Recent Updates on Mouse Models for Human Immunodeficiency, Influenza, and Dengue Viral Infections
Abstract
1. Introduction
2. Human Immuno Deficiency Virus
2.1. Knockout Mouse Models of HIV
2.2. Transgenic Mouse Models of HIV
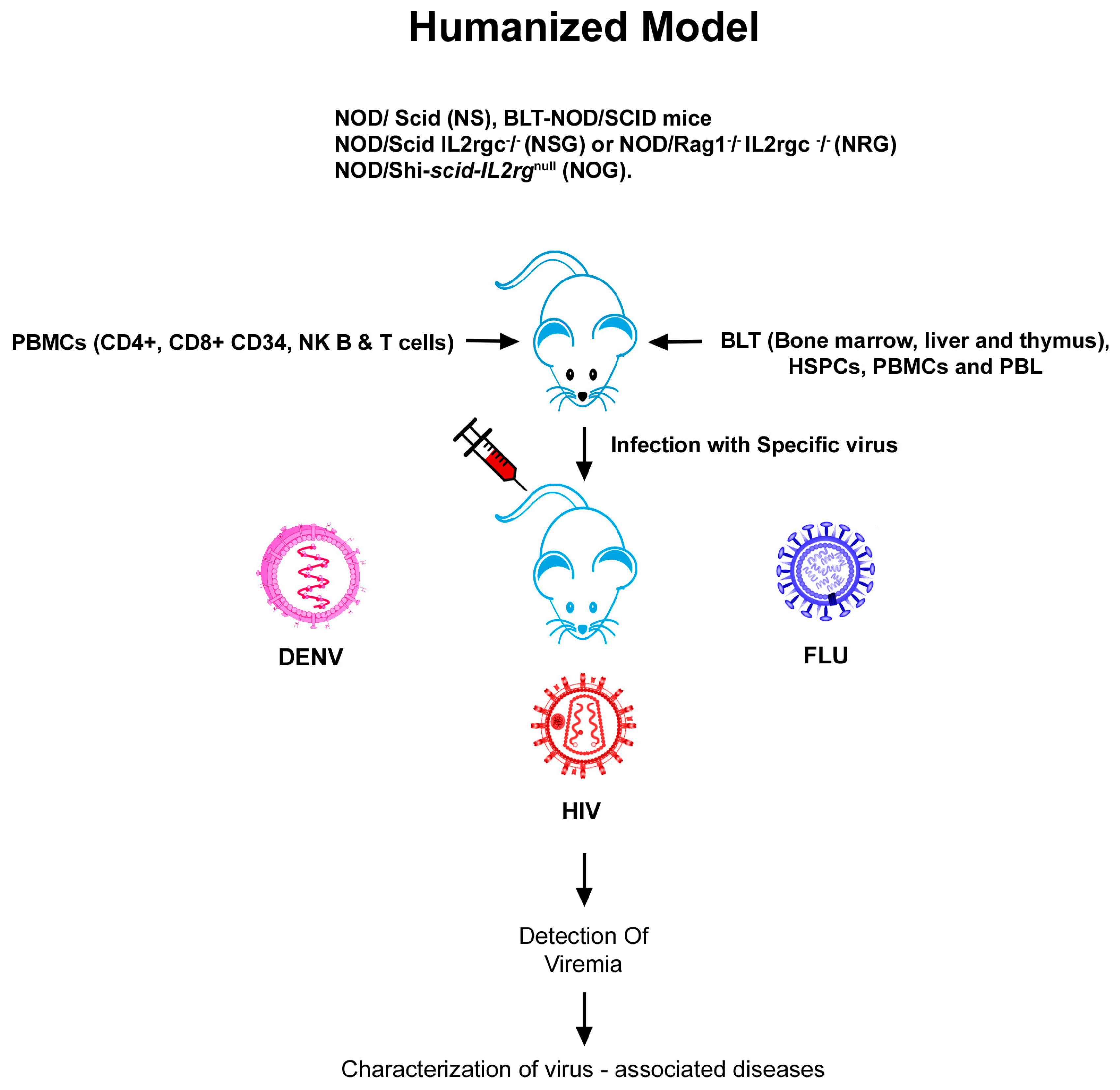
2.3. Humanized Mouse Models of HIV
3. Influenza Virus
3.1. Knockout Mouse Models of Influenza A
3.2. Transgenic Mouse Models of Influenza A
3.3. Humanized Mouse Models of Influenza A
4. Dengue Virus (DENV)
4.1. Knockout Mouse Models of DENV
4.2. Transgenic Mouse Models of DENV
4.3. Humanized Mouse Models of DENV
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Global HIV and AIDS Statistics-2018 Fact Sheet. Available online: http://www.unaids.org/en/resources/fact-sheet (accessed on 28 August 2018).
