Protection of Phage Applications in Crop Production: A Patent Landscape
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset Scientific Publications
2.2. Patent Search, Legal Status, and Geographical Distribution
2.3. Categorization of Patent Documents and Scientific Publications
3. Results
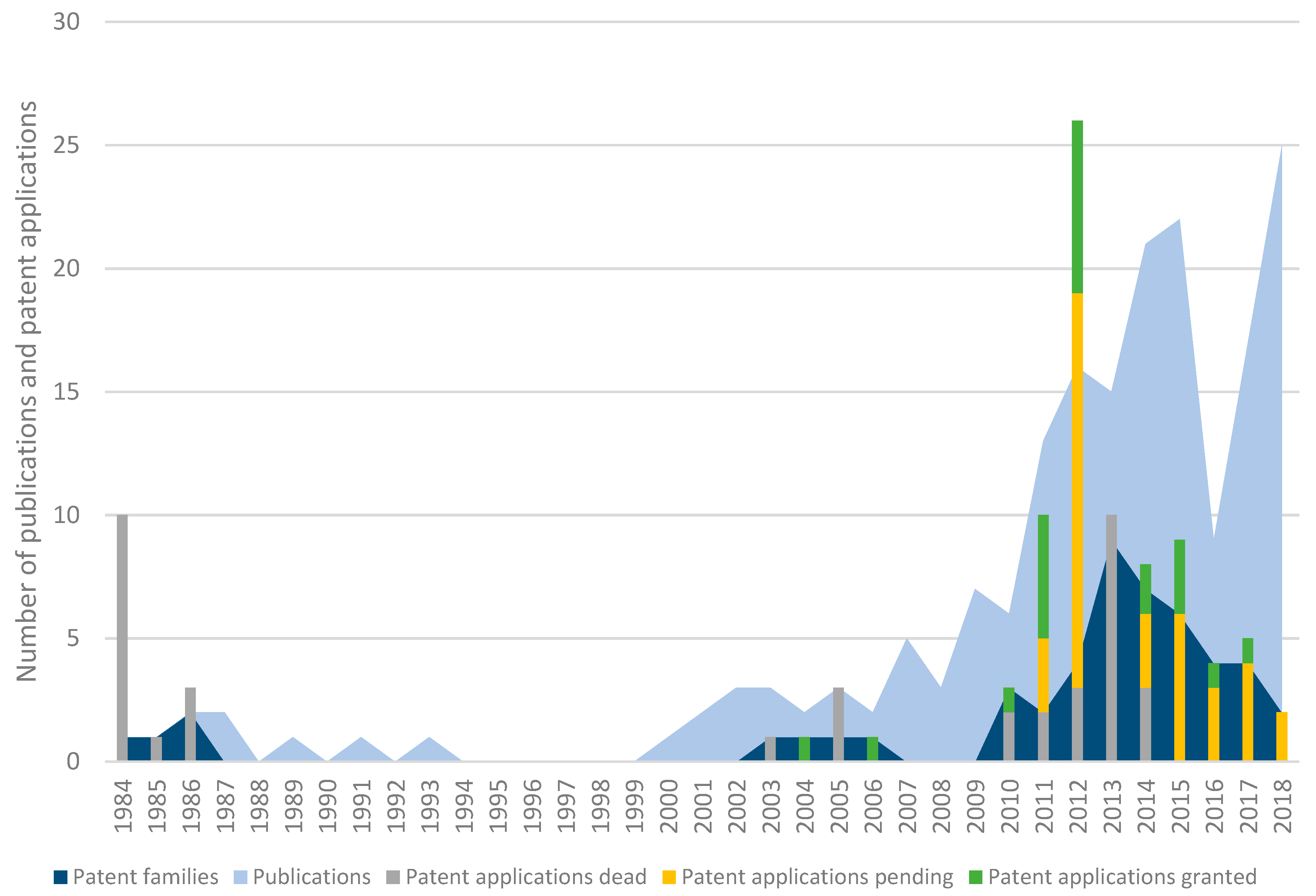
3.1. Patent Search, Scientific Papers, and Legal Status
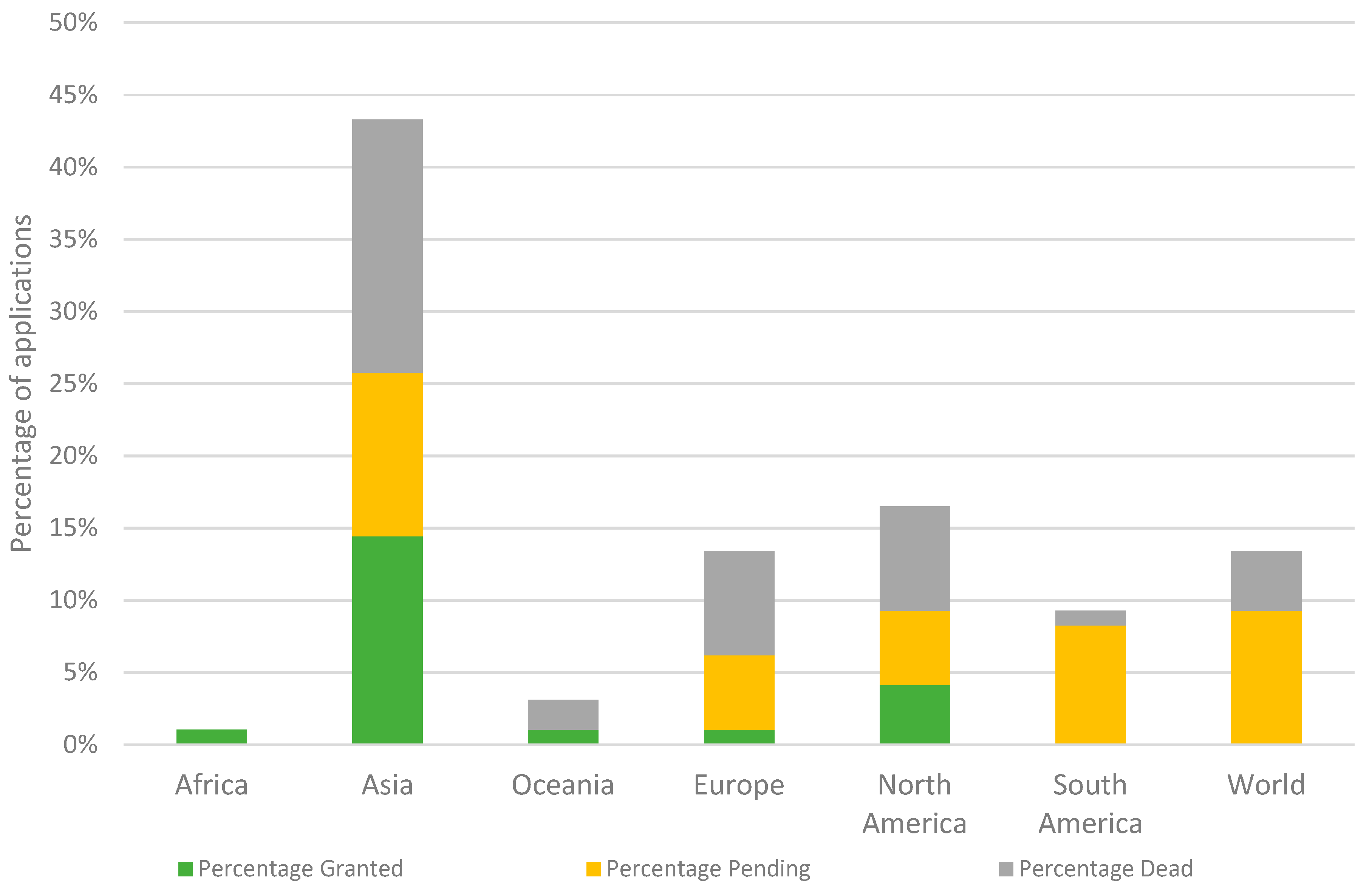
3.2. Applicants and Geographical Distribution
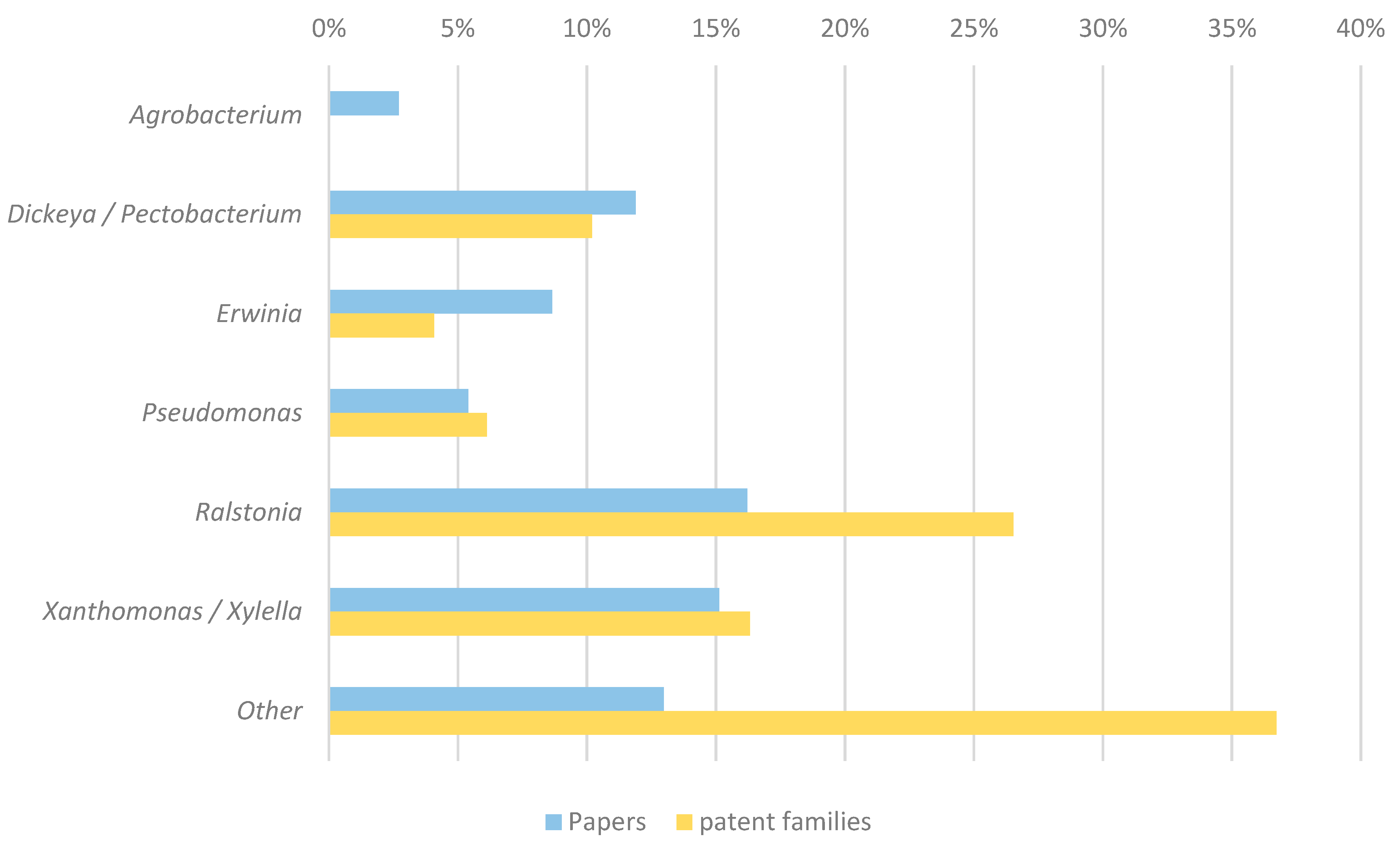
3.3. Categorization of Patents and Scientific Publications
