Mikania Micrantha Wilt Virus Alters Insect Vector’s Host Preference to Enhance Its Own Spread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Plants
2.3. MMWV Inoculation Procedures
2.4. Choice Tests
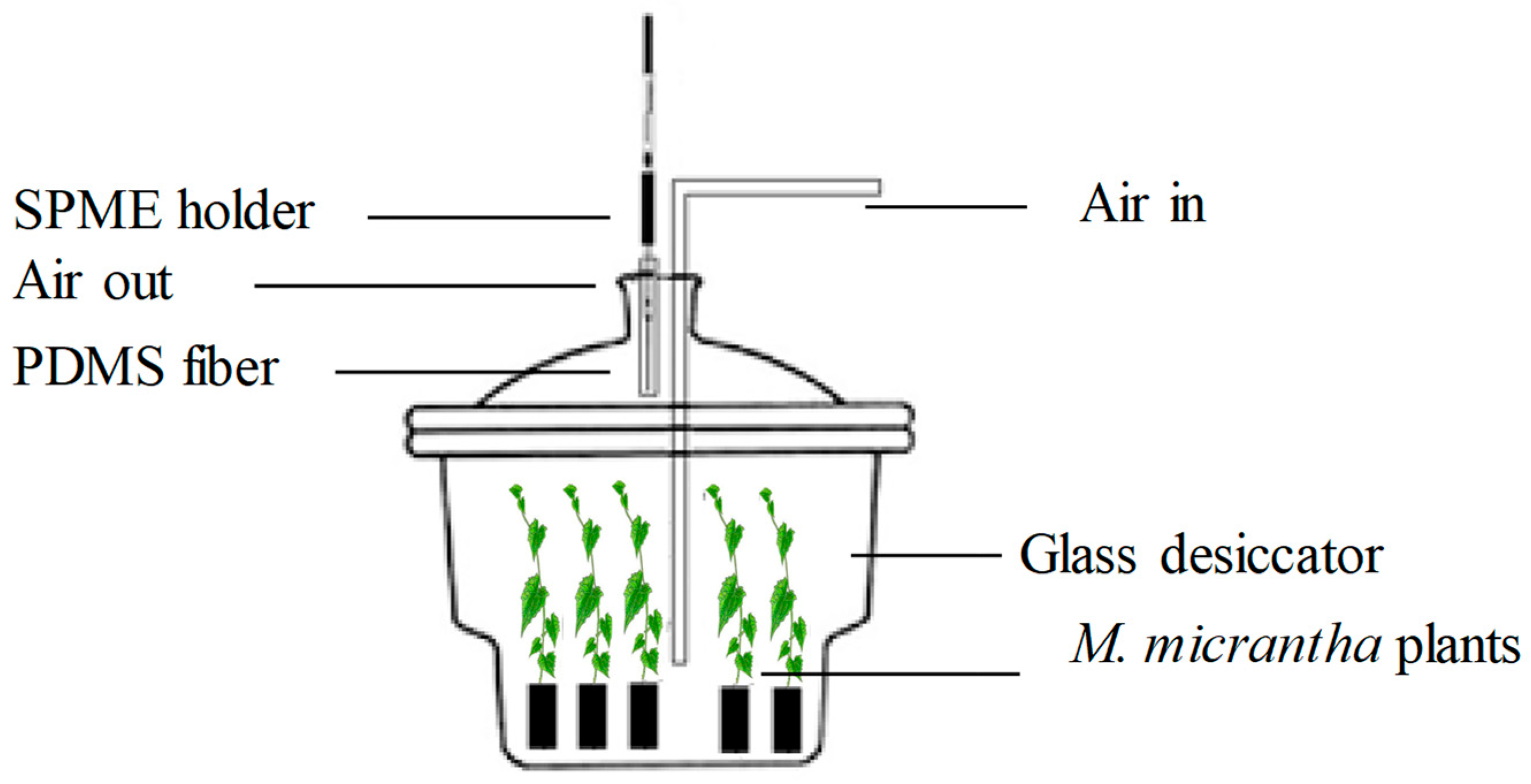
2.5. Collection of Plant Volatiles
2.6. Volatile Analysis
2.7. Population Growth of M. persicae
2.8. Data Analysis
3. Results
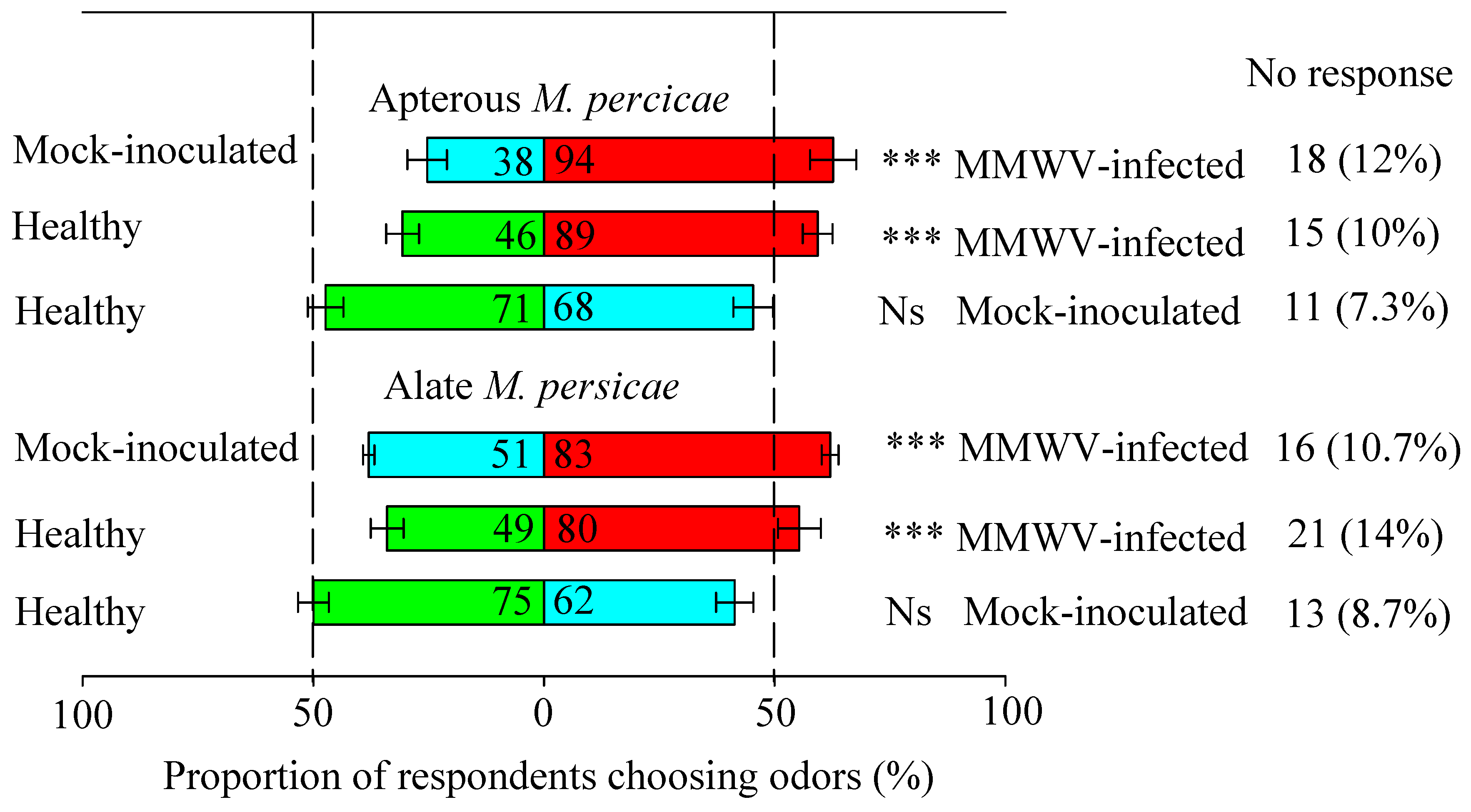
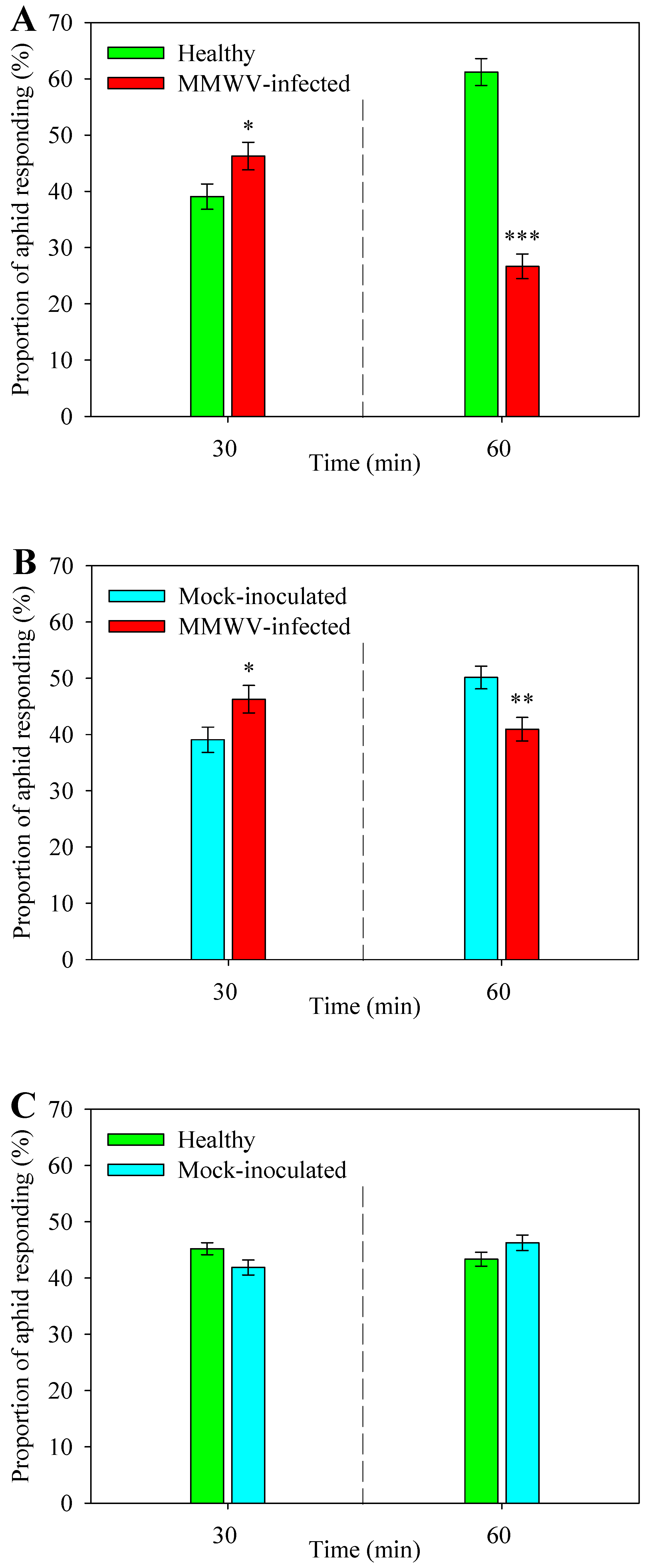
3.1. Odor from MMWV-Infected Plants is More Attractive to Aphids
3.2. Aphid Distribution in Preference for Contact Cues
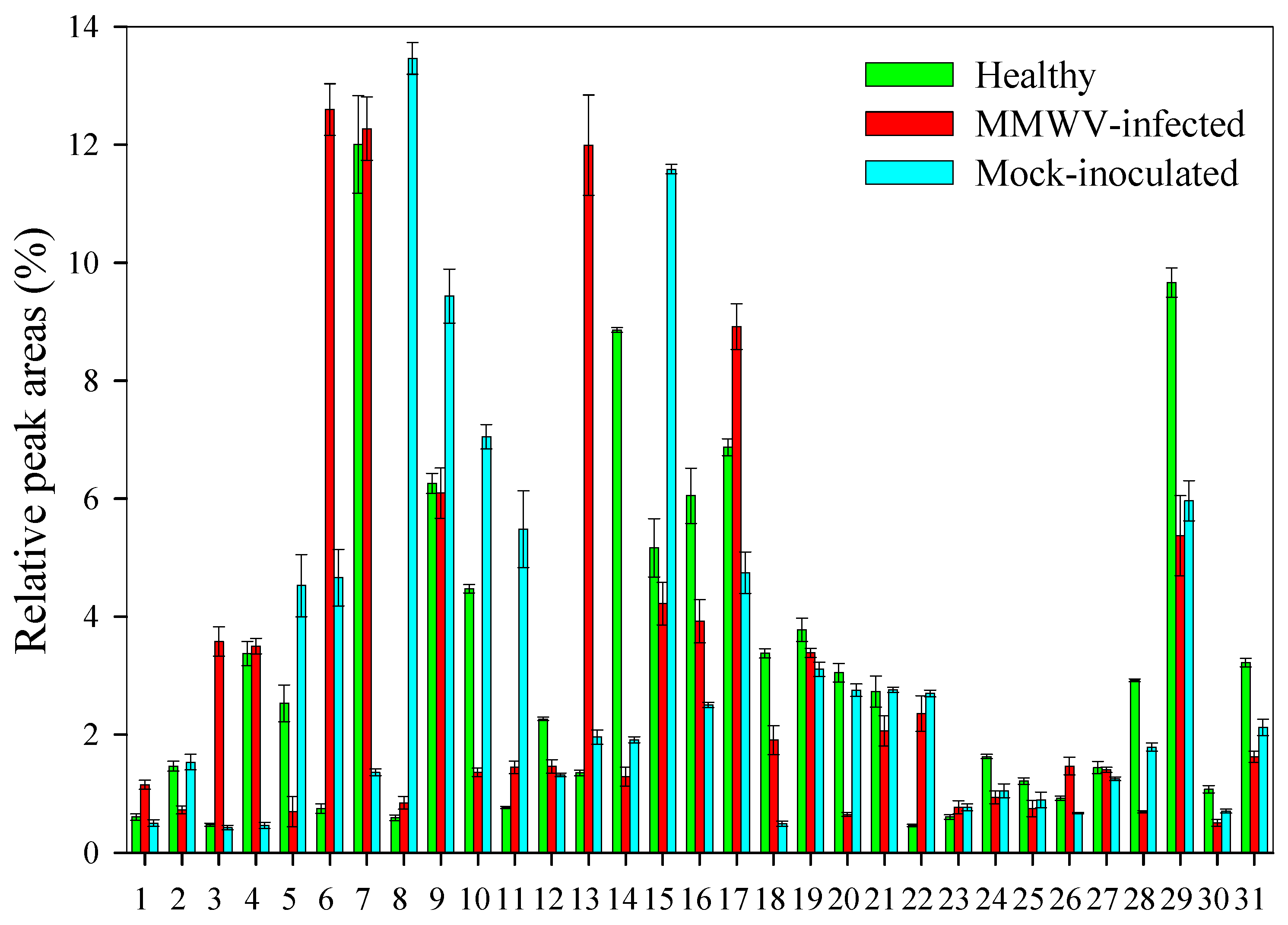
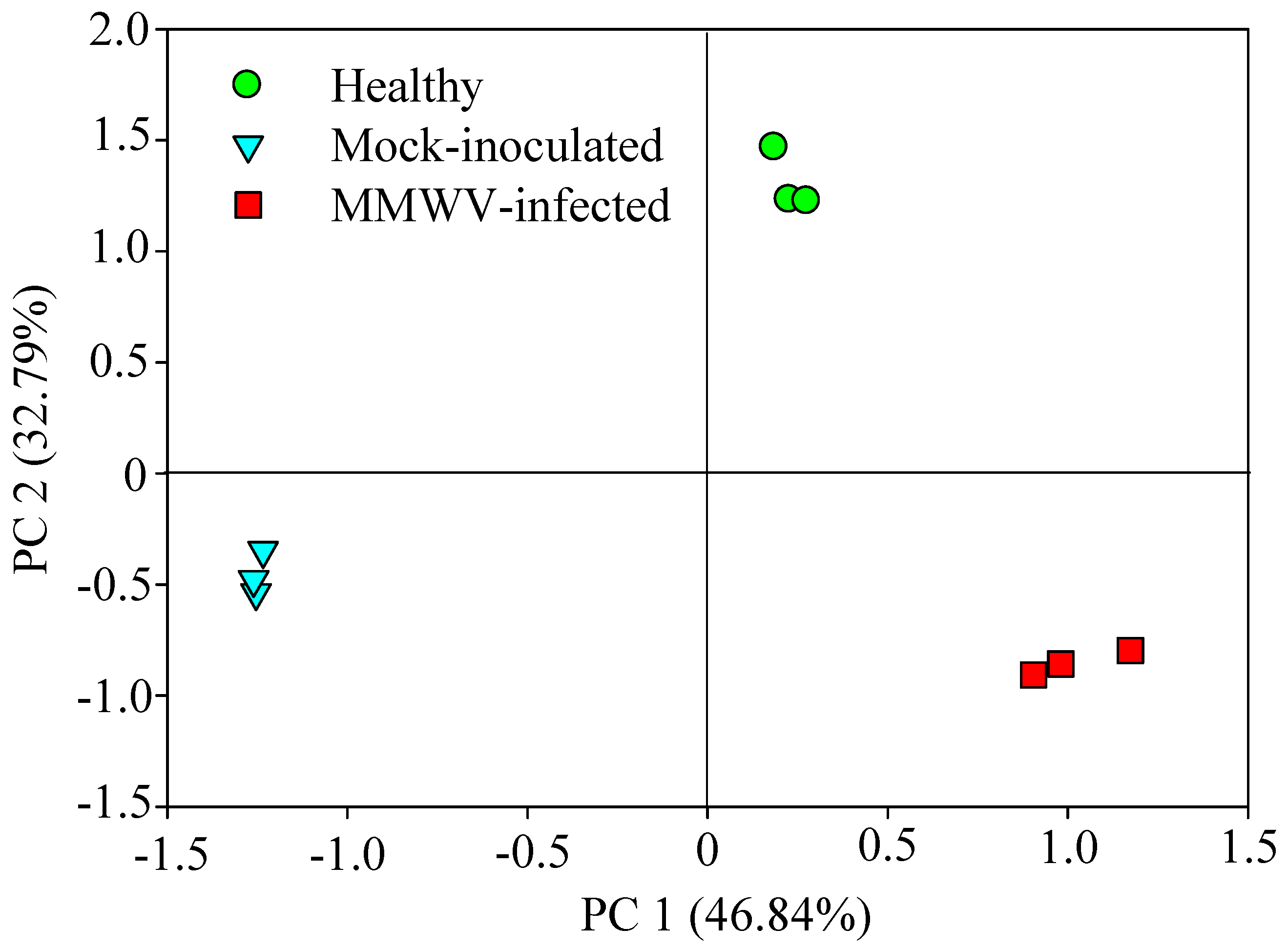
3.3. Chemical Analysis of Volatiles
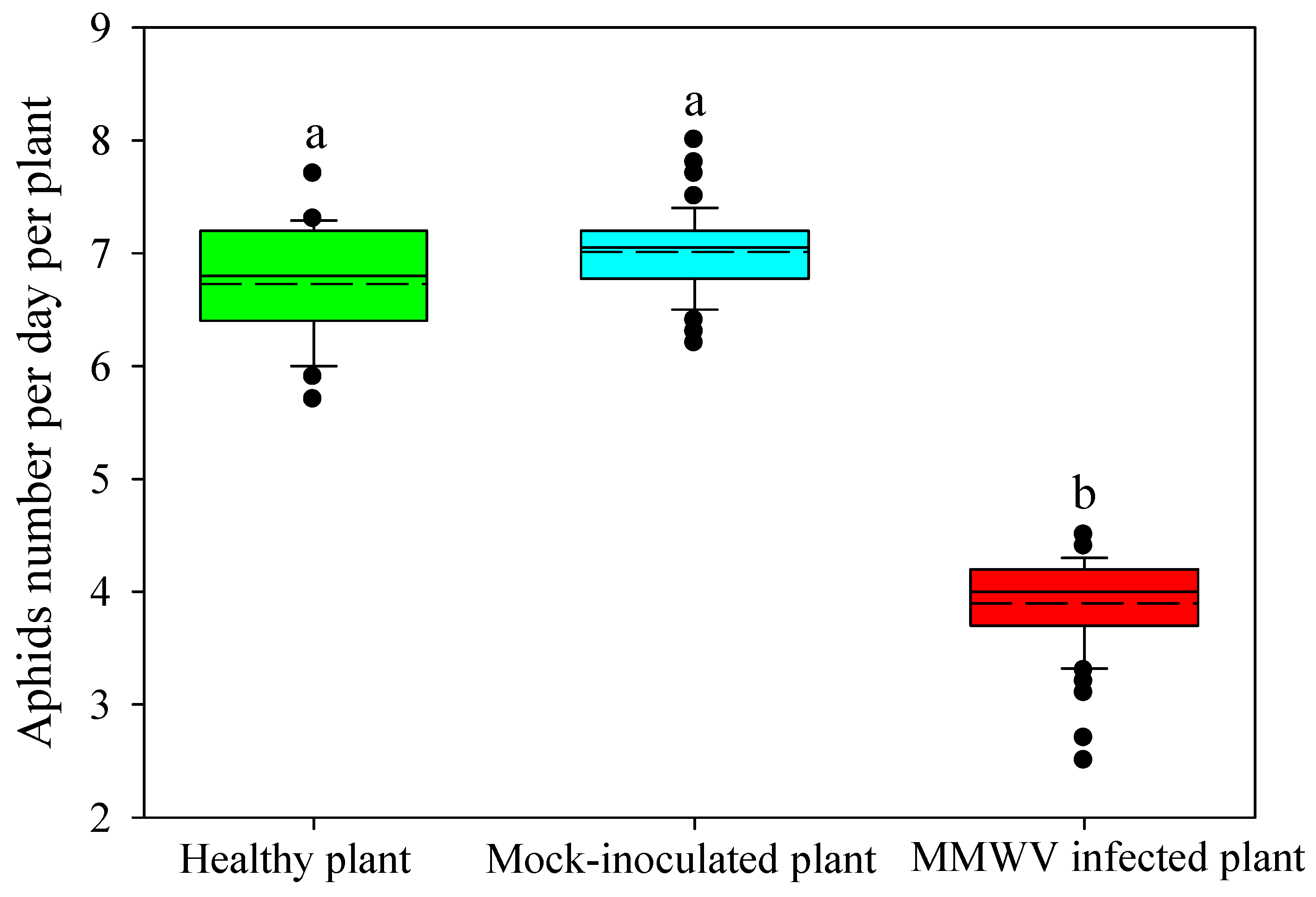
3.4. Effect of MMWV Infection on the Development of the M. persicae Population
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.Y.; Ye, W.H.; Cao, H.L.; Feng, H.L. Mikania micrantha H.B.K. in China—An overview. Weed Res. 2004, 44, 42–49. [Google Scholar] [CrossRef]
- Wang, R.L.; Ding, L.W.; Sun, Q.Y.; Li, J.; Xu, Z.F.; Peng, S.L. Genome sequence and characterization of a new virus infecting Mikania micrantha H.B.K. Arch. Virol. 2008, 153, 1765–1770. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Xu, G.; Clements, D.R.; Jin, G.; Chen, A.; Zhang, F.; Kato-Noguchi, H. Suppression of the invasive plant mile-a-minute (Mikania micrantha) by local crop sweet potato (Ipomoea batatas) by means of higher growth rate and competition for soil nutrients. BMC Ecol. 2015, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yuan, K.; Zhang, S.; Wang, R. Influence of Mikania micrantha wilt virus on growth, reproduction and allelopathic potential of its host. Allelopath. J. 2015, 35, 87–96. [Google Scholar]
- Huang, F.; Peng, S.; Chen, B.; Liao, H.; Huang, Q.; Lin, Z.; Liu, G. Rapid evolution of dispersal-related traits during range expansion of an invasive vine Mikania micrantha. Oikos 2015, 124, 1023–1030. [Google Scholar] [CrossRef]
- Ng, J.C.K.; Falk, B.W. Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu. Rev. Phytopathol. 2006, 44, 183–212. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479, 278–289. [Google Scholar] [CrossRef]
- Cassone, B.J.; Wijeratne, S.; Michel, A.P.; Stewart, L.R.; Chen, Y.; Yan, P.; Redinbaugh, M.G. Virus-independent and common transcriptome responses of leafhopper vectors feeding on maize infected with semi-persistently and persistent propagatively transmitted viruses. BMC Genom. 2014, 15, 133. [Google Scholar] [CrossRef]
- Mauck, K.; Bosque-Pérez, N.A.; Eigenbrode, S.D.; De Moraes, C.M.; Mescher, M.C. Transmission mechanisms shape pathogen effects on host-vector interactions: Evidence from plant viruses. Funct. Ecol. 2012, 26, 1162–1175. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Effects of pathogens on sensory-mediated interactions between plants and insect vectors. Curr. Opin. Plant Biol. 2016, 32, 53–61. [Google Scholar] [CrossRef]