- Nair, H.; Brooks, W.A.; Katz, M.; Roca, A.; Berkley, J.A.; Madhi, S.A.; Simmerman, J.M.; Gordon, A.; Sato, M.; Howie, S.; et al. Global burden of respiratory infections due to seasonal influenza in young children: A systematic review and meta-analysis. Lancet 2011, 378, 1917–1930. [Google Scholar] [CrossRef]
- Barrila, J.; Radtke, A.L.; Crabbé, A.; Sarker, S.F.; Herbst-Kralovetz, M.M.; Ott, C.M.; Nickerson, C.A. Organotypic 3D cell culture models: Using the rotating wall vessel to study host-pathogen interactions. Nat. Rev. Microbiol. 2010, 8, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Bot, A.; Casares, S.; Bot, S.; Von Boehmer, H.; Bona, C. Cellular mechanisms involved in protection against influenza virus infection in transgenic mice expressing a TCR receptor specific for class II hemagglutinin peptide in CD4+ and CD8+ T cells. J. Immunol. 1998, 160, 4500–4507. [Google Scholar] [PubMed]
- Kirberg, J.; Baron, A.; Jakob, S.; Rolink, A.; Karjalainen, K.; Von Boehmer, H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J. Exp. Med. 1994, 180, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Rall, G.F.; Lawrence, D.M.; Patterson, C.E. The application of transgenic and knockout mouse technology for the study of viral pathogenesis. Virology 2000, 271, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Mombaerts, P.; Iacomini, J.; Johnson, R.S.; Herrup, K.; Tonegawa, S.; Papaioannou, V.E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 1992, 68, 869–877. [Google Scholar] [CrossRef]
- Shinkai, Y.; Rathbun, G.; Lam, K.P.; Oltz, E.M.; Stewart, V.; Mendelsohn, M.; Charron, J.; Datta, M.; Young, F.; Stall, A.M.; et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 1992, 68, 855–867. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Hertzog, P.J.; Holland, K.A.; Sumarsono, S.H.; Tymms, M.J.; Hamilton, J.A.; Whitty, G.; Bertoncello, I.; Kola, I. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc. Natl. Acad. Sci. USA 1995, 92, 11284–11288. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef]
- Meraz, M.A.; White, J.M.; Sheehan, K.C.; Bach, E.A.; Rodig, S.J.; Dighe, A.S.; Kaplan, D.H.; Riley, J.K.; Greenlund, A.C.; Campbell, D.; et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 1996, 84, 431–442. [Google Scholar] [CrossRef]
- Matsuyama, T.; Kimura, T.; Kitagawa, M.; Pfeffer, K.; Kawakami, T.; Watanabe, N.; Kundig, T.M.; Amakawa, R.; Kishihara, K.; Wakeham, A.; et al. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell 1993, 75, 83–97. [Google Scholar] [CrossRef]
- Kennedy, M.K.; Glaccum, M.; Brown, S.N.; Butz, E.A.; Viney, J.L.; Embers, M.; Matsuki, N.; Charrier, K.; Sedger, L.; Willis, C.R.; et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000, 91, 771–780. [Google Scholar] [CrossRef]
- Theocharides, A.P.; Rongvaux, A.; Fritsch, K.; Flavell, R.A.; Manz, M.G. Humanized hemato-lymphoid system mice. Haematologica 2016, 101, 5–19. [Google Scholar] [CrossRef]
