The Dengue ED3 Dot Assay, a Novel Serological Test for the Detection of Denguevirus Type-Specific Antibodies and Its Application in a Retrospective Seroprevalence Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Viruses
2.3. Serum Specimens
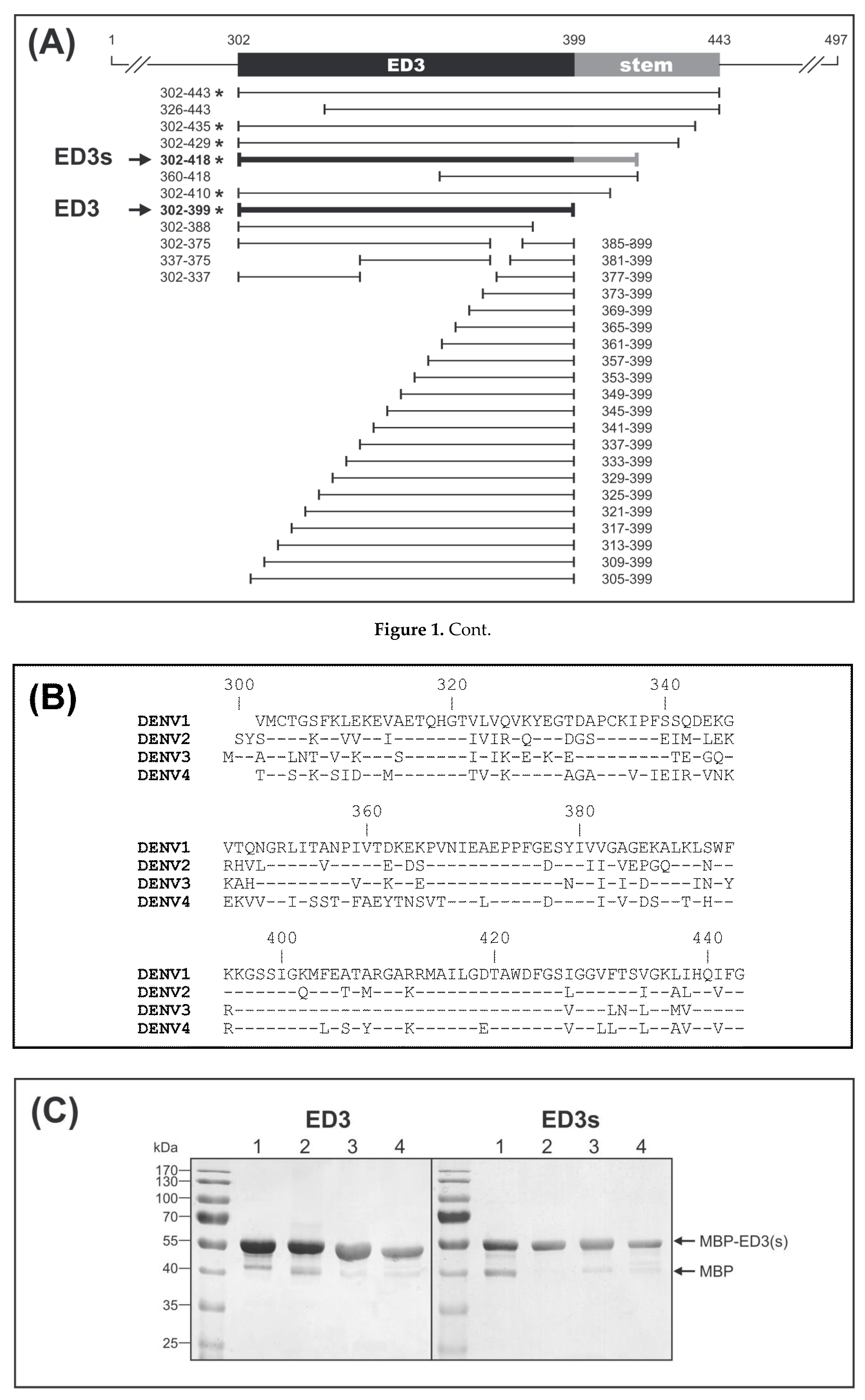
2.4. Maltose Binding Protein-ED3 Fusion Proteins
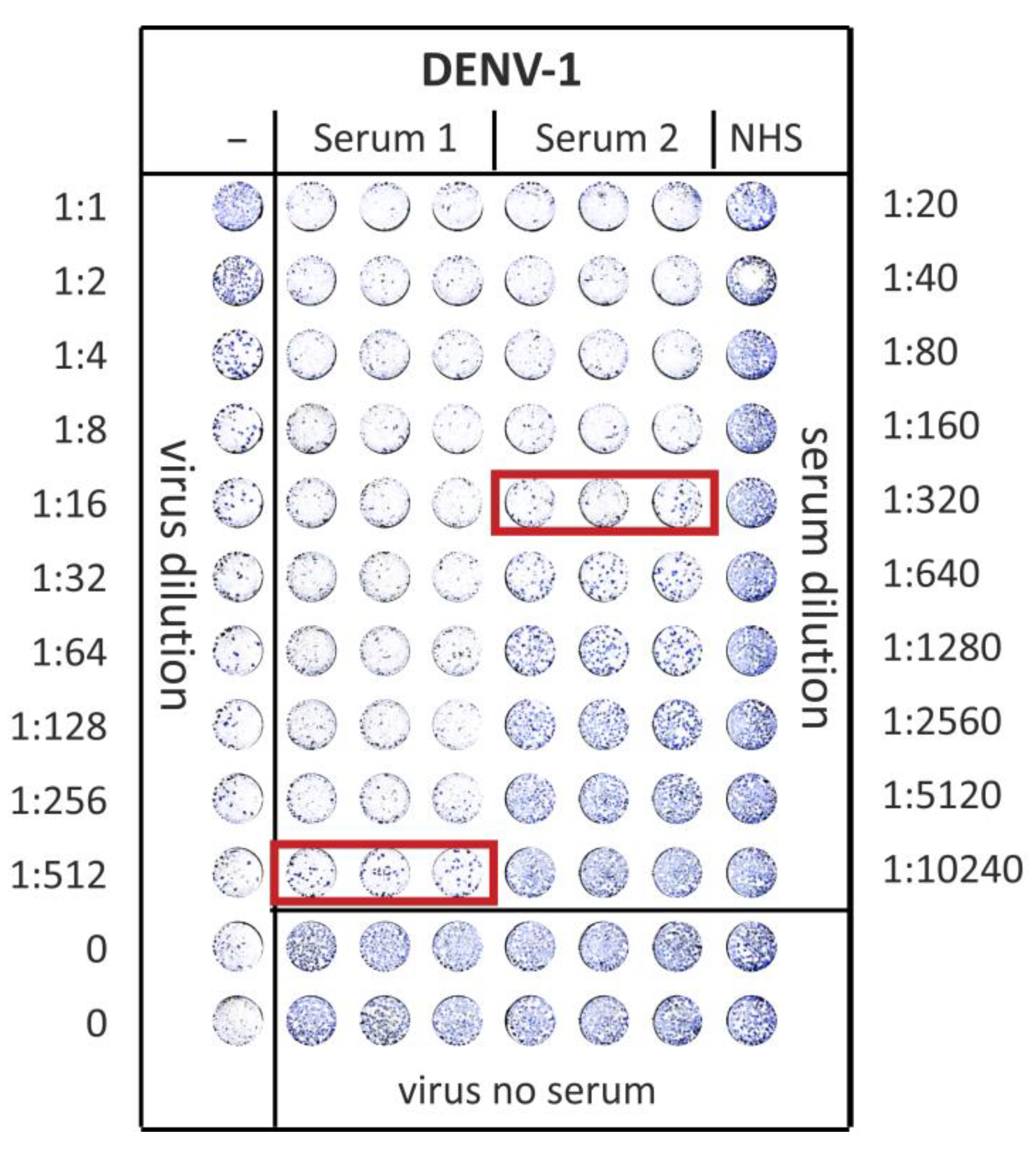
2.5. Foci Reduction Neutralization Test (FRNT)
2.6. ED3 Enzyme-Linked Immunosorbent Assay (ELISA)
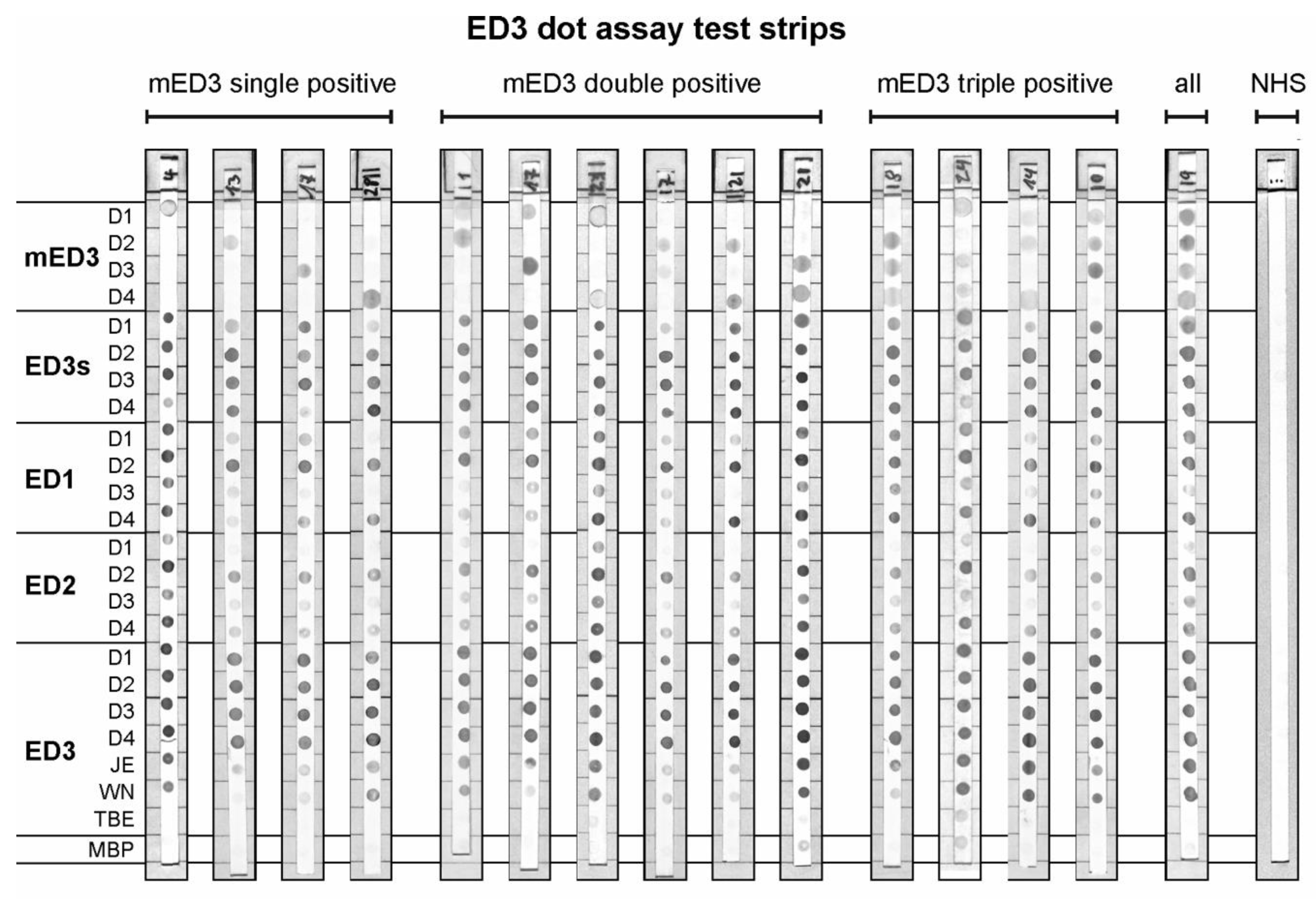
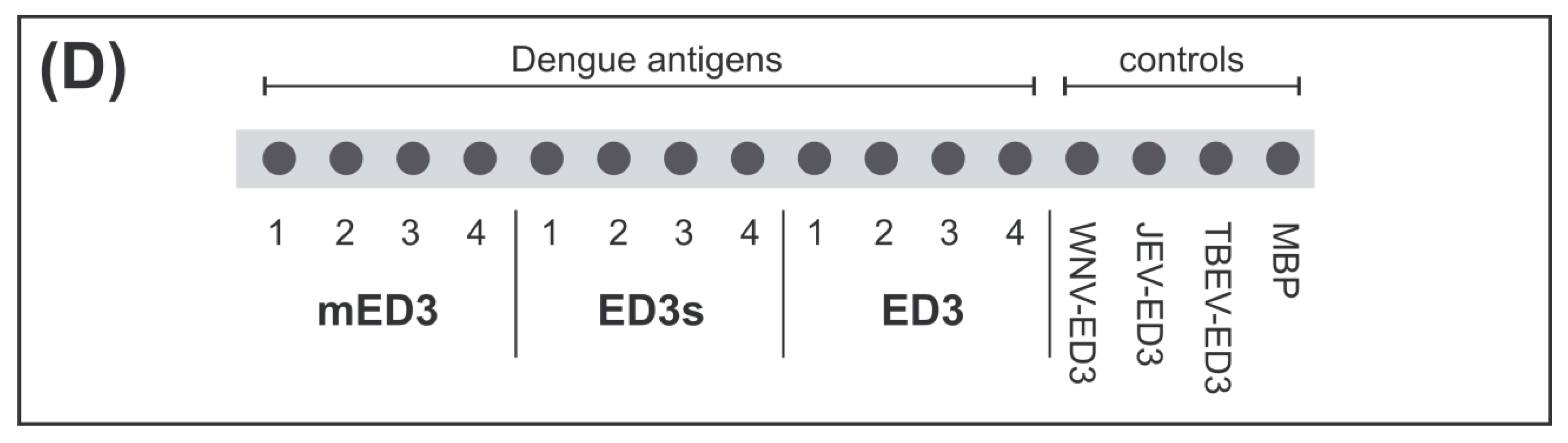
2.7. ED3 Dot Assay
3. Results
3.1. Recombinant ED3 Antigens
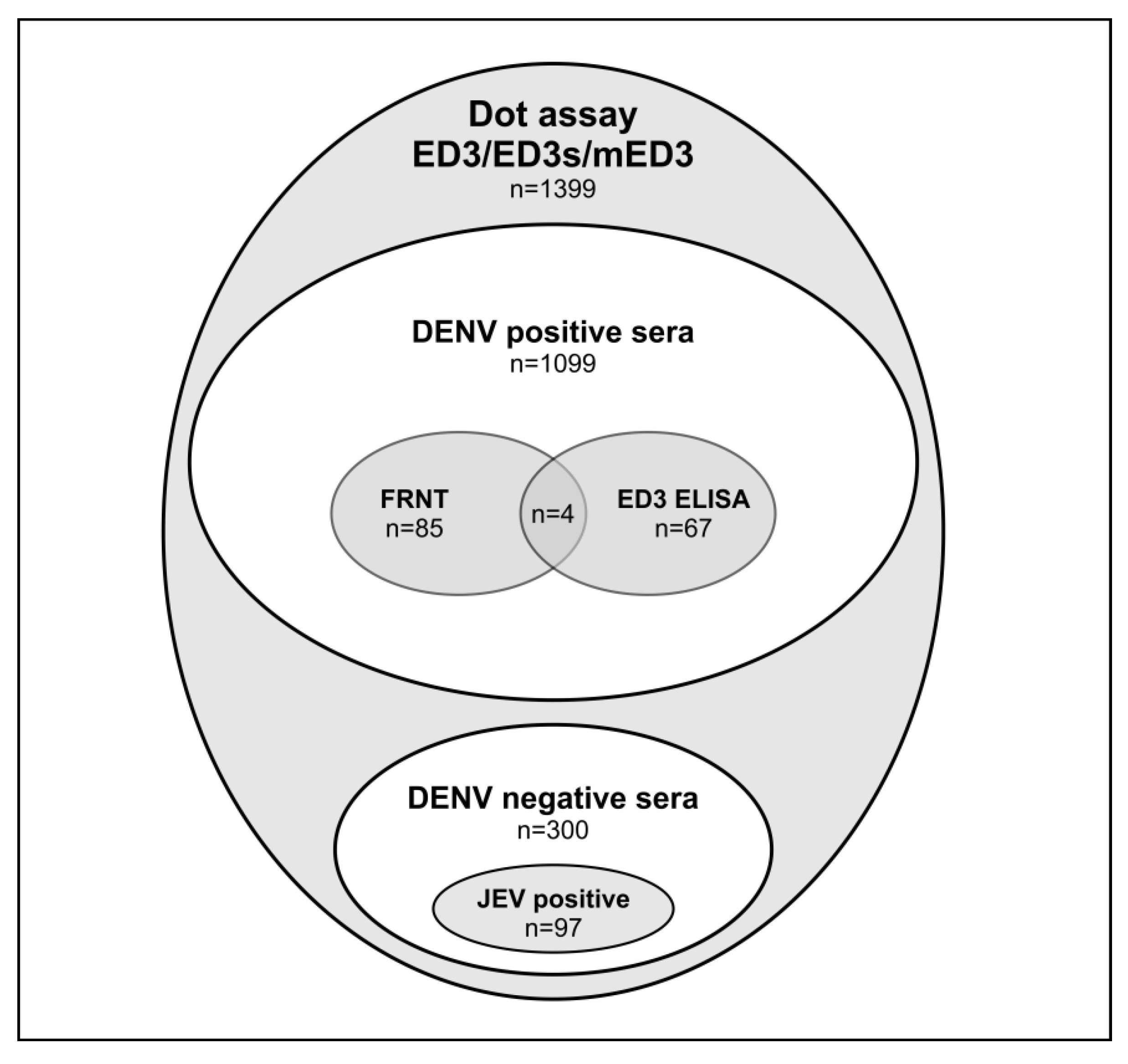
3.2. Sera Used in the Study
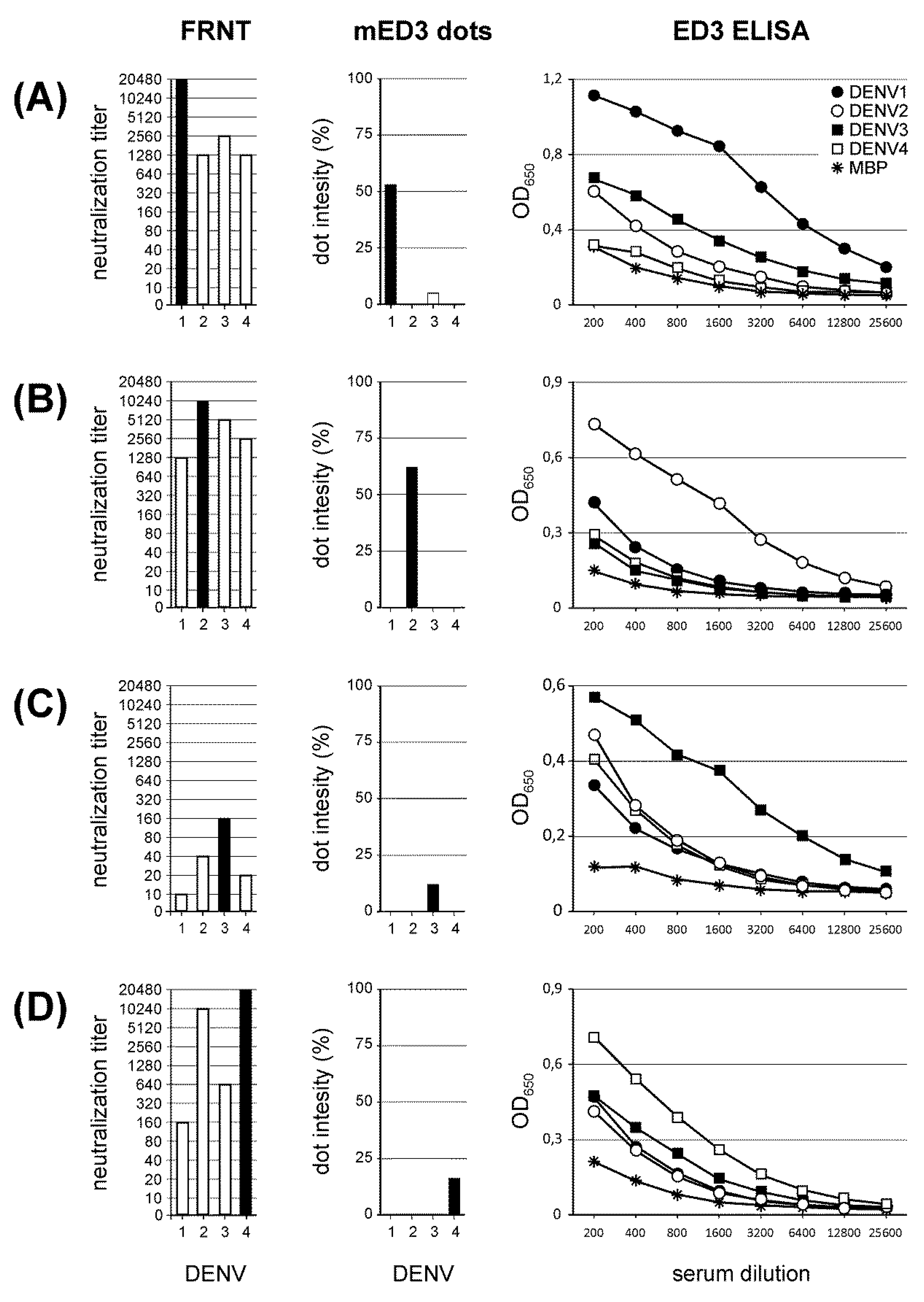
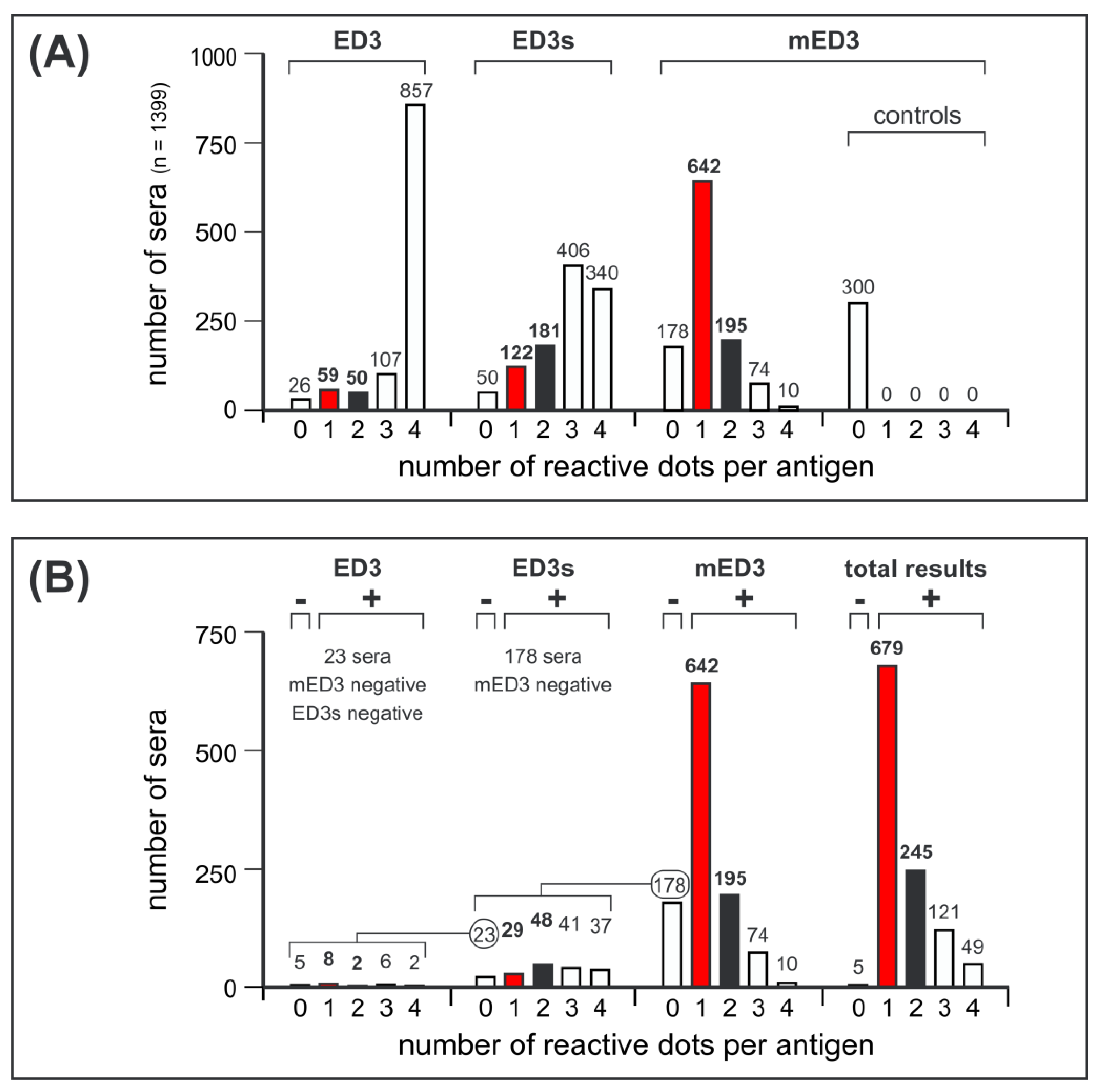
3.3. ED3 Dot Assay Responses and Comparison to Serotype-Specifc Results Obtained by FRNT and ED3 ELISA
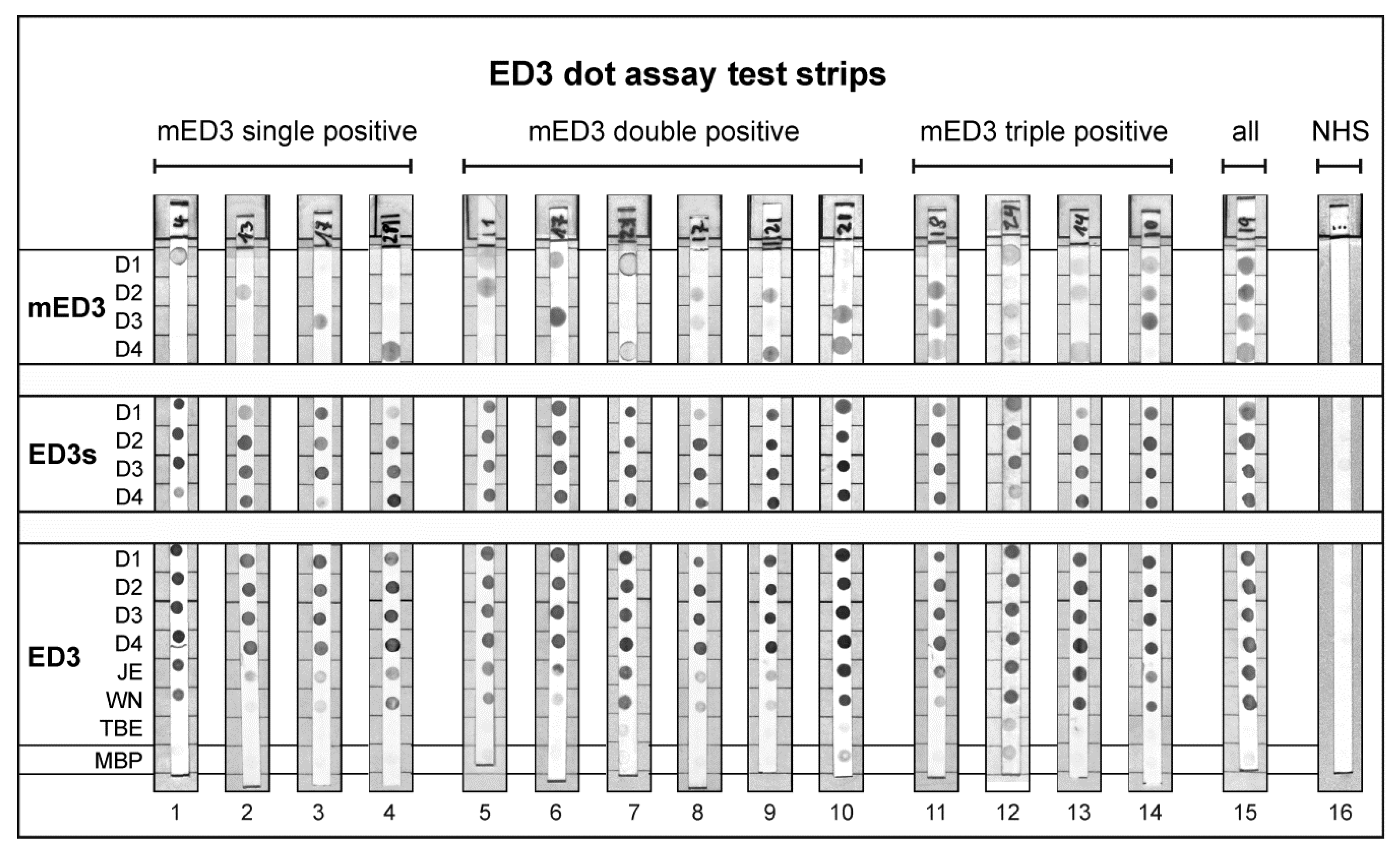
3.4. Reactivity of ED3 Antigens on Test Strips
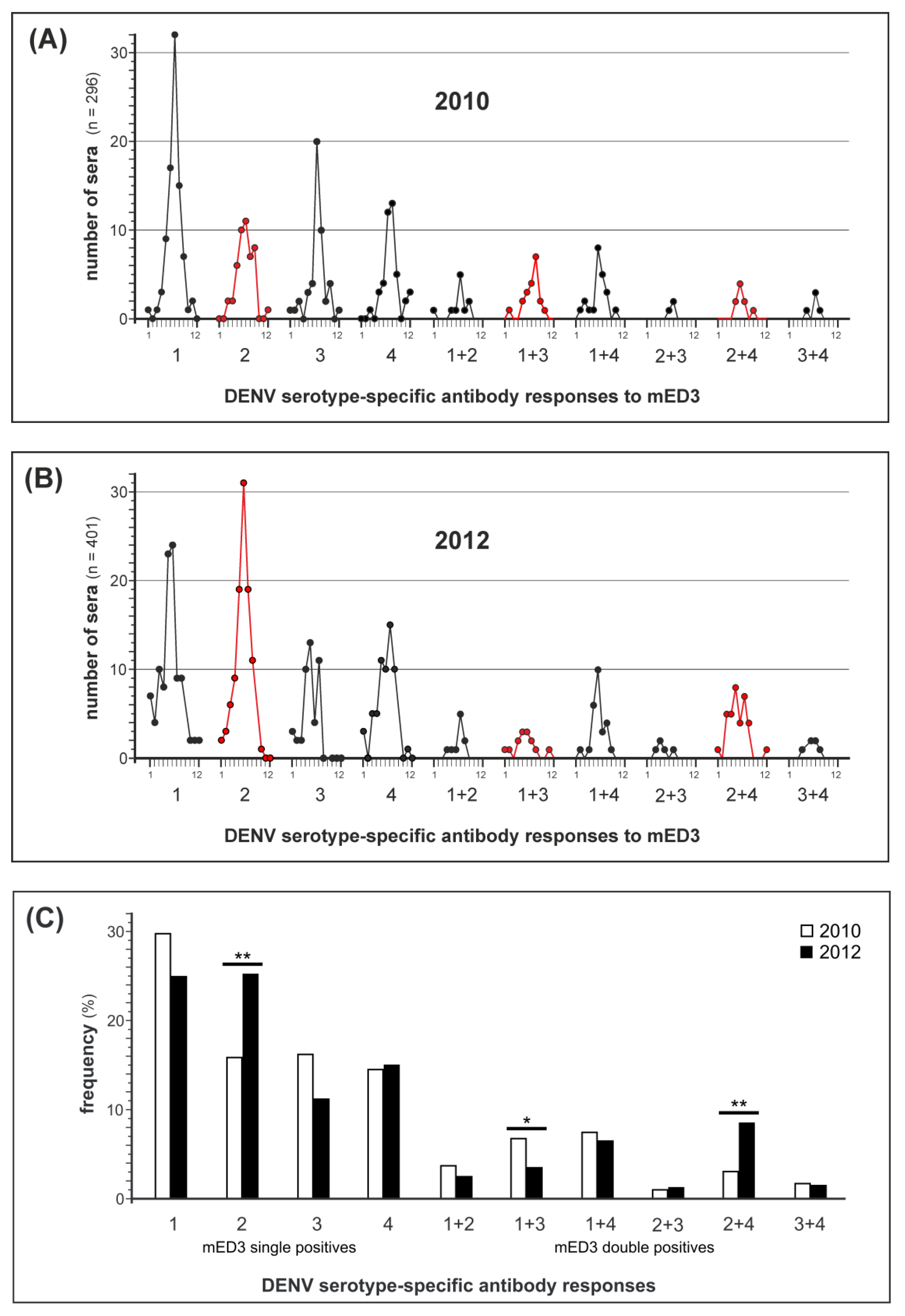
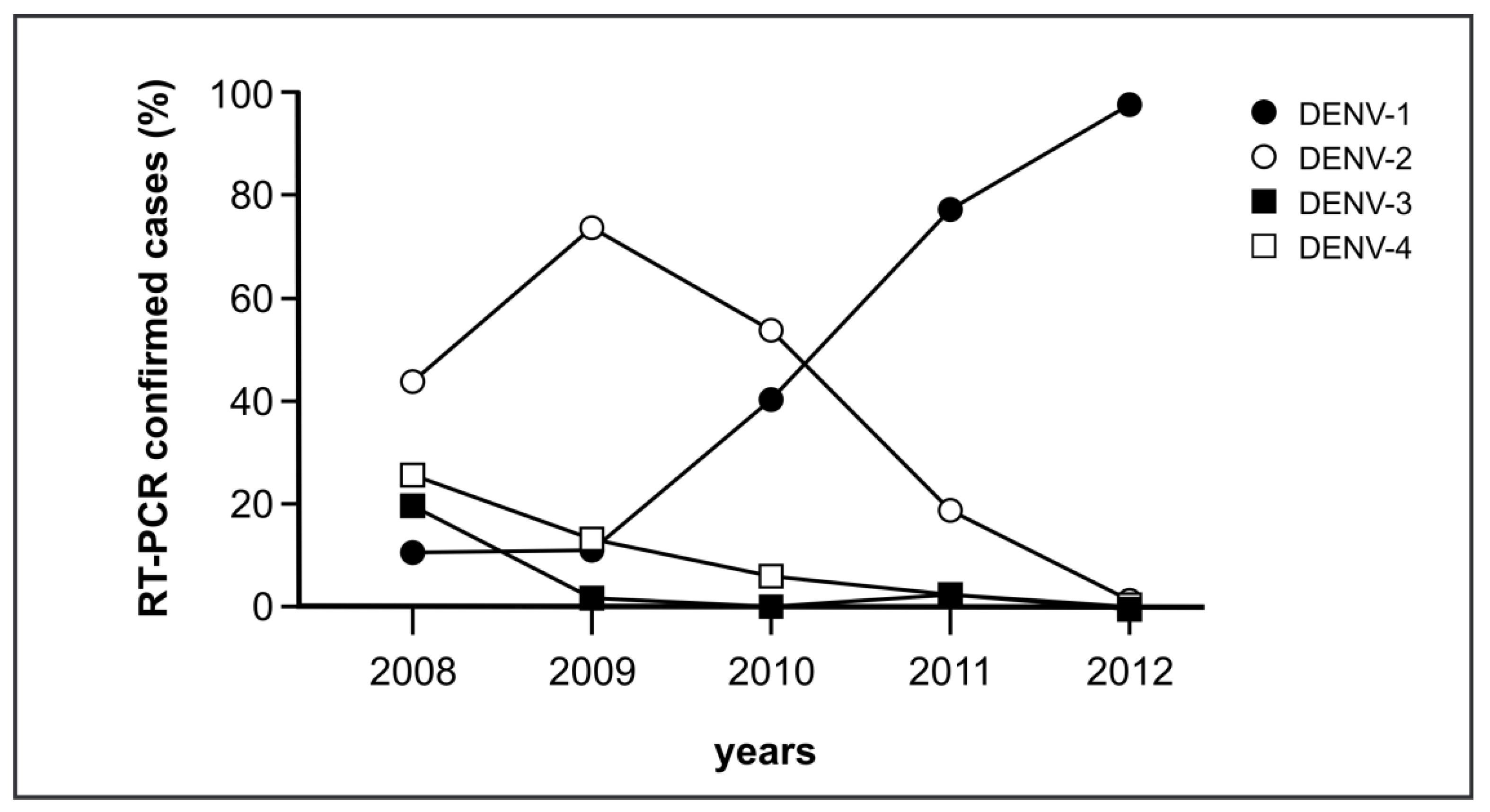
3.5. Retrospective Analysis of the DENV Serotype-Specific Antibody Response in CAMBODIA in 2010 and 2012
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Waggoner, J.J.; Balmaseda, A.; Gresh, L.; Sahoo, M.K.; Montoya, M.; Wang, C.; Abeynayake, J.; Kuan, G.; Pinsky, B.A.; Harris, E. Homotypic Dengue Virus Reinfections in Nicaraguan Children. J. Infect. Dis. 2016, 214, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Alvarez, M.; Rodriguez-Roche, R.; Bernardo, L.; Montes, T.; Vazquez, S.; Morier, L.; Alvarez, A.; Gould, E.A.; Kourí, G.; et