- Mauck, K.E.; Chesnais, Q.; Shapiro, L.R. Evolutionary determinants of host and vector manipulation by plant viruses. In Advances in Virus Research, 1st ed.; Malmstrom, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 101, pp. 189–250. [Google Scholar]
- Chesnais, Q.; Couty, A.; Uzest, M.; Brault, V.; Ameline, A. Plant infection by two different viruses induce contrasting changes of vectors fitness and behavior. Insect Sci. 2019, 26, 86–96. [Google Scholar] [CrossRef]
- Jiu, M.; Zhou, X.P.; Tong, L.; Xu, J.; Yang, X.; Wan, F.H.; Liu, S.S. Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS ONE 2007, 2, e182. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef]
- Eckel, R.V.W.; Lampert, E.P. Relative attractiveness of tobacco etch virus-infected and healthy flue-cured tobacco plants to aphids. J. Econ. Entomol. 1996, 89, 1017–1027. [Google Scholar] [CrossRef]
- Ingwell, L.L.; Eigenbrode, S.D.; Bosque-Pérez, N.A. Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2012, 2, 578. [Google Scholar] [CrossRef]
- Dietzgen, R.G.; Mann, K.S.; Johnson, K.N. Plant virus-insect vector interactions: Current and potential future research directions. Viruses 2016, 8, 303. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Bosque-Pérez, N.A.; Davis, T.S. Insect-borne plant pathogens and their vectors: Ecology, evolution, and complex interactions. Ann. Rev. Entomol. 2017, 63, 169–191. [Google Scholar] [CrossRef]
- Rajabaskar, D.; Bosque-Pérez, N.A.; Eigenbrode, S.D. Preference by a virus vector for infected plants is reversed after virus acquisition. Virus Res. 2014, 186, 32–37. [Google Scholar] [CrossRef]
- Wang, H.; Xu, D.; Pu, L.; Zhou, G. Southern rice black-streaked dwarf virus alters insect vectors’ host orientation preferences to enhance spread and increase rice ragged stunt virus co-infection. Phytopathology 2013, 104, 196–201. [Google Scholar] [CrossRef]
- Wang, R.L.; Chen, Y.; Zhang, H.; Guan, A.M.; Wang, Z.Y.; Gong, X.; Yin, Y.; Zeng, R.S. Host range, transmitting vector, and damage characteristics of Mikania micrantha wilt virus. Chin. J. Ecol. 2013, 32, 72–77. [Google Scholar]
- Wu, D.; Qi, T.; Li, W.X.; Tian, H.; Gao, H.; Wang, J.; Ge, J.; Yao, R.; Ren, C.; Wang, X.B.; et al. Viral effector protein manipulates host hormone signaling to attract insect vectors. Cell Res. 2017, 27, 402–415. [Google Scholar] [CrossRef]
- Ninkovic, V.; Feng, Y.; Olsson, U.; Pettersson, J. Ladybird footprints induce aphid avoidance behavior. Biol. Control. 2013, 65, 63–71. [Google Scholar] [CrossRef]