- Ishikawa, F.; Yasukawa, M.; Lyons, B.; Yoshida, S.; Miyamoto, T.; Yoshimoto, G.; Watanabe, T.; Akashi, K.; Shultz, L.D.; Harada, M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chainnull mice. Blood 2005, 106, 1565–1573. [Google Scholar] [CrossRef]
- Lai, F.; Chen, Q. Humanized Mouse Models for the Study of Infection and Pathogenesis of Human Viruses. Viruses 2018, 10, 643. [Google Scholar] [CrossRef]
- Brehm, M.A.; Bortell, R.; Verma, M.; Shultz, L.D.; Greiner, D.L. Humanized mice in translational immunology. Transl. Immunol. Mech. Pharmacol. Approaches 2016, 285–326. [Google Scholar]
- Shultz, L.D.; Brehm, M.A.; Garcia-Martinez, J.V.; Greiner, D.L. Humanized mice for immune system investigation: Progress, promise and challenges. Nat. Rev. Immunol. 2012, 12, 786–798. [Google Scholar] [CrossRef]
- Walsh, N.; Kenney, L.; Jangalwe, S.; Aryee, K.E.; Greiner, D.L.; Brehm, M.A.; Shultz, L.D. Humanized mouse models of clinical disease. Annu. Rev. Pathol. 2017, 12, 187–215. [Google Scholar] [CrossRef]
- King, M.A.; Covassin, L.; Brehm, M.A.; Racki, W.; Pearson, T.; Leif, J.; Laning, J.; Fodor, W.; Foreman, O.; Burzenski, L.; et al. Hu-PBL-NOD-SCID IL2rgnull mouse model of xenogeneic graft-versus-host-like disease and the role of host MHC. Clin. Exp. Immunol. 2009, 157, 104–118. [Google Scholar] [CrossRef]
- Melkus, M.W.; Estes, J.D.; Padgett-Thomas, A.; Gatlin, J.; Denton, P.W.; Othieno, F.A.; Wege, A.K.; Haase, A.T.; Garcia, J.V. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 2006, 12, 1316–1322. [Google Scholar] [CrossRef]
- Hatziioannou, T.; Evans, D.T. Animal models for HIV/AIDS research. Nat. Rev. Microbiol. 2012, 10, 852–867. [Google Scholar] [CrossRef]
- Wittmann, S.; Behrendt, R.; Eissmann, K.; Volkmann, B.; Thomas, D.; Ebert, T.; Cribier, A.; Benkirane, M.; Hornung, V.; Bouzas, N.F.; et al. Phosphorylation of murine SAMHD1 regulates its antiretroviral activity. Retrovirology 2015, 12, 103. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Maelfait, J.; Bridgeman, A.; Rigby, R.; Hayward, B.; Liberatore, R.A.; Bieniasz, P.D.; Towers, G.J.; Moita, L.F.; Crow, Y.J.; et al. SAMHD1-dependent retroviral control and escape in mice. EMBO J. 2013, 32, 2454–2462. [Google Scholar] [CrossRef]
- Behrendt, R.; Schumann, T.; Gerbaulet, A.; Nguyen, L.A.; Schubert, N.; Alexopoulou, D.; Berka, U.; Lienenklaus, S.; Peschke, K.; Gibbert, K.; et al. Mouse SAMHD1 has antiretroviral activity and suppresses a spontaneous cell-intrinsic antiviral response. Cell Rep. 2013, 4, 689–696. [Google Scholar] [CrossRef]
- Okeoma, C.M.; Lovsin, N.; Peterlin, B.M.; Ross, S.R. APOBEC3 inhibits mouse mammary tumour virus replication in vivo. Nature 2007, 445, 927. [Google Scholar] [CrossRef]
- Saitoh, T.; Satoh, T.; Yamamoto, N.; Uematsu, S.; Takeuchi, O.; Kawai, T.; Akira, S. Antiviral protein Viperin promotes Toll-like receptor 7-and Toll-like receptor 9-mediated type I interferon production in plasmacytoid dendritic cells. Immunity 2011, 34, 352–363. [Google Scholar] [CrossRef]