al. Neutralizing antibodies after infection with dengue 1 virus. Emerg. Infect. Dis. 2007, 13, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Imrie, A.; Meeks, J.; Gurary, A.; Sukhbaatar, M.; Truong, T.T.; Cropp, C.B.; Effler, P. Antibody to dengue 1 detected more than 60 years after infection. Viral Immunol. 2007, 20, 672–675. [Google Scholar] [CrossRef]
- Zompi, S.; Montoya, M.; Pohl, M.O.; Balmaseda, A.; Harris, E. Dominant cross-reactive B cell response during secondary acute dengue virus infection in humans. PLoS Negl. Trop. Dis. 2012, 6, e1568. [Google Scholar] [CrossRef]
- Mathew, A.; West, K.; Kalayanarooj, S.; Gibbons, R.V.; Srikiatkhachorn, A.; Green, S.; Libraty, D.; Jaiswal, S.; Rothman, A.L. B-cell responses during primary and secondary dengue virus infections in humans. J. Infect. Dis. 2011, 204, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef]
- Halstead, S.B.; O’Rourke, E.J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J. Exp. Med. 1977, 146, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Tadano, M.; Saito, M.; Maneekarn, N.; Sittisombut, N.; Sirisanthana, V.; Poneprasert, B.; Fukunaga, T. Studies on Serological Cross-Reaction in Sequential Flavivirus Infections. Microbiol. Immunol. 1994, 38, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Karabatsos, N.; Dalrymple, J.M.; Shope, R.E.; Porterfield, J.S.; Westaway, E.G.; Brandt, W.E. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 1989, 70, 37–43. [Google Scholar] [CrossRef]