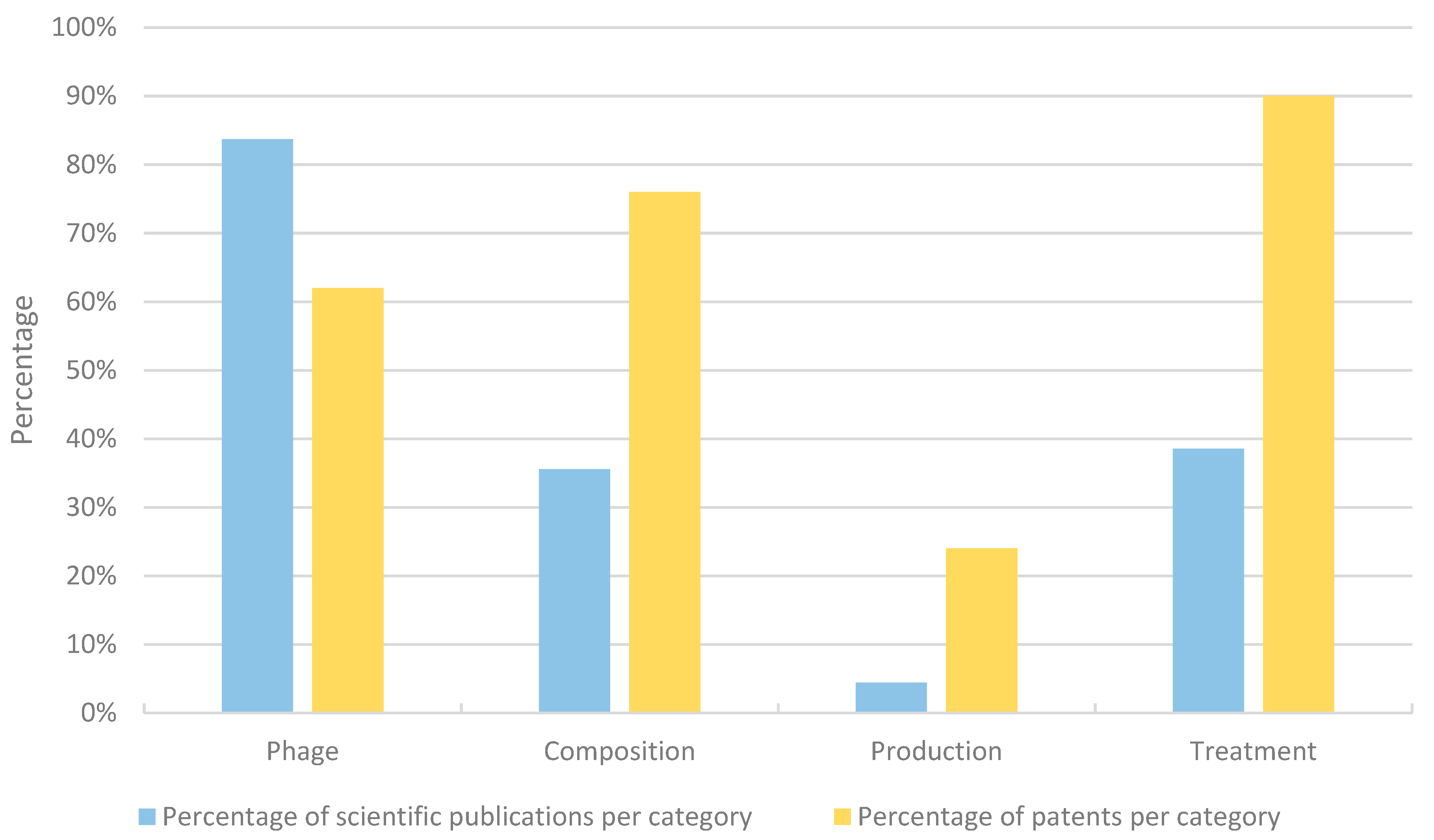
3.4. Claim and Abstract Analysis
4. Discussion
4.1. Despite a Growing Interest in Phage Biocontrol, Patenting Activities Remain Limited
4.2. Efforts in Asia to Protect Phage Biocontrol Preparations
4.3. Scientific Publications and Patent Documents Show a Correlation in Terms of Targeted Pathogens
4.4. Granted Patents Include Broad Claims
4.5. Phages and Other Viruses as Part of an Integrated Pest Management Strategy
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Twort, F.W. An Investigation On The Nature Of Ultra-Microscopic Viruses. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef]
