Hypopituitarism after Orthohantavirus Infection: What is Currently Known?
Abstract
1. Introduction
2. Pathophysiology of Hypopituitarism
3. Clinical Consequences of Hypopituitarism
4. Hemorrhagic Complications in Orthohantavirus Infection
5. Clinical Characteristics of Hypopituitarism Due to Orthohantavirus Infection
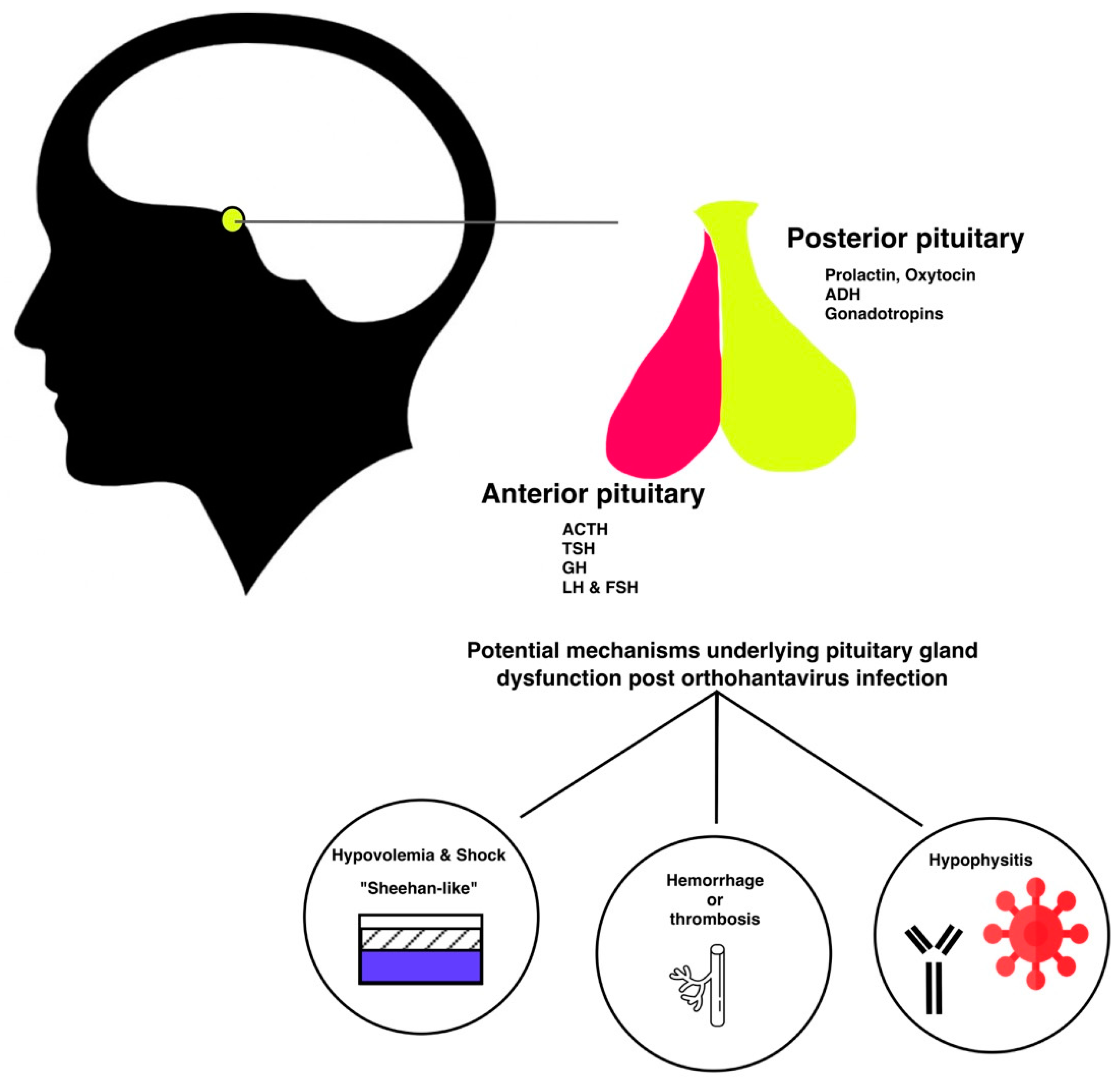
6. Pathophysiology of Orthohantavirus Associated Hypopituitarism
7. Discussion
Author Contributions
Conflicts of Interest
References
- Vapalahti, O.; Mustonen, J.; Lundkvist, Å.; Henttonen, H.; Plyusnin, A.; Vaheri, A. Hantavirus infections in Europe. Lancet Infect. Dis. 2003, 3, 653–661. [Google Scholar] [CrossRef]
- Peters, C.J.; Simpson, G.L.; Levy, H. Spectrum of orthohantavirus infection: Hemorrhagic fever with renal syndrome and orthohantavirus pulmonary syndrome. Annu. Rev. Med. 1999, 50, 531. [Google Scholar] [CrossRef]
- Jonsson, C.B.; Figueiredo, L.T.M.; Vapalahti, O. A Global Perspective on Orthohantavirus Ecology, Epidemiology, and Disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar] [CrossRef]
- Park, K.H.; Kang, Y.U.; Kang, S.-J.; Jung, Y.-S.; Jang, H.-C.; Jung, S.-I. Experience with Extrarenal Manifestations of Hemorrhagic Fever with Renal Syndrome in a Tertiary Care Hospital in South Korea. Am. J. Trop. Med. Hyg. 2011, 84, 229–233. [Google Scholar] [CrossRef][Green Version]
- Goeijenbier, M.; Verner-Carlsson, J.; van Gorp, E.C.M.; Rockx, B.; Koopmans, M.P.G.; Lundkvist, A.; van der Giessen, J.W.; Reusken, C.B. Seoul orthohantavirus in brown rats in the Netherlands: Implications for physicians. Neth. J. Med. 2015, 73, 155–160. [Google Scholar]
- Steer, A. Pathology of hemorrhagic fever: A comparison of the findings—1951 and 1952. Am. J. Pathol. 1955, 31, 201. [Google Scholar]
- Lukes, R.J. The pathology of thirty-nine fatal cases of epidemic hemorrhagic fever. Am. J. Med. 1954, 15, 639–650. [Google Scholar] [CrossRef]
- Lim, T.H.; Chang, K.H.; Han, M.C.; Chang, Y.B.; Lim, S.M.; Yu, Y.S.; Chun, Y.H.; Lee, J.S. Pituitary atrophy in Korean (epidemic) hemorrhagic fever: CT correlation with pituitary function and visual field. Am. J. Neuroradiol. 1986, 7, 633–637. [Google Scholar]
- Valtonen, M.; Kaupilla, M.; Kotilainen, P.; Lähdevirta, J.; Svartbäck, C.M.; Kosunen, O.; Nurminen, J.; Sarkinnen, H.; Brummer-Korvinkontio, M. Four fatal cases of nephropathia epidemica. Scand. J. Infect. Dis. 1995, 27, 515–517. [Google Scholar] [CrossRef]
- Vance, N.F.; Brittenham, M. Hypopituitarism. NEJM 1994, 330, 1651–1662. [Google Scholar] [CrossRef]