- Halstead, S.B. Which Dengue Vaccine Approach Is the Most Promising, and Should We Be Concerned about Enhanced Disease after Vaccination? There Is Only One True Winner. Cold Spring Harb. Perspect. Biol. 2017, 10, a029520. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Artsob, H.; Pelegrino, J.L.; Buchy, P.; Cardosa, M.J.; Devi, S.; Enria, D.A.; Farrar, J.; Gubler, D.J.; Guzman, M.G.; et al. Evaluation of diagnostic tests: Dengue. Nat. Rev. Microbiol. 2010, 8, S30–S38. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Zhou, Y.; Olivarez, N.P.; Broadwater, A.H.; de Silva, A.M.; Crowe, J.E. Persistence of Circulating Memory B Cell Clones with Potential for Dengue Virus Disease Enhancement for Decades following Infection. J. Virol. 2012, 86, 2665–2675. [Google Scholar] [CrossRef]
- Rouvinski, A.; Guardado-Calvo, P.; Barba-Spaeth, G.; Duquerroy, S.; Vaney, M.-C.; Kikuti, C.M.; Sanchez, M.E.N.; Dejnirattisai, W.; Wongwiwat, W.; Haouz, A.; et al. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 2015, 520, U109–U260. [Google Scholar] [CrossRef]
- Putnak, J.R.; De La Barrera, R.; Burgess, T.; Pardo, J.; Dessy, F.; Gheysen, D.; Lobet, Y.; Green, S.; Endy, T.P.; Thomas, S.J.; et al. Comparative evaluation of three assays for measurement of dengue virus neutralizing antibodies. Am. J. Trop. Med. Hyg. 2008, 79, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Salje, H.; Rodríguez-Barraquer, I.; Rainwater-Lovett, K.; Nisalak, A.; Thaisomboonsuk, B.; Thomas, S.J.; Fernandez, S.; Jarman, R.G.; Yoon, I.