- D’Herelle, F. Roux On an invisible microbe antagonistic toward dysenteric bacilli: Brief note by Mr. F. D’Herelle, presented by Mr. Roux. Res. Microbiol. 2007, 158, 553–554. [Google Scholar] [PubMed]
- Pirnay, J.P.; De Vos, D.; Verbeken, G.; Merabishvili, M.; Chanishvili, N.; Vaneechoutte, M.; Zizi, M.; Laire, G.; Lavigne, R.; Huys, I.; et al. The phage therapy paradigm: Prêt-à-porter or sur-mesure? Pharm. Res. 2011, 28, 934–937. [Google Scholar] [CrossRef]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and bacterial plant diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef]
- Mallmann, W.; Hemstreet, C. Isolation ofan inhibitory substance from plants. Agric. Res. 1924, 28, 599–602. [Google Scholar]
- Okabe, N.; Goto, M. Bacteriophages of Plant Pathogens. Annu. Rev. Phytopathol. 1963, 1, 397–418. [Google Scholar] [CrossRef]
- Strange, R.; Scott, P.R. Plant disease: A threat to global food security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Inagro IWT—Beheersing bacteriële pathogeen opkweek kolen-prei. Available online: http://leden.inagro.be/Wie-is-Inagro/Projecten/project/13960 (accessed on 27 December 2018).