- Agarwala, B.K.; Choudhury, P.R. Host races of the cotton aphid, Aphis gossypii, in asexual populations from wild plants of taro and brinjal. J. Insect Sci. 2013, 13, 1–13. [Google Scholar] [CrossRef]
- Fereres, A.; Peñaflor, M.F.G.V.; Favaro, C.F.; Azevedo, K.E.X.; Landi, C.H.; Maluta, N.K.P.; Bento, J.M.S.; Lopes, J.R.S. Tomato infection by whitefly-transmitted circulative and non-circulative viruses induce contrasting changes in plant volatiles and vector behaviour. Viruses 2016, 8, 225. [Google Scholar] [CrossRef]
- Jahan, S.H.; Lee, G.S.; Lee, S.; Lee, K.Y. Upregulation of probing- and feeding-related behavioural frequencies in Bemisia tabaci upon acquisition of Tomato yellow leaf curl virus. Pest Manag. Sci. 2014, 70, 1497–1502. [Google Scholar] [CrossRef]
- Mauck, K.E.; Kenney, J.; Chesnais, Q. Progress and challenges in identifying molecular mechanisms underlying host and vector manipulation by plant viruses. Curr. Opin. Insect Sci. 2019, 33, 7–18. [Google Scholar] [CrossRef]
- Fereres, A.; Shukle, R.H.; Araya, J.E.; Foster, J.E. Probing and feeding behavior of Sitobion avenae (F.) (Horn., Aphididae) on three wheat cultivars infected with barley yellow dwarf virus. J. Appl. Entomol. 1990, 109, 29–36. [Google Scholar] [CrossRef]
- De Vos, M.; Jander, G. Volatile communication in plant-aphid interactions. Curr. Opin. Plant Biol. 2010, 13, 366–371. [Google Scholar] [CrossRef]