-K.; Cummings, D.A.T. Variability in dengue titer estimates from plaque reduction neutralization tests poses a challenge to epidemiological studies and vaccine development. PLoS Negl. Trop. Dis. 2014, 8, e2952. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Kuhn, R.J. Structural proteomics of dengue virus. Curr. Opin. Microbiol. 2008, 11, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Wongwiwat, W.; Supasa, S.; Zhang, X.; Dai, X.; Rouvinski, A.; Jumnainsong, A.; Edwards, C.; Quyen, N.T.H.; Duangchinda, T.; et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat. Immunol. 2015, 16, 170–177. [Google Scholar] [CrossRef]
- Crill, W.D.; Hughes, H.R.; Delorey, M.J.; Chang, G.-J.J. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS ONE 2009, 4, e4991. [Google Scholar] [CrossRef] [PubMed]
- Gromowski, G.D.; Barrett, A.D.T. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology 2007, 366, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Sukupolvi-Petty, S.; Austin, S.K.; Purtha, W.E.; Oliphant, T.; Nybakken, G.E.; Schlesinger, J.J.; Roehrig, J.T.; Gromowski, G.D.; Barrett, A.D.; Fremont, D.H.; et al. Type- and Subcomplex-Specific Neutralizing Antibodies against Domain III of Dengue Virus Type 2 Envelope Protein Recognize Adjacent Epitopes. J. Virol. 2007, 81, 12816–12826. [Google Scholar] [CrossRef]