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, D.A. Genetics of Bactericide. Annu. Rev. Phytopathol. 1990, 28, 201–219. [Google Scholar] [CrossRef]
- Frampton, R.A.; Pitman, A.R.; Fineran, P.C. Advances in bacteriophage-mediated control of plant pathogens. Int. J. Microbiol. 2012, 2012, 326452. [Google Scholar] [CrossRef]
- Pietrzak, U.; McPhail, D.C. Copper accumulation, distribution and fractionation in vineyard soils of Victoria, Australia. Geoderma 2004, 122, 151–166. [Google Scholar] [CrossRef]
- Copping, L.G.; Duke, S.O. Natural products that have been used commercially as crop protection agents. Pest Manag. Sci. 2007, 63, 524–554. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield Trends Are Insufficient to Double Global Crop Production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, J.A. A Conceptual Framework for Integrated Pest Management. Trends Plant Sci. 2017, 22, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.D. The Cost of New Agrochemical Product Discovery, Development and Resgistration in 1995, 2000, 2005–8 and 2010 to 2014. R&D Expenditure in 2014 and Expectations for 2019. A Consultancy Study for CropLife International, CropLife America and the European Crop; Pathhead: Midlothian, UK, 2016. [Google Scholar]
- European Crop Protection Registering Plant Protection Products in the EU. Available online: https://www.ecpa.eu/sites/default/files/7450_Registrationbrochure_3.pdf%0Ahttp://www.ecpa.eu/files/attachments/20110125_PPPBrochure_ECPA.pdf (accessed on 5 February 2019).
- WIPO—Protecting Your Inventions Abroad: Frequently Asked Questions About the Patent Cooperation Treaty (PCT). Available online: https://www.wipo.int/pct/en/faqs/faqs.html (accessed on 12 March 2019).
- Todd, K. The Promising Viral Threat To Bacterial Resistance: The Uncertain Patentability of Phage Therapeutics and the Necessity of Alternative Incentives. J. D. Expect. 2019, 68, 767–805. [Google Scholar]
- Williams, H.L. How do patents affect research investments? Annu. Rev. Econ. 2017, 9, 441–469. [Google Scholar] [CrossRef]
- Pirnay, J.-P.; Verbeken, G.; Rose, T.; Jennes, S.; Zizi, M.; Huys, I.; Lavigne, R.; Merabishvili, M.; Vaneechoutte, M.; Buckling, A.; et al. Introducing yesterday’s phage therapy in today’s medicine. Future Virol. 2012, 7, 379–390. [Google Scholar] [CrossRef]
- Verbeken, G. Towards An Adequate Regulatory Framework For Bacteriophage Therapy; KU Leuven and Royal Military Academy: Leuven, Belgium, 2015. [Google Scholar]
- Verbeure, B.; Matthijs, G.; Van Overwalle, G. Analysing DNA patents in relation with diagnostic genetic testing. Eur. J. Hum. Genet. 2006, 14, 26–33. [Google Scholar] [CrossRef]
- Huys, I.; Berthels, N.; Matthijs, G.; Van Overwalle, G. Legal uncertainty in the area of genetic diagnostic testing. Nat. Biotechnol. 2009, 27, 903–909. [Google Scholar] [CrossRef]
- McKenna, F.; El-Tarabily, K.A.; Hardy, G.S.J.; Dell, B. Novel in vivo use of a polyvalent Streptomyces phage to disinfest Streptomyces scabies-infected seed potatoes. Plant Pathol. 2001, 50, 666–675. [Google Scholar] [CrossRef]
- EPO—Basic Definitions. Available online: https://www.epo.org/searching-for-patents/helpful-resources/first-time-here/definitions.html (accessed on 14 February 2019).