- Swiecki, M.; Wang, Y.; Gilfillan, S.; Lenschow, D.J.; Colonna, M. Cutting edge: Paradoxical roles of BST2/tetherin in promoting type I IFN response and viral infection. J. Immunol. 2012, 1103145. [Google Scholar] [CrossRef]
- Bloch, N.; Glasker, S.; Sitaram, P.; Hofmann, H.; Shepard, C.N.; Schultz, M.L.; Kim, B.; Landau, N.R. A highly active isoform of lentivirus restriction factor SAMHD1 in mouse. J. Biol. Chem. 2017, 292, 1068–1080. [Google Scholar] [CrossRef]
- Browning, J.; Horner, J.W.; Pettoello-Mantovani, M.; Raker, C.; Yurasov, S.; DePinho, R.A.; Goldstein, H. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. Proc. Natl. Acad. Sci. USA 1997, 94, 14637–14641. [Google Scholar] [CrossRef]
- Seay, K.; Qi, X.; Zheng, J.H.; Zhang, C.; Chen, K.; Dutta, M.; Deneroff, K.; Ochsenbauer, C.; Kappes, J.C.; Littman, D.R.; et al. Mice transgenic for CD4-specific human CD4, CCR5 and cyclin T1 expression: A new model for investigating HIV-1 transmission and treatment efficacy. PLoS ONE 2013, 8, 63537. [Google Scholar] [CrossRef]
- Keppler, O.T.; Welte, F.J.; Ngo, T.A.; Chin, P.S.; Patton, K.S.; Tsou, C.L.; Abbey, N.W.; Sharkey, M.E.; Grant, R.M.; You, Y.; et al. Progress toward a human CD4/CCR5 transgenic rat model for de novo infection by human immunodeficiency virus type 1. J. Exp. Med. 2002, 195, 719–736. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.M.; Abramczuk, J.W.; Pezen, D.S.; Rutledge, R.; Belcher, J.H.; Hakim, F.; Shearer, G.; Lamperth, L.; Travis, W.; Fredrickson, T. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science 1988, 242, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Dickie, P.; Gazzinelli, R.; Chang, L.J. Models of HIV type 1 proviral gene expression in wild-type HIV and MLV/HIV transgenic mice. AIDS Res. Hum. Retrovir. 1996, 12, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Dickie, P.; Ramsdell, F.; Notkins, A.L.; Venkatesan, S. Spontaneous and inducible epidermal hyperplasia in transgenic mice expressing HIV-1 Nef. Virology 1993, 197, 431–438. [Google Scholar] [CrossRef]
- Hanna, Z.; Kay, D.G.; Cool, M.; Jothy, S.; Rebai, N.; Jolicoeur, P. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J. Virol. 1998, 72, 121–132. [Google Scholar] [PubMed]
- Rahim, M.M.A.; Chrobak, P.; Hu, C.; Hanna, Z.; Jolicoeur, P. Adult AIDS-like disease in a novel inducible human immunodeficiency virus type 1 Nef transgenic mouse model: CD4+ T-cell activation is Nef dependent and can occur in the absence of lymphophenia. J. Virol. 2009, 83, 11830–11846. [Google Scholar] [CrossRef] [PubMed]
- Hanna, Z.; Weng, X.; Kay, D.G.; Poudrier, J.; Lowell, C.; Jolicoeur, P. The pathogenicity of human immunodeficiency virus (HIV) type 1 Nef in CD4C/HIV transgenic mice is abolished by mutation of its SH3-binding domain, and disease development is delayed in the absence of Hck. J. Virol. 2001, 75, 9378–9392. [Google Scholar] [CrossRef] [PubMed]