- Schneider, H.J.; Aimaretti, G.; Kreitschmann-Andermahr, I.; Stalla, G.-K.; Ghigo, E. Hypopituitarism. Lancet 2007, 369, 1461–1470. [Google Scholar] [CrossRef]
- Briet, C.; Salenave, S.; Bonneville, J.-F.; Laws, E.R.; Chanson, P. Pituitary Apoplexy. Endocr. Rev. 2015, 36, 622–645. [Google Scholar] [CrossRef]
- Di Ieva, A.; Weckman, A.; Di Michele, J.; Rotondo, F.; Grizzi, F.; Kovacs, K.; Cusimano, M.D. Microvascular morphometrics of the hypophysis and pituitary tumors: From bench to operating theatre. Microvasc. Res. 2013, 89, 7–14. [Google Scholar] [CrossRef]
- Kovacs, K. Sheehan syndrome. Lancet 2003, 361, 520–522. [Google Scholar] [CrossRef]
- Laway, B.; Mir, S. Pregnancy and pituitary disorders: Challenges in diagnosis and management. Indian J. Endocrinol. Metab. 2013, 17, 996. [Google Scholar] [CrossRef]
- Bellastella, G.; Maiorino, M.I.; Bizzarro, A.; Giugliano, D.; Esposito, K.; Bellastella, A.; De Bellis, A. Revisitation of autoimmune hypophysitis: Knowledge and uncertainties on pathophysiological and clinical aspects. Pituitary 2016, 19, 625–642. [Google Scholar] [CrossRef]
- Tarvainen, M.; Mäkelä, S.; Mustonen, J.; Jaatinen, P. Autoimmune polyendocrinopathy and hypophysitis after Puumala orthohantavirus infection. Endocrinol. Diabetes Metab. Case Rep. 2016. [Google Scholar] [CrossRef]
- Garg, M.; Brar, K.; Suryanarayana, K. Adult hypopituitarism: Are we missing or is it clinical lethargy? Indian J. Endocrinol. Metab. 2011, 15, 170. [Google Scholar] [CrossRef]
- Chung, T.T.; Monson, J.P. Hypopituitarism. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Fliers, E. Clinical Neuroendocrinology, 124th ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Chen, J.P.; Cosgriff, T.M. Hemorrhagic fever virus-induced changes in hemostasis and vascular biology. Blood Coagul. Fibrinolysis 2000, 11, 461–483. [Google Scholar] [CrossRef]
- Lee, M. Coagulopathy in patients with hemorrhagic fever with renal syndrome. J. Korean Med. Sci. 1987, 4, 201–211. [Google Scholar] [CrossRef]
- Mackow, E.R.; Gavrilovskaya, I.N. Orthohantavirus regulation of endothelial cell functions. Thromb. Haemost. 2009. [Google Scholar] [CrossRef]
- Laine, O.; Mäkelä, S.; Mustonen, J.; Helminen, M.; Vaheri, A.; Lassila, R.; Joutsi-Korhonen, L. Platelet ligands and ADAMTS13 during Puumala orthohantavirus infection and associated thrombocytopenia. Blood Coagul. Fibrinolysis 2011, 22, 468–472. [Google Scholar] [CrossRef]
- Mustonen, J.; Mäkelä, S.; Outinen, T.; Laine, O.; Jylhävä, J.; Arstila, P.T.; Hurme, M.; Vaheri, A. The pathogenesis of nephropathia epidemica: New knowledge and unanswered questions. Antivir. Res. 2013, 100, 589–604. [Google Scholar] [CrossRef]