- Braverman, M. United States. In Use and Regulation of Microbial Pesticides in Representative Jurisdictions Worldwide; 2015; Available online: https://www.IOBC-Global.org (accessed on 14 February 2019).
- Wang, B.; Li, Z. Use and Regulation of Biopesticides in China. In Use And Regulation Of Microbial Pesticides In Representative Jurisdictions Worldwide; 2015; Available online: https://www.IOBC-Global.org (accessed on 14 February 2019).
- Kim, J.J.; Lee, S.G.; Lee, S.; Jee, H.-J. South Korea. In Use and Regulation of Microbial Pesticides in Representative Jurisdictions Worldwide; 2015; Available online: https://www.IOBC-Global.org (accessed on 14 February 2019).
- Rombouts, S.; Volckaert, A.; Venneman, S.; Declercq, B.; Vandenheuvel, D.; Allonsius, C.N.; Van Malderghem, C.; Jang, H.B.; Briers, Y.; Noben, J.P.; et al. Characterization of novel bacteriophages for biocontrol of bacterial blight in leek caused by Pseudomonas syringae pv. porri. Front. Microbiol. 2016, 7, 1–15. [Google Scholar] [CrossRef]
- EPA PRIA Fee Category Table—Registration Division—New Active Ingredients. Available online: https://www.epa.gov/pria-fees/pria-fee-category-table-registration-division-new-active-ingredients (accessed on 15 February 2019).
- Barbosa, C.; Venail, P.; Holguin, A.V.; Vives, M.J. Co-Evolutionary Dynamics of the Bacteria Vibrio sp. CV1 and Phages V1G, V1P1, and V1P2: Implications for Phage Therapy. Microb. Ecol. 2013, 66, 897–905. [Google Scholar] [CrossRef]
- European Commission. Guidance Document For The Assessment Of The Equivalence Of Technical Grade Active Ingredients For Identical Microbial Strains Or Isolates Approved Under Regulation (EC) No 1107/2009; European Commission Health and Food safety: Brussels, Belgium, 2014. [Google Scholar]
- Adriaenssens, E.M.; Rodney Brister, J. How to name and classify your phage: An informal guide. Viruses 2017, 9, 70. [Google Scholar] [CrossRef]
- Van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- AgriPhageTM Product Info AgriPhage. Available online: https://www.agriphage.com/product-info/ (accessed on 9 February 2019).
- Enviroinvest Zrt. Erwiphage PLUS. Available online: http://www.erwiphage.com/ (accessed on 12 March 2019).
- APS Biocontrol Ltd. Biolyse Products. Available online: https://www.apsbiocontrol.com/products (accessed on 12 March 2019).
- Erstling, J.; Strom, R. Korea’s Patent Policy and Its Impact on Economic Development: A Model for Emerging Countries. San Diego Int. Law J. 2009, 11, 441–481. [Google Scholar]
- Long, C.X.; Wang, J. China’s patent promotion policies and its quality implications. Sci. Pub. Policy 2018, 46, 91–104. [Google Scholar] [CrossRef]
- APPPC; FAO. Plant Protion Profiles from Asia-Pacific countries—Chapter 3 Intergrated Pest Management. Available online: http://www.fao.org/docrep/010/ag123e/AG123E22.htm (accessed on 13 February 2019).
- Álvarez, B.; Biosca, E.G. Bacteriophage-Based Bacterial Wilt Biocontrol for an Environmentally Sustainable Agriculture. Front. Plant Sci. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Ryan, R.P.; Vorhölter, F.J.; Potnis, N.; Jones, J.B.; Van Sluys, M.A.; Bogdanove, A.J.; Dow, J.M. Pathogenomics of Xanthomonas: Understanding bacterium-plant interactions. Nat. Rev. Microbiol. 2011, 9, 344–355. [Google Scholar] [CrossRef]
- Janse, J.D.; Obradovic, A. Xylella Fastidiosa: Its Biology, Diagnosis, Control And Risks. J. Plant Pathol. 2010, 92, S1.35–S1.48. [Google Scholar]
- Xin, X.F.; Kvitko, B.; He, S.Y. Pseudomonas syringae: What it takes to be a pathogen. Nat. Rev. Microbiol. 2018, 16, 316–328. [Google Scholar] [CrossRef]
- WIPO. IP and Business: Quality Patents: Claiming what Counts. Available online: https://www.wipo.int/wipo_magazine/en/2006/01/article_0007.html (accessed on 14 February 2019).