- Hanna, Z.; Priceputu, E.; Kay, D.G.; Poudrier, J.; Chrobak, P.; Jolicoeur, P. In vivo mutational analysis of the N-terminal region of HIV-1 Nef reveals critical motifs for the development of an AIDS-like disease in CD4C/HIV transgenic mice. Virology 2004, 327, 273–286. [Google Scholar] [CrossRef]
- Thaney, V.E.; Sanchez, A.B.; Fields, J.A.; Minassian, A.; Young, J.W.; Maung, R.; Kaul, M. Transgenic mice expressing HIV-1 envelope protein gp120 in the brain as an animal model in neuroAIDS research. J. Neurovirol. 2018, 1, 1–2. [Google Scholar] [CrossRef]
- Maung, R.; Hoefer, M.M.; Sanchez, A.B.; Sejbuk, N.E.; Medders, K.E.; Desai, M.K.; Catalan, I.C.; Dowling, C.C.; De Rozieres, C.M.; Garden, G.A.; et al. CCR5 knockout prevents neuronal injury and behavioral impairment induced in a transgenic mouse model by a CXCR4-using HIV-1 glycoprotein 120. J. Immunol. 2014, 1302915. [Google Scholar] [CrossRef]
- Putatunda, R.; Zhang, Y.; Li, F.; Yang, X.F.; Barbe, M.F.; Hu, W. Adult neurogenic deficits in HIV-1 Tg26 transgenic mice. J. Neuroinflamm. 2018, 1, 287. [Google Scholar] [CrossRef]
- Denton, P.W.; Sogaard, O.S.; Tolstrup, M. Using animal models to overcome temporal, spatial and combinatorial challenges in HIV persistence research. J. Transl. Med. 2016, 14, 44. [Google Scholar] [CrossRef]
- Akkina, R. New generation humanized mice for virus research: Comparative aspects and future prospects. Virology 2013, 435, 14–28. [Google Scholar] [CrossRef]
- Choudhary, S.K.; Archin, N.M.; Cheema, M.; Dahl, N.P.; Garcia, J.V.; Margolis, D.M. Latent HIV-1 infection of resting CD4+ T cells in the humanized Rag2−/− γc−/− mouse. J. Virol. 2012, 86, 114–120. [Google Scholar] [CrossRef]
- Marsden, M.D.; Kovochich, M.; Suree, N.; Shimizu, S.; Mehta, R.; Cortado, R.; Bristol, G.; An, D.S.; Zack, J.A. HIV latency in the humanized BLT mouse. J. Virol. 2012, 86, 339–347. [Google Scholar] [CrossRef]
- Honeycutt, J.B.; Sheridan, P.A.; Matsushima, G.K.; Garcia, J.V. Humanized mouse models for HIV-1 infection of the CNS. J. Neurovirol. 2015, 21, 301–309. [Google Scholar] [CrossRef]
- Olesen, R.; Swanson, M.D.; Kovarova, M.; Nochi, T.; Chateau, M.; Honeycutt, J.B.; Long, J.M.; Denton, P.W.; Hudgens, M.G.; Richardson, A.; et al. ART influences HIV persistence in the female reproductive tract and cervicovaginal secretions. J. Clin. Investig. 2016, 126, 892–904. [Google Scholar] [CrossRef]
- Lavender, K.J.; Pace, C.; Sutter, K.; Messer, R.J.; Pouncey, D.L.; Cummins, N.W.; Natesampillai, S.; Zheng, J.; Goldsmith, J.; Widera, M.; et al. An advanced BLT-humanized mouse model for extended HIV-1 cure studies. Aids 2018, 32, 1–10. [Google Scholar] [CrossRef]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.; et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011, 365, 493–505. [Google Scholar] [CrossRef]
- Brooks, D.G.; Kitchen, S.G.; Kitchen, C.M.; Scripture-Adams, D.D.; Zack, J.A. Generation of HIV latency during thymopoiesis. Nat. Med. 2001, 7, 459–464. [Google Scholar] [CrossRef]
- Brehm, M.A.; Wiles, M.V.; Greiner, D.L.; Shultz, L.D. Generation of improved humanized mouse models for human infectious diseases. J. Immunol. 2014, 410, 3–17. [Google Scholar] [CrossRef]