- Wu, Y.; Davis, T.S.; Eigenbrode, S.D. Aphid behavioral responses to virus-infected plants are similar despite divergent fitness effects. Entomol. Exp. Appl. 2014, 153, 246–255. [Google Scholar] [CrossRef]
- Su, Q.; Preisser, E.L.; Zhou, X.M.; Xie, W.; Liu, B.M.; Wang, S.L.; Wu, Q.J.; Zhang, Y.J. Manipulation of host quality and defense by a plant virus improves performance of whitefly vectors. J. Econ. Entomol. 2015, 108, 11–19. [Google Scholar] [CrossRef]
- Dáder, B.; Then, C.; Berthelot, E.; Ducousso, M.; Ng, J.C.K.; Drucker, M. Insect transmission of plant viruses: Multilayered interactions optimize viral propagation. Insect Sci. 2017, 24, 929–946. [Google Scholar] [CrossRef]
- Tan, X.L.; Chen, J.L.; Benelli, G.; Desneux, N.; Yang, X.Q.; Liu, T.X.; Ge, F. Pre-infestation of tomato plants by aphids modulates transmission-acquisition relationship among whiteflies, Tomato yellow leaf curl virus (TYLCV) and plants. Front. Plant Sci. 2017, 8, 1597. [Google Scholar] [CrossRef]
- Wosula, E.N.; Davis, J.A.; Clark, C.A. Population dynamics of three aphid species (Hemiptera: Aphididae) on four Ipomoea spp. Infected or non-infected with sweetpotato potyviruses. J. Econ. Entomol. 2013, 106, 1566–1573. [Google Scholar] [CrossRef]
- Callaway, R.M.; Thelen, G.C.; Rodriguez, A.; Holben, W.E. Soil biota and exotic plant invasion. Nature 2004, 427, 31–733. [Google Scholar] [CrossRef]