- Schofield, D.A.; Bull, C.T.; Rubio, I.; Wechter, W.P.; Westwater, C.; Molineux, I.J. Development of an engineered bioluminescent reporter phage for detection of bacterial blight of crucifers. Appl. Environ. Microbiol. 2012, 78, 3592–3598. [Google Scholar] [CrossRef] [PubMed]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Genera | Patent Number | Applicant | Filing Year | Type of Claim | |||
|---|---|---|---|---|---|---|---|
| Phage | Composition | Production | Treatment | ||||
| Other | US9539343 | Fixed Phage (GB) | 2012 | 1 | 1 | ||
| CN103747792 | Fixed Phage (GB) | 2012 | 1 | 1 | |||
| JP6230994 | Fixed Phage (GB) | 2012 | 1 | 1 | |||
| US9278141 | Fixed Phage (GB) | 2013 | 5 | ||||
| CN104630154 | University of Zhejiang (CN) | 2015 | 1 | 1 | 1 | 2 | |
| KR101887987 | Pukyong National University (KR) | 2016 | 1 | 1 | 1 | ||
| Dickeya/Pectobacterium | KR101368328 | Rural development Administration (KR) | 2010 | 1 | 1 | ||
| KR101790019 | Rural development administration (KR) | 2014 | 1 | 1 | 1 | ||
| KR101797463 | Rural development Administration (KR) | 2014 | 1 | 1 | 1 | ||
| KR101891298 | Seoul National University (KR) | 2016 | 1 | 2 | 1 | ||
| Pseudomonas | KR101584214 | Chungbuk National University (KR) | 2012 | 1 | 1 | ||
| Ralstonia | JP4532959 | Sanin Kensetsu Kogyo (JP) | 2004 | 3 | 2 | 2 | |
| JP4862154 | University of Hiroshima (JP) | 2006 | 1 | 1 | 3 | ||
| US9380786 | University of Hiroshima (JP) | 2012 | 1 | ||||
| JP5812466 | University of Hiroshima (JP) | 2011 | 1 | 3 | |||
| CN104542717 | Guizhou Tobacco science research institute (CN) | 2015 | 1 | 1 | |||
| ES2592352 | University of Valencia (ES) | 2015 | 1 | 1 | 2 | ||
| Xanthomonas/Xylella | AU2013331060 | Texas A&M University (US) | 2013 | 1 | 1 | 2 | 4 |
| JP6391579 | Texas A&M University (US) | 2013 | 1 | 1 | 2 | 2 | |
| US9357785 | Texas A&M University (US) | 2013 | 1 | ||||
| ZA201502230 | Texas A&M University (US) | 2013 | 1 | 1 | 2 | 2 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holtappels, D.; Lavigne, R.; Huys, I.; Wagemans, J. Protection of Phage Applications in Crop Production: A Patent Landscape. Viruses 2019, 11, 277. https://doi.org/10.3390/v11030277
Holtappels D, Lavigne R, Huys I, Wagemans J. Protection of Phage Applications in Crop Production: A Patent Landscape. Viruses. 2019; 11(3):277. https://doi.org/10.3390/v11030277
Chicago/Turabian StyleHoltappels, Dominique, Rob Lavigne, Isabelle Huys, and Jeroen Wagemans. 2019. "Protection of Phage Applications in Crop Production: A Patent Landscape" Viruses 11, no. 3: 277. https://doi.org/10.3390/v11030277
APA StyleHoltappels, D., Lavigne, R., Huys, I., & Wagemans, J. (2019). Protection of Phage Applications in Crop Production: A Patent Landscape. Viruses, 11(3), 277. https://doi.org/10.3390/v11030277