- Karpel, M.E.; Boutwell, C.L.; Allen, T.M. BLT humanized mice as a small animal model of HIV infection. Curr. Opin. Virol. 2015, 13, 75–80. [Google Scholar] [CrossRef]
- Akkina, R.; Allam, A.; Balazs, A.B.; Blankson, J.N.; Burnett, J.C.; Casares, S.; Garcia, J.V.; Hasenkrug, K.J.; Kashanchi, F.; Kitchen, S.G.; et al. Improvements and limitations of humanized mouse models for HIV research: NIH/NIAID “meet the experts” 2015 workshop summary. AIDS Res. Hum. Retrovir. 2016, 32, 109–119. [Google Scholar] [CrossRef]
- Horwitz, J.A.; Halper-Stromberg, A.; Mouquet, H.; Gitlin, A.D.; Tretiakova, A.; Eisenreich, T.R.; Malbec, M.; Gravemann, S.; Billerbeck, E.; Dorner, M.; et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc. Natl. Acad. Sci. USA 2013, 110, 16538–16543. [Google Scholar] [CrossRef]
- Kovochich, M.; Marsden, M.D.; Zack, J.A. Activation of latent HIV using drug-loaded nanoparticles. PLoS ONE 2011, 6, e18270. [Google Scholar] [CrossRef]
- Denton, P.W.; Olesen, R.; Choudhary, S.K.; Archin, N.M.; Wahl, A.; Swanson, M.D.; Chateau, M.; Nochi, T.; Krisko, J.F.; Spagnuolo, R.A.; et al. Generation of HIV latency in humanized BLT mice. J. Virol. 2012, 86, 630–634. [Google Scholar] [CrossRef]
- Arainga, M.; Edagwa, B.; Mosley, R.L.; Poluektova, L.Y.; Gorantla, S.; Gendelman, H.E. A mature macrophage is a principal HIV-1 cellular reservoir in humanized mice after treatment with long acting antiretroviral therapy. Retrovirology 2017, 14, 17. [Google Scholar] [CrossRef]
- Honeycutt, J.B.; Wahl, A.; Archin, N.; Choudhary, S.; Margolis, D.; Garcia, J.V. HIV-1 infection, response to treatment and establishment of viral latency in a novel humanized T cell-only mouse (TOM) model. Retrovirology 2013, 10, 121. [Google Scholar] [CrossRef]
- Honeycutt, J.B.; Thayer, W.O.; Baker, C.E.; Ribeiro, R.M.; Lada, S.M.; Cao, Y.; Cleary, R.A.; Hudgens, M.G.; Richman, D.D.; Garcia, J.V. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat. Med. 2017, 23, 638–643. [Google Scholar] [CrossRef]
- Metcalf Pate, K.A.; Pohlmeyer, C.W.; Walker-Sperling, V.E.; Foote, J.B.; Najarro, K.M.; Cryer, C.G.; Salgado, M.; Gama, L.; Engle, E.L.; Shirk, E.N.; et al. A murine viral outgrowth assay to detect residual HIV type 1 in patients with undetectable viral loads. J. Infect. Dis. 2015, 212, 1387–1396. [Google Scholar] [CrossRef]
- Yuan, Z.; Kang, G.; Lu, W.; Li, Q. Reactivation of HIV-1 proviruses in immune-compromised mice engrafted with human VOA- negative CD4+ T cells. J. Virus Erad. 2017, 3, 61–65. [Google Scholar]
- Satheesan, S.; Li, H.; Burnett, J.C.; Takahashi, M.; Li, S.; Wu, S.X.; Synold, T.W.; Rossi, J.J.; Zhou, J. HIV replication and latency in a humanized NSG mouse model during suppressive oral combinational ART. J. Virol. 2018, JVI-02118. [Google Scholar]
- WHO: Influenza (Seasonal). Available online: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 12 October 2018).