- Gromowski, G.D.; Barrett, N.D.; Barrett, A.D.T. Characterization of dengue virus complex-specific neutralizing epitopes on envelope protein domain III of dengue 2 virus. J. Virol. 2008, 82, 8828–8837. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Gromowski, G.D.; Li, L.; Schuh, A.J.; Lee, J.C.; Barrett, A.D.T. Characterization of dengue complex-reactive epitopes on dengue 3 virus envelope protein domain III. Virology 2009, 384, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Batra, G.; Nemani, S.K.; Tyagi, P.; Swaminathan, S.; Khanna, N. Evaluation of envelope domain III-based single chimeric tetravalent antigen and monovalent antigen mixtures for the detection of anti-dengue antibodies in human sera. BMC Infect. Dis. 2011, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.A.; Paixão, V.F.; Oliveira, M.D.; Honda, E.R.; Oliveira, L.L.; da Silva, C.C.; De Paula, S.O. Dengue-1 envelope protein domain III produced in Pichia pastoris: Potential use for serological diagnosis. Protein Expr. Purif. 2013, 92, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Pang, Z.; Guan, C.; Qi, J.; Li, D. Dengue virus envelope domain III protein based on a tetravalent antigen secreted from insect cells: Potential use for serological diagnosis. Virus Res. 2015, 201, 73–78. [Google Scholar] [CrossRef]
- Ludolfs, D.; Schilling, S.; Altenschmidt, J.; Schmitz, H. Serological differentiation of infections with dengue virus serotypes 1 to 4 by using recombinant antigens. J. Clin. Microbiol. 2002, 40, 4317–4320. [Google Scholar] [CrossRef]