- Goeijenbier, M.; van Wissen, M.; van de Weg, C.; Jong, E.; Gerdes, V.E.A.; Meijers, J.C.M.; Brandjes, D.P.M.; van Gorp, E.C.M. Review: Viral infections and mechanisms of thrombosis and bleeding. J. Med. Virol. 2012, 84, 1680–1696. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, M.; Han, Q.; Fang, J.; Zhao, Q.; Zhang, N. Intensity of Platelet β3 Integrin in Patients with Hemorrhagic Fever with Renal Syndrome and Its Correlation with Disease Severity. Viral Immunol. 2008, 21, 255–262. [Google Scholar] [CrossRef]
- Hepojoki, J.; Vaheri, A.; Strandin, T. The fundamental role of endothelial cells in orthohantavirus pathogenesis. Front. Microbiol. 2014, 5, 727. [Google Scholar] [CrossRef]
- Gavrilovskaya, I.N.; Gorbunova, E.E.; Mackow, E.R. Pathogenic Orthohantaviruses Direct the Adherence of Quiescent Platelets to Infected Endothelial Cells. J. Virol. 2010, 84, 4832–4839. [Google Scholar] [CrossRef]
- Assinger, A. Platelets and Infection—An Emerging Role of Platelets in Viral Infection. Front. Immunol. 2014, 5, 649. [Google Scholar] [CrossRef]
- Goeijenbier, M.; Meijers, J.C.M.; Anfasa, F.; Roose, J.M.; van de Weg, C.A.M.; Bakhtiari, K.; Henttonen, H.; Vaheri, A.; Osterhaus, A.D.M.E.; van Gorp, E.C.M.; et al. Effect of Puumala orthohantavirus infection on human umbilical vein endothelial cell hemostatic function: Platelet interactions, increased tissue factor expression and fibrinolysis regulator release. Front. Microbiol. 2015, 6, 220. [Google Scholar] [CrossRef]
- Zapata, J.C.; Cox, D.; Salvato, M.S. The role of platelets in the pathogenesis of viral hemorrhagic fevers. PLoS Negl. Trop. Dis. 2014, 8, e2858. [Google Scholar] [CrossRef]
- Lütteke, N.; Raftery, M.J.; Lalwani, P.; Lee, M.-H.; Giese, T.; Voigt, S.; Bannert, N.; Schulze, H.; Krüger, D.H.; Schönrich, G. Switch to high-level virus replication and HLA class I upregulation in differentiating megakaryocytic cells after infection with pathogenic orthohantavirus. Virology 2010, 405, 70–80. [Google Scholar] [CrossRef]
- Khaiboullina, S.F.; Morzunov, S.P.; St. Jeor, S.C.; Rizvanov, A.A.; Lombardi, V.C. Orthohantavirus Infection Suppresses Thrombospondin-1 Expression in Cultured Endothelial Cells in a Strain-Specific Manner. Front. Microbiol. 2016, 7, 1077. [Google Scholar] [CrossRef]
- Strandin, T.; Hepojoki, J.; Laine, O.; Mäkelä, S.; Klingström, J.; Lundkvist, A.; Julkunen, I.; Mustonen, J.; Vaheri, A. Interferons Induce STAT1-Dependent Expression of Tissue Plasminogen Activator, a Pathogenicity Factor in Puumala Hantavirus Disease. J. Infect. Dis. 2016, 213, 1632–1641. [Google Scholar] [CrossRef]