- Mallia, P.; Johnston, S.L. Influenza infection and COPD. Int. J. Chron Obstruct. Pulmon. Dis. 2007, 2, 55. [Google Scholar] [CrossRef]
- Kim, M.H.; Song, W.J.; Yang, M.S.; Lee, S.H.; Kwon, J.W.; Kim, S.H.; Kang, H.R.; Park, H.W.; Cho, Y.J.; Cho, S.H.; et al. Clinical course of asthma patients with H1N1 influenza infection and oseltamivir. Minerva Med. 2018, 109, 7–14. [Google Scholar]
- Barnes, M.; Heywood, A.E.; Mahimbo, A.; Rahman, B.; Newall, A.T.; Macintyre, C.R. Acute myocardial infarction and influenza: A meta-analysis of case–control studies. Heart 2015, 101, 1738–1747. [Google Scholar] [CrossRef]
- Stanwell-Smith, R.; Parker, A.M.; Chakraverty, P.; Soltanpoor, N.; Simpson, C.N. Possible association of influenza A with fetal loss: Investigation of a cluster of spontaneous abortions and stillbirths. Commun. Dis. Rep. CDR Rev. 1994, 4, 28–32. [Google Scholar]
- Palese, P. Influenza: Old and new threats. Nat. Med. 2004, 10, S82. [Google Scholar] [CrossRef]
- Horimoto, T.; Kawaoka, Y. Pandemic threat posed by avian influenza A viruses. Clin. Microbiol. Rev. 2001, 14, 129–149. [Google Scholar] [CrossRef]
- Guarnaccia, T.; Carolan, L.A.; Maurer-Stroh, S.; Lee, R.T.; Job, E.; Reading, P.C.; Petrie, S.; McCaw, J.M.; McVernon, J.; Hurt, A.C.; et al. Antigenic drift of the pandemic 2009 A (H1N1) influenza virus in A ferret model. PLoS Pathog. 2013, 9, 1003354. [Google Scholar] [CrossRef]
- Margine, I.; Krammer, F. Animal models for influenza viruses: Implications for universal vaccine development. Pathogens 2014, 3, 845–874. [Google Scholar] [CrossRef]
- Blazejewska, P.; Koscinski, L.; Viegas, N.; Anhlan, D.; Ludwig, S.; Schughart, K. Pathogenicity of different PR8 influenza A virus variants in mice is determined by both viral and host factors. Virology 2011, 412, 36–45. [Google Scholar] [CrossRef]
- Srivastava, B.; Błażejewska, P.; Heßmann, M.; Bruder, D.; Geffers, R.; Mauel, S.; Gruber, A.D.; Schughart, K. Host genetic background strongly influences the response to influenza a virus infections. PLoS ONE 2009, 4, e4857. [Google Scholar] [CrossRef]
- Schmitz, N.; Kurrer, M.; Bachmann, M.F.; Kopf, M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 2005, 79, 6441–6448. [Google Scholar] [CrossRef]
- Liu, B.; Mori, I.; Hossain, M.J.; Dong, L.; Takeda, K.; Kimura, Y. Interleukin-18 improves the early defence system against influenza virus infection by augmenting natural killer cell-mediated cytotoxicity. J. Gen. Virol. 2004, 85, 423–428. [Google Scholar] [CrossRef]
- Thomas, P.G.; Dash, P.; Aldridge, J.R., Jr.; Ellebedy, A.H.; Reynolds, C.; Funk, A.J.; Martin, W.J.; Lamkanfi, M.; Webby, R.J.; Boyd, K.L.; et al. NLRP3 (NALP3/CIAS1/Cryopyrin) mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009, 30, 566. [Google Scholar] [CrossRef]
- Perrone, L.A.; Szretter, K.J.; Katz, J.M.; Mizgerd, J.P.; Tumpey, T.M. Mice lacking both TNF and IL-1 receptors exhibit reduced lung inflammation and delay in onset of death following infection with a highly virulent H5N1 virus. J. Infect. Dis. 2010, 202, 1161–1170. [Google Scholar] [CrossRef]
- Gally, F.; Kosmider, B.; Weaver, M.R.; Pate, K.M.; Hartshorn, K.L.; Oberley-Deegan, R.E. FABP5 deficiency enhances susceptibility to H1N1 influenza A virus-induced lung inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, 64–72. [Google Scholar] [CrossRef]