- De Alwis, R.; Smith, S.a.; Olivarez, N.P.; Messer, W.B.; Huynh, J.P.; Wahala, W.M.P.B.; White, L.J.; Diamond, M.S.; Baric, R.S.; Crowe, J.E.; et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc. Natl. Acad. Sci. USA 2012, 109, 7439–7444. [Google Scholar] [CrossRef]
- Emmerich, P.; Mika, A.; Schmitz, H. Detection of serotype-specific antibodies to the four dengue viruses using an immune complex binding (ICB) ELISA. PLoS Negl. Trop. Dis. 2013, 7, e2580. [Google Scholar] [CrossRef] [PubMed]
- Libraty, D.H.; Zhang, L.; Obcena, A.; Brion, J.D.; Capeding, R.Z. Anti-dengue virus envelope protein domain III IgG ELISA among infants with primary dengue virus infections. Acta Trop. 2015, 142, 103–107. [Google Scholar] [CrossRef]
- Blessmann, J.; Van Linh, P.; Nu, P.A.T.; Thi, H.D.; Muller-Myhsok, B.; Buss, H.; Tannich, E. Epidemiology of amebiasis in a region of high incidence of amebic liver abscess in central Vietnam. Am. J. Trop. Med. Hyg. 2002, 66, 578–583. [Google Scholar] [CrossRef]
- Klint, J.K.; Senff, S.; Saez, N.J.; Seshadri, R.; Lau, H.Y.; Bende, N.S.; Undheim, E.A.B.; Rash, L.D.; Mobli, M.; King, G.F. Production of Recombinant Disulfide-Rich Venom Peptides for Structural and Functional Analysis via Expression in the Periplasm of E. coli. PLoS ONE 2013, 8, e63865. [Google Scholar] [CrossRef] [PubMed]
- Institut Pasteur du Cambodge Virology Unit. Public Health and Training Activities Report 2015; Institut Pasteur du Cambodge: Phnom Penh, Cambodia, 2016; Available online: http://www.pasteur-kh.org/wp-content/uploads/2015/12/Annual-report-2015_Virology-Unit_Public-Health_20160922vf-1.pdf (accessed on 2 February 2017).