- Sarıgüzel, N.; Hofmann, J.; Canpolat, A.T.; Türk, A.; Ettinger, J.; Atmaca, D.; Akyar, I.; Yücel, S.; Arıkan, E.; Uyar, Y.; et al. Dobrava Orthohantavirus Infection Complicated by Panhypopituitarism, Istanbul, Turkey, 2010. Emerg. Infect. Dis. 2012, 18, 1180–1183. [Google Scholar] [CrossRef]
- Suh, D.C.; Park, J.S.; Park, S.-K.; Lee, H.K.; Chang, K.H. Pituitary hemorrhage as a complication of hantaviral disease. Am. J. Neuroradiol. 1995, 16, 175–178. [Google Scholar]
- Forslund, T.; Saltevo, J.; Anttinen, J.; Auvinen, S.; Brummer-Korvenkonio, M.; Korhonen, A.; Poutiainen, M. Complications of nephropathia epidemica: Three cases. J. Int. Med. 1992, 232, 87–90. [Google Scholar] [CrossRef]
- Jost, C.; Krausse, R.; Graninger, W.; Weber, K. Transient hypopituitarism in a patient with nephropathia epidemica. BMJ Case Rep. 2009. [Google Scholar] [CrossRef]
- Settergen, B.; Boman, J.; Linderholm, M.; Wiström, J.; Hägg, E.; Arvidsson, P.A. A case of nephropathia epidemica associated with panhypopituitarism and nephrotic syndrome. Nephron 1992, 61, 234–235. [Google Scholar] [CrossRef]
- Kim, N.H.; Cho, J.G.; Ahn, Y.K.; Lee, S.U.; Kim, K.H.; Kim, W.; Jeong, M.H.; Park, J.C.; Kang, J.C. A case of torsades des pointes associated with hypopituitarism due to hemorrhagic fever with renal syndrome. J. Korean Med. Sci. 2001, 16, 355–358. [Google Scholar] [CrossRef]
- Stojanovic, M.; Pekic, S.; Cvijovic, G.; Miljic, D.; Doknic, M.; Nikolic-Djurovic, M.; Micic, D.; Hrvacevic, R.; Nesic, V.; Popovic, V. High Risk of Hypopituitarism in Patients Who Recovered from Hemorrhagic Fever with Renal Syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 2722–2728. [Google Scholar] [CrossRef]
- Pekic, S.; Cvijovic, G.; Stojanovic, M.; Kendereski, A.; Micic, D.; Popovic, V. Hypopituitarism as a late complication of hemorrhagic fever. Endocrine 2005, 26, 79–82. [Google Scholar] [CrossRef]
- Hautala, T.; Sironen, T.; Vapalahti, O.; Pääkkö, E.; Särkioja, T.; Salmela, P.I.; Vaheri, A.; Plyusnin, A.; Kauma, H. Hypophyseal hemorrhage and panhypopituitarism during Puumala virus infection: Magnetic resonance imaging and detection of viral antigen in the hypophysis. Clin. Infect. Dis. 2002, 35, 96–101. [Google Scholar] [CrossRef]
- Hullinghorst, R.L.; Steer, A. Pathology of epidemic hemorrhagic fever. Ann. Intern. Med. 1953, 38, 77–101. [Google Scholar]
- Laine, O.; Joutsi-Korhonen, L.; Lassila, R.; Huhtala, H.; Vaheri, A.; Mäkelä, S.; Mustonen, J. Elevated thrombopoietin and platelet indices confirm active thrombopoiesis but fail to predict clinical severity of puumala orthohantavirus infection. Medicine 2016, 95, e5689. [Google Scholar] [CrossRef]
