.; Wharton, S.A.; Daniels, R.S.; Bennett, M.S.; McCauley, J.W.; Collins, P.J.; Walker, P.A.; Skehel, J.J.; et al. Receptor binding by an H7N9 influenza virus from humans. Nature 2013, 499, 496–499. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, W.; Wang, F.; Qi, J.; Wu, Y.; Song, H.; Gao, F.; Bi, Y.; Zhang, Y.; Fan, Z.; et al. Structures and receptor binding of hemagglutinins from human-infecting H7N9 influenza viruses. Science 2013, 342, 243–247. [Google Scholar] [CrossRef]
- Bao, L.; Bi, Y.; Wong, G.; Qi, W.; Li, F.; Lv, Q.; Wang, L.; Liu, F.; Yang, Y.; Zhang, C.; et al. Diverse biological characteristics and varied virulence of H7N9 from wave 5. Emerg. Microbes Infect. 2019, 8, 94–102. [Google Scholar] [CrossRef]
- Imai, M.; Watanabe, T.; Kiso, M.; Nakajima, N.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Hatta, M.; Yamada, S.; Ito, M.; Sakai-Tagawa, Y.; et al. A highly pathogenic avian H7N9 influenza virus isolated from a human is lethal in some ferrets infected via respiratory droplets. Cell Host Microbe 2017, 22, 615–626. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, Y.; Zhang, W.; Qi, J.; Gao, G.F. Enabling the “host jump”: structural determinants of receptor-binding specificity in influenza A viruses. Nat. Rev. Microbiol. 2014, 12, 822–831. [Google Scholar] [CrossRef]
- Huang, L.; Guo, X.; Tu, Y. Identification of surface glycan chain types of A549 cells and hep-2 cells and their binding to H5N1 avian influenza virus. Basic Clin. Med. 2010, 30, 1318–1324. [Google Scholar]
- Hsu, A.C.; Barr, I.; Hansbro, P.M.; Wark, P.A. Human influenza is more effective than avian influenza at antiviral suppression in airway cells. Am. J. Respir. Cell Mol. Biol. 2011, 44, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Kirkeby, S.; Martel, C.J.; Aasted, B. Infection with human H1N1 influenza virus affects the expression of sialic acids of metaplastic mucous cells in the ferret airways. Virus Res. 2009, 144, 225–232. [Google Scholar] [CrossRef] [PubMed]
| Protein | Amino Acid No. | Anhui | Anhui FR | Anhui CK | Th005 | Th005 FR | Th005 CK | Function | Reference(s) | |
|---|---|---|---|---|---|---|---|---|---|---|
| HA | Cleavage | —— | —— | —— | KRIA | KRIA | KRIA | Avian pathogenicity | [13,14] | |
| 48 | I | I | I | V | V | V | Increase human-type receptor affinity | [15] | ||
| 57 | R | R | R | K | K | K | ||||
| 122 | A | A | A | P | P | P | Influence human immune response | [16] | ||
| 128 | S | S | S | N | N | N | Increase human-type receptor affinity | [17] | ||
| 135 | A | A | A | V | V | V | Modulate receptor affinity | [18] | ||
| 133 | N | N | D | N | N | N | Increase avian-type receptor affinity | [16,19] | ||
| 158-159 | D | N | D | N | N | N | ||||
| 226 | L | L | L | Q | Q | Q | Alter receptor specificity | [20] | ||
| NA | 145 | G | G | G | E | E | E | Glycosylation site | [21] | |
| 357 | A | A | A | D | D | D | Influence neighboring NA tetramers | |||
| PB1 | 525 | V | V | V | I | I | I | Influence replication in varied species | [22] | |
| PB2 | 191 | K | K | K | E | E | E | Influence polymerase activity | [23] | |
| 292 | V | V | V | I | I | I | Influence replication in human | [22] | ||
| 379 | R | R | R | R | S | R | Influence replication in mouse cells | [24,25,26] | ||
| 382 | I | I | N | I | I | I | ||||
| 383 | Q | Q | S | Q | Q | Q | ||||
| 511 | V | V | I | I | I | I | ||||
| 526 | K | K | R | R | R | R | Enhance the 627K and 701N function | |||
| 535 | M | M | L | L | L | L | Restore the polymerase activity | |||
| 559 | N | N | T | T | T | T | Influence polymerase activity | |||
| 570 | M | M | I | I | I | I | ||||
| 647 | I | I | I | M | M | M | Contribute to the phenotype | [22,24] | ||
| PA | 55 | D | D | K | D | D | D | Host signature amino acids | [26] | |
| 409 | N | S | N | N | N | N | ||||
| NS1 | 41 | M | K | K | K | K | K | Not Clear | [22] | |
| 124 | M | M | M | V | V | V | ||||
| M2 | 24 | E | E | E | D | D | D | Not Clear | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, S.; Qi, F.; Li, F.; Lv, Q.; Wang, G.; Wang, S.; Jiang, J.; Wang, L.; Bao, L.; Qin, C. Human-Derived A/Guangdong/Th005/2017 (H7N9) Exhibits Extremely High Replication in the Lungs of Ferrets and Is Highly Pathogenic in Chickens. Viruses 2019, 11, 494. https://doi.org/10.3390/v11060494
Gong S, Qi F, Li F, Lv Q, Wang G, Wang S, Jiang J, Wang L, Bao L, Qin C. Human-Derived A/Guangdong/Th005/2017 (H7N9) Exhibits Extremely High Replication in the Lungs of Ferrets and Is Highly Pathogenic in Chickens. Viruses. 2019; 11(6):494. https://doi.org/10.3390/v11060494
Chicago/Turabian StyleGong, Shuran, Feifei Qi, Fengdi Li, Qi Lv, Guanpeng Wang, Shunyi Wang, Jing Jiang, Lin Wang, Linlin Bao, and Chuan Qin. 2019. "Human-Derived A/Guangdong/Th005/2017 (H7N9) Exhibits Extremely High Replication in the Lungs of Ferrets and Is Highly Pathogenic in Chickens" Viruses 11, no. 6: 494. https://doi.org/10.3390/v11060494
APA StyleGong, S., Qi, F., Li, F., Lv, Q., Wang, G., Wang, S., Jiang, J., Wang, L., Bao, L., & Qin, C. (2019). Human-Derived A/Guangdong/Th005/2017 (H7N9) Exhibits Extremely High Replication in the Lungs of Ferrets and Is Highly Pathogenic in Chickens. Viruses, 11(6), 494. https://doi.org/10.3390/v11060494