- Fahimi, H.; Sadeghizadeh, M.; Hassan, Z.M.; Auerswald, H.; Schreiber, M. Immunogenicity of a novel tetravalent dengue envelope protein domain III-based antigen in mice. EXCLI J. 2018, 17, 1054–1068. [Google Scholar] [PubMed]
- Sabchareon, A.; Wallace, D.; Sirivichayakul, C.; Limkittikul, K.; Chanthavanich, P.; Suvannadabba, S.; Jiwariyavej, V.; Dulyachai, W.; Pengsaa, K.; Wartel, T.A.; et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet 2012, 380, 1559–1567. [Google Scholar] [CrossRef]
- Fonseca, B.A.; Khoshnood, K.; Shope, R.E.; Mason, P.W. Flavivirus type-specific antigens produced from fusions of a portion of the E protein gene with the Escherichia coli trpE gene. Am. J. Trop. Med. Hyg. 1991, 44, 500–508. [Google Scholar] [CrossRef]
- Simmons, M.; Nelson, W.M.; Wu, S.J.; Hayes, C.G. Evaluation of the protective efficacy of a recombinant dengue envelope B domain fusion protein against dengue 2 virus infection in mice. Am. J. Trop. Med. Hyg. 1998, 58, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, R.J.; Cui, T.; Xu, Q.; Fu, J.; Chan, Y.C. The production of recombinant dengue virus E protein using Escherichia coli and Pichia pastoris. J. Virol. Methods 1997, 69, 159–169. [Google Scholar] [CrossRef]
- Lazaro-Olán, L.; Mellado-Sánchez, G.; García-Cordero, J.; Escobar-Gutiérrez, A.; Santos-Argumedo, L.; Gutiérrez-Castañeda, B.; Cedillo-Barrón, L. Analysis of antibody response in human dengue patients from the Mexican coast using recombinant antigens. Vector Borne Zoonotic Dis. 2008, 8, 69–79. [Google Scholar] [CrossRef]
- Reddy, P.B.J.; Pattnaik, P.; Tripathi, N.K.; Srivastava, A.; Rao, P.V.L. Expression, purification and evaluation of diagnostic potential and immunogenicity of dengue virus type 3 domain III protein. Protein Pept. Lett. 2012, 19, 509–519. [Google Scholar] [CrossRef]
- Staropoli, I.; Clément, J.M.; Frenkiel, M.P.; Hofnung, M.; Deubel, V. Dengue virus envelope glycoprotein can be secreted from insect cells as a fusion with the maltose-binding protein. J. Virol. Methods 1996, 56, 179–189. [Google Scholar] [CrossRef]
- Wahala, W.M.P.B.; Kraus, A.A.; Haymore, L.B.; Accavitti-Loper, M.A.; de Silva, A.M. Dengue virus neutralization by human immune sera: Role of envelope protein domain III-reactive antibody. Virology 2009, 392, 103–113. [Google Scholar] [CrossRef]
- Zidane, N.; Dussart, P.; Bremand, L.; Villani, M.E.; Bedouelle, H. Thermodynamic stability of domain III from the envelope protein of flaviviruses and its improvement by molecular design. Protein Eng. Des. Sel. 2013, 26, 389–399. [Google Scholar] [CrossRef]
- Zidane, N.; Dussart, P.; Bremand, L.; Bedouelle, H. Cross-reactivities between human IgMs and the four serotypes of dengue virus as probed with artificial homodimers of domain-III from the envelope proteins. BMC Infect. Dis. 2013, 13, 302. [Google Scholar] [CrossRef]
- Wellems, T.E.; Howard, R.J. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 1986, 83, 6065–6069. [Google Scholar] [CrossRef]
- Lazo, L.; Izquierdo, A.; Suzarte, E.; Gil, L.; Valdés, I.; Marcos, E.; Álvarez, M.; Romero, Y.; Guzmán, M.G.; Guillén, G.; et al. Evaluation in mice of the immunogenicity and protective efficacy of a tetravalent subunit vaccine candidate against dengue virus. Microbiol. Immunol. 2014, 58, 219–226. [Google Scholar] [CrossRef]
- Coconi-Linares, N.; Ortega-Dávila, E.; López-González, M.; García-Machorro, J.; García-Cordero, J.; Steinman, R.M.; Cedillo-Barrón, L.; Gómez-Lim, M.A. Targeting of envelope domain III protein of DENV type 2 to DEC-205 receptor elicits neutralizing antibodies in mice. Vaccine 2013, 31, 2366–2371. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, L.; Izquierdo, A.; Alvarez, M.; Rosario, D.; Prado, I.; López, C.; Martínez, R.; Castro, J.; Santana, E.; Hermida, L.; et al. Immunogenicity and protective efficacy of a recombinant fusion protein containing the domain III of the dengue 1 envelope protein in non-human primates. Antivir. Res. 2008, 80, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.E.; Choi, J.L.; Harrison, S.C. Structure of a Dengue Virus Envelope Protein Late-Stage Fusion Intermediate. J. Virol. 2013, 87, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Volk, D.E.; Lee, Y.-C.; Li, X.; Thiviyanathan, V.; Gromowski, G.D.; Li, L.; Lamb, A.R.; Beasley, D.W.C.; Barrett, A.D.T.; Gorenstein, D.G. Solution structure of the envelope protein domain III of dengue-4 virus. Virology 2007, 364, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wuu, A.; Basu, R.; Holbrook, M.R.; Barrett, A.D.T.; Lee, J.C. Solution structure and structural dynamics of envelope protein domain III of mosquito- and tick-borne flaviviruses. Biochemistry 2004, 43, 9168–9176. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.O.S.; Caliri, A. Stereochemical features of the envelope protein Domain III of dengue virus reveals putative antigenic site in the five-fold symmetry axis. Biochim. Biophys. Acta 2013, 1834, 221–230. [Google Scholar] [CrossRef]
- Saotome, T.; Nakamura, S.; Islam, M.M.; Nakazawa, A.; Dellarole, M.; Arisaka, F.; Kidokoro, S.; Kuroda, Y. Unusual Reversible Oligomerization of Unfolded Dengue Envelope Protein Domain 3 at High Temperatures and Its Abolition by a Point Mutation. Biochemistry 2016, 55, 4469–4475. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO) Regional Office for South-East Asia. Dengue: Guidelines for Diagnosis, Treatment, Prevention, and Control; WHO and the Special Programme for Research and Training in Tropical Diseases (TDR): WHO Press: Geneva, Switzerland, 2009. [Google Scholar]