- Outinen, T.K.; Laine, O.K.; Mäkelä, S.; Pörsti, I.; Huhtala, H.; Vaheri, A.; Mustonen, J. Thrombocytopenia associates with the severity of inflammation and variables reflecting capillary leakage in Puumala Orthohantavirus infection, an analysis of 546 Finnish patients. Infect. Dis. 2016, 48, 682–687. [Google Scholar] [CrossRef]
- Partanen, T.; Koivikko, M.; Leisti, P.; Salmela, P.; Pääkkö, E.; Karttunen, A.; Sintonen, H.; Risteli, L.; Hautala, N.; Vapalahti, O.; et al. Hautala, Long-term hormonal follow-up after human Puumala orthohantavirus infection. Clin. Endocrinol. 2016, 84, 85–91. [Google Scholar] [CrossRef]
- Mäkelä, S.; Jaatinen, P.; Miettinen, M.; Salmi, J.; Ala-Houhala, I.; Huhtala, H.; Hurme, M.; Pörsti, I.; Vaheri, A.; Mustonen, J. Hormonal deficiencies during and after Puumala orthohantavirus infection. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 705–713. [Google Scholar] [CrossRef]
- Jin, H.Y.; Kang, S.M.; Kim, S.Y.; Park, J.H.; Baek, H.S.; Park, T.S. A Case of Graves’ Disease Combined with Hantaan Virus Infection. J. Korean Med. Sci. 2009, 24, 158. [Google Scholar] [CrossRef][Green Version]
| Hormone Deficiency | Signs and Symptoms |
|---|---|
| Growth hormone (GH) | Increased body fat; reduced muscle mass and strength; reduced stamina and psychological problems, e.g., depression or concentration loss; dyslipidemia; atherosclerosis |
| Luteinizing and follicle-stimulating hormone (LH and FSH) | Sub- or infertility, loss of libido Men: impotence; testicle atrophy; loss of facial, body, and pubic hair; reduced muscle mass; osteoporosis Women: a- or oligomenorrhea, dyspareunia, osteoporosis |
| Thyroid-stimulation hormone (TSH) | Cold intolerance, weight gain, fatigue, hair loss, constipation, hoarse voice |
| Adrenocorticotropic hormone (ACTH) | (Orthostatic) hypotension, hypoglycemia, fatigue, muscle weakness |
| Prolactin (PL) | Postpartum failure of lactation |
| Antidiuretic hormone (ADH) | Polydipsia and polyuria |
| Serotype | Country | No. | Diagnostics | Endocrine Disturbances | Time to Detection | Duration | Outcome | Ref |
|---|---|---|---|---|---|---|---|---|
| Puumala | Finland | 1 | NE-IFAT titre | Panhypopituarism | 5 years | NR | Survived | Forslund et al., 1992 [38] |
| Puumala | Sweden | 1 | Specific Puumala IgM and IgG | Panhypopituitarism | 6 months | NR | Survived | Settergen et al., 1992 [40] |
| NR | Korea | 1 | Serologic antibody testing | Panhypopituitarism | Day 20 a | NR | Survived | Suh et al., 1994 [37] |