- Dunn, A.M.; Torchin, M.E.; Hatcher, M.J.; Kotanen, P.M.; Blumenthal, D.M.; Byers, J.E.; Coon, C.A.C.; Frankel, V.M.; Holt, R.D.; Hufbauer, R.A.; et al. Indirect effects of parasites in invasions. Funct. Ecol. 2012, 26, 1262–1274. [Google Scholar] [CrossRef]
- Harding, D.P.; Raizada, M.N. Controlling weeds with fungi, bacteria and viruses: A review. Front. Plant Sci. 2015, 6, 659. [Google Scholar] [CrossRef]
- Valles, S.M.; Porter, S.D. Dose response of red imported fire ant colonies to Solenopsis invicta virus 3. Arch. Virol. 2015, 160, 2407–2413. [Google Scholar] [CrossRef]
- Arora, A.K.; Douglas, A.E. Hype or opportunity? Using microbial symbionts in novel strategies for insect pest control. J. Insect Physiol. 2017, 103, 10–17. [Google Scholar] [CrossRef]
- Valles, S.M.; Hashimoto, Y. Isolation and characterization of Solenopsis invicta virus 3, a new positive-strand RNA virus infecting the red imported fire ant, Solenopsis invicta. Virology 2009, 388, 354–361. [Google Scholar] [CrossRef]
- Diaz, R.; Manrique, V.; Hibbard, K.; Fox, A.; Roda, A.; Gandolfo, D.; Medal, J.; Hight, S.; Overholt, W.A. Successful biological control of tropical soda apple (Solanales: Solanaceae) in Florida: A review of key program components. Florida Entomol. 2014, 97, 179–190. [Google Scholar] [CrossRef]