- Halstead, S.B.; Rojanasuphot, S.; Sangkawibha, N. Original antigenic sin in dengue. Am. J. Trop. Med. Hyg. 1983, 32, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martínez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Vong, S.; Khieu, V.; Glass, O.; Ly, S.; Duong, V.; Huy, R.; Ngan, C.; Wichmann, O.; Letson, G.W.; Margolis, H.S.; et al. Dengue incidence in urban and rural Cambodia: Results from population-based active fever surveillance, 2006–2008. PLoS Negl. Trop. Dis. 2010, 4, e903. [Google Scholar] [CrossRef]
- Vatti, A.; Monsalve, D.M.; Pacheco, Y.; Chang, C.; Anaya, J.-M.; Gershwin, M.E. Original antigenic sin: A comprehensive review. J. Autoimmun. 2017, 83, 12–21. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Fonville, J.M.; Gromowski, G.D.; Bustos Arriaga, J.; Green, A.; James, S.L.; Lau, L.; Montoya, M.; Wang, C.; VanBlargan, L.A.; et al. Dengue viruses cluster antigenically but not as discrete serotypes Suppl. Science 2015, 349, 1338–1343. [Google Scholar] [CrossRef]
- Brien, J.D.; Austin, S.K.; Sukupolvi-Petty, S.; O’Brien, K.M.; Johnson, S.; Fremont, D.H.; Diamond, M.S. Genotype-specific neutralization and protection by antibodies against dengue virus type 3. J. Virol. 2010, 84, 10630–10643. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.; Holmes, E.C.; Zhang, C.; Mammen, M.P.; Nimmannitya, S.; Kalayanarooj, S.; Boots, M. Cross-protective immunity can account for the alternating epidemic pattern of dengue virus serotypes circulating in Bangkok. Proc. Natl. Acad. Sci. USA 2006, 103, 14234–14239. [Google Scholar] [CrossRef] [PubMed]

| Serotype Specificity by ED3 Dot Assay | No. Sera Tested | Serotype Identified by FRNT | Matching Results (%) | ||||
|---|---|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | ||||
| DENV-1 | 31 | 29 | 2 | 0 | 0 | 29 | (93.5) |
| DENV-2 | 25 | 0 | 25 | 0 | 0 | 25 | (100) |
| DENV-3 | 19 | 0 | 3 | 15 | 1 | 15 | (79) |
| DENV-4 | 10 | 0 | 4 | 0 | 6 | 6 | (60) |
| total | 85 | 75 | (88) | ||||
| Serotype Specificity by ED3 Dot Assay | No. Sera Tested | Serotype Identified by ED3 ELISA | Matching Results (%) | ||||
|---|---|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | ||||
| DENV-1 | 25 | 23 | 0 | 2 | 0 | 23 | (92) |
| DENV-2 | 22 | 0 | 22 | 0 | 0 | 22 | (100) |
| DENV-3 | 12 | 2 | 0 | 10 | 0 | 10 | (83) |
| DENV-4 | 8 | 0 | 1 | 0 | 7 | 7 | (87.5) |
| total | 67 | 62 | (92.5) | ||||
| Serum mED3 positive for DENV | RT-PCR Serotype | Days After Onset of Fever | IgM MAC-ELISA | HIA | |||
|---|---|---|---|---|---|---|---|
| DENV | JEV | DENV-2 | DENV-3 | JEV | |||
| 1 | DENV-1 | 9 | positive | positive | 0 | 80 | 0 |
| 2 | DENV-1 | 8 | positive | negative | 10,240 | 5120 | 2560 |
| 3 | DENV-1 | 7 | positive | positive | 10,240 | 20,480 | 20,480 |
| 4 | DENV-2 | 8 | positive | positive | 1280 | 1280 | 1280 |
| 1 + 2 | DENV-1 | 8 | positive | negative | 2560 | 2560 | 1280 |
| 1 + 3 | DENV-1 | 8 | positive | positive | 5120 | 10,240 | 5120 |
| 1 + 4 | DENV-1 | 7 | n.d. | n.d. | 160 | 320 | 320 |
| 2 + 3 | DENV-1 | 8 | positive | positive | 20,480 | 20,480 | 20,480 |
| 2 + 4 | DENV-1 | 7 | positive | positive | 10,240 | 5120 | 2560 |
| 3 + 4 | DENV-1 | 9 | positive | positive | 20,480 | 20,480 | 10,240 |
| 2 + 3 + 4 | DENV-1 | 6 | positive | positive | 5120 | 5120 | 2560 |
| 1 + 3 + 4 | DENV-1 | 7 | nd | nd | 80 | 40 | 80 |
| 1 + 2 + 4 | DENV-1 | 7 | positive | positive | 10,240 | 20,480 | 5120 |
| 1 + 2 + 3 | DENV-1 | 7 | positive | positive | 10,240 | 10,240 | 1280 |
| 1 + 2 + 3 + 4 | DENV-1 | 7 | positive | positive | 20,480 | 20,480 | 20,480 |
| NHS | negative | - | negative | negative | negative | negative | negative |
| 2010 | |||||||
| Serotype by RT-PCR | No. Sera Tested | Serotype-Specific Antibody Response Identified by mED3 * | Matching Results (%) | ||||
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | ||||
| DENV-1 | 21 | 13 | 4 | 1 | 3 | 13 | (61,9) |
| DENV-2 | 19 | 12 | 0 | 3 | 4 | 0 | (0) |
| DENV-3 | 15 | 9 | 3 | 0 | 3 | 0 | (0) |
| DENV-4 | 4 | 3 | 1 | 0 | 0 | 0 | (0) |
| total | 59 | 37 | 8 | 4 | 10 | 13 | (22,0) |
| 2012 | |||||||
| Serotype by RT-PCR | No. Sera Tested | Serotype-Specific Antibody Response Identified by mED3 * | Matching Results (%) | ||||
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | ||||
| DENV-1 | 276 | 84 | 95 | 41 | 56 | 84 | (30,4) |
| DENV-2 | 5 | 2 | 0 | 1 | 2 | 0 | (0) |
| DENV-3 | 0 | 0 | 0 | 0 | 0 | 0 | (0) |
| DENV-4 | 1 | 0 | 1 | 0 | 0 | 0 | (0) |
| total | 282 | 86 | 96 | 42 | 58 | 84 | (29,8) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auerswald, H.; Klepsch, L.; Schreiber, S.; Hülsemann, J.; Franzke, K.; Kann, S.; Y, B.; Duong, V.; Buchy, P.; Schreiber, M. The Dengue ED3 Dot Assay, a Novel Serological Test for the Detection of Denguevirus Type-Specific Antibodies and Its Application in a Retrospective Seroprevalence Study. Viruses 2019, 11, 304. https://doi.org/10.3390/v11040304
Auerswald H, Klepsch L, Schreiber S, Hülsemann J, Franzke K, Kann S, Y B, Duong V, Buchy P, Schreiber M. The Dengue ED3 Dot Assay, a Novel Serological Test for the Detection of Denguevirus Type-Specific Antibodies and Its Application in a Retrospective Seroprevalence Study. Viruses. 2019; 11(4):304. https://doi.org/10.3390/v11040304
Chicago/Turabian StyleAuerswald, Heidi, Leonard Klepsch, Sebastian Schreiber, Janne Hülsemann, Kati Franzke, Simone Kann, Bunthin Y, Veasna Duong, Philippe Buchy, and Michael Schreiber. 2019. "The Dengue ED3 Dot Assay, a Novel Serological Test for the Detection of Denguevirus Type-Specific Antibodies and Its Application in a Retrospective Seroprevalence Study" Viruses 11, no. 4: 304. https://doi.org/10.3390/v11040304
APA StyleAuerswald, H., Klepsch, L., Schreiber, S., Hülsemann, J., Franzke, K., Kann, S., Y, B., Duong, V., Buchy, P., & Schreiber, M. (2019). The Dengue ED3 Dot Assay, a Novel Serological Test for the Detection of Denguevirus Type-Specific Antibodies and Its Application in a Retrospective Seroprevalence Study. Viruses, 11(4), 304. https://doi.org/10.3390/v11040304