| Puumala | Finland | 4 | NR | NR | - | - | Deceased | Valtonen et al., 1995 b [9] |
| NR | Korea | 1 | NR | Panhypopituitarism | 13 years | NR | Survived | Kim et al., 2001 [41] |
| Puumala b Puumala Puumala | Finland Finland Finland | 1 1 1 | Serologic antibody testing Serologic antibody testing Serologic antibody testing | NR Panhypopituitarism Panhypopituitarism | - 5 months Day 7 a | - NR NR | Deceased Survived Survived | Hautala et al., 2002 [44] |
| NR Puumala Puumala | Serbia Serbia Serbia | 1 1 1 | Serologic antibody testing Serologic antibody testing Serologic antibody testing | Panhypopituitarism Panhypopituitarism ACTH, FSH, LH and GH | 1.5 years 2 years 2 years | NR NR NR | Survived Survived Survived | Pekic et al., 2005 [42] |
| NR NR NR NR | Serbia Serbia Serbia Serbia | 3 2 1 5 | Indirect immunofluorescent assay Indirect immunofluorescent assay Indirect immunofluorescent assay Indirect immunofluorescent assay | GH FSH, LH ACTH ≥4 axes | >6 months >6 months >6 months 0.5–11 yrs | NR NR NR NR | Survived Survived Survived Survived | Stojanovic et al., 2008 [42] |
| Puumala | Austria | 1 | Specific Puumala IgM | Panhypopituitarism | Acute a | 5 months | Survived | Jost et al., 2009 [39] |
| Dobrova | Turkey | 1 | Serologic antibody testing | Panhypopituitarism | Day 19 a | 16 months | Survived | Sariguzel et al., 2010 [36] |
| Puumala | Finland | 1 | Specific Puumala IgM and IgG | TSH, FSH, LH, ADH | 6 months | Ongoing | Survived | Tarvainen et al., 2016 [17] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhoelan, S.; Langerak, T.; Noack, D.; van Schinkel, L.; van Nood, E.; van Gorp, E.C.M.; Rockx, B.; Goeijenbier, M. Hypopituitarism after Orthohantavirus Infection: What is Currently Known? Viruses 2019, 11, 340. https://doi.org/10.3390/v11040340
Bhoelan S, Langerak T, Noack D, van Schinkel L, van Nood E, van Gorp ECM, Rockx B, Goeijenbier M. Hypopituitarism after Orthohantavirus Infection: What is Currently Known? Viruses. 2019; 11(4):340. https://doi.org/10.3390/v11040340
Chicago/Turabian StyleBhoelan, Soerajja, Thomas Langerak, Danny Noack, Linda van Schinkel, Els van Nood, Eric C.M. van Gorp, Barry Rockx, and Marco Goeijenbier. 2019. "Hypopituitarism after Orthohantavirus Infection: What is Currently Known?" Viruses 11, no. 4: 340. https://doi.org/10.3390/v11040340
APA StyleBhoelan, S., Langerak, T., Noack, D., van Schinkel, L., van Nood, E., van Gorp, E. C. M., Rockx, B., & Goeijenbier, M. (2019). Hypopituitarism after Orthohantavirus Infection: What is Currently Known? Viruses, 11(4), 340. https://doi.org/10.3390/v11040340