- Kollmann, J.; Bañuelos, M.J.; Nielsen, S.L. Effects of virus infection on growth of the invasive alien impatiens glandulifera. Preslia 2007, 79, 33–44. [Google Scholar]
- Faillace, C.A.; Lorusso, N.S.; Duffy, S. Overlooking the smallest matter: Viruses impact biological invasions. Ecol. Lett. 2017, 20, 524–538. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.-L.; Zhu-Salzman, K.; Elzaki, M.E.A.; Huang, Q.-Q.; Chen, S.; Ma, Z.-H.; Liu, S.-W.; Zhang, J.-E. Mikania Micrantha Wilt Virus Alters Insect Vector’s Host Preference to Enhance Its Own Spread. Viruses 2019, 11, 336. https://doi.org/10.3390/v11040336
Wang R-L, Zhu-Salzman K, Elzaki MEA, Huang Q-Q, Chen S, Ma Z-H, Liu S-W, Zhang J-E. Mikania Micrantha Wilt Virus Alters Insect Vector’s Host Preference to Enhance Its Own Spread. Viruses. 2019; 11(4):336. https://doi.org/10.3390/v11040336
Chicago/Turabian StyleWang, Rui-Long, Keyan Zhu-Salzman, Mohammed Esmail Abdalla Elzaki, Qiao-Qiao Huang, Shi Chen, Zhi-Hui Ma, Shi-Wei Liu, and Jia-En Zhang. 2019. "Mikania Micrantha Wilt Virus Alters Insect Vector’s Host Preference to Enhance Its Own Spread" Viruses 11, no. 4: 336. https://doi.org/10.3390/v11040336
APA StyleWang, R.-L., Zhu-Salzman, K., Elzaki, M. E. A., Huang, Q.-Q., Chen, S., Ma, Z.-H., Liu, S.-W., & Zhang, J.-E. (2019). Mikania Micrantha Wilt Virus Alters Insect Vector’s Host Preference to Enhance Its Own Spread. Viruses, 11(4), 336. https://doi.org/10.3390/v11040336