- Huang, C.H.; Chen, C.J.; Yen, C.T.; Yu, C.P.; Huang, P.N.; Kuo, R.L.; Lin, S.J.; Chang, C.K.; Shih, S.R. Caspase-1 deficient mice are more susceptible to influenza A virus infection with PA variation. J. Infect. Dis. 2013, 208, 1898–1905. [Google Scholar] [CrossRef]
- Watanabe, T.; Watanabe, S.; Maher, E.A.; Neumann, G.; Kawaoka, Y. Pandemic potential of avian influenza A (H7N9) viruses. Trends Microbiol. 2014, 22, 623–631. [Google Scholar] [CrossRef]
- Neumann, G.; Kawaoka, Y. Transmission of influenza A viruses. Virology 2015, 479, 234–246. [Google Scholar] [CrossRef]
- Haller, O.; Staeheli, P.; Schwemmle, M.; Kochs, G. Mx GTPases: Dynamin-like antiviral machines of innate immunity. Trends Microbiol. 2015, 23, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Deeg, C.M.; Hassan, E.; Mutz, P.; Rheinemann, L.; Gotz, V.; Magar, L.; Schilling, M.; Kallfass, C.; Nurnberger, C.; Soubies, S.; et al. In vivo evasion of MxA by avian influenza viruses requires human signature in the viral nucleoprotein. J. Exp. Med. 2017, 214, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, C.; Yang, Z.; Chi, X.; Zhang, J.; Chen, J.L. Targeted disruption of influenza A virus hemagglutinin in genetically modified mice reduces viral replication and improves disease outcome. Sci. Rep. 2016, 6, 23746. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, G.; Garulli, B.; Sciaraffia, E.; Facchini, M.; Donatelli, I.; Castrucci, M.R. A heat-inactivated H7N3 vaccine induces cross-reactive cellular immunity in HLA-A2. 1 transgenic mice. Virol. J. 2016, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, G.; Sciaraffia, E.; Facchini, M.; Gubinelli, F.; Soprana, E.; Panigada, M.; Bernasconi, V.; Garulli, B.; Siccardi, A.; Donatelli, I.; et al. Protective immunity against influenza in HLA-A2 transgenic mice by modified vaccinia virus Ankara vectored vaccines containing internal influenza proteins. Pathog. Glob. Health 2017, 111, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Moise, L.; Tassone, R.; Latimer, H.; Terry, F.; Levitz, L.; Haran, J.P.; Ross, T.M.; Boyle, C.; Martin, W.D.; De Groot, A.S. Immunization with cross-conserved H1N1 influenza CD4+ T-cell epitopes lowers viral burden in HLA DR3 transgenic mice. Hum. Vaccin Immunother. 2013, 9, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.M.; Poon, L.L.; Guan, Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J. Clin. Virol. 2009, 45, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Konigshofer, Y.; Chien, Y.H. γδ T cells—Innate immune lymphocytes. Curr. Opin. Immunol. 2006, 18, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Eberl, M.; Moser, B. Monocytes and γδ T cells: Close encounters in microbial infection. Trends Immunol. 2009, 30, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, M.; O’brien, R.L.; Born, W.K. γδ T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.W.; Lau, Y.L.; Peiris, J.S. Use of Humanized mice to study antiviral activity of human γδ. Hong Kong Med. J. 2014, 20, 4–6. [Google Scholar] [PubMed]
- Richards, K.A.; Topham, D.; Chaves, F.A.; Sant, A.J. Cutting edge: CD4 T cells generated from encounter with seasonal influenza viruses and vaccines have broad protein specificity and can directly recognize naturally generated epitopes derived from the live pandemic H1N1 virus. J. Immunol. 2010, 1001395. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Bernstein, D.I.; Winokur, P.; Rupp, R.; Anderson, E.; Rouphael, N.; Dickey, M.; Stapleton, J.T.; Edupuganti, S.; Spearman, P.; et al. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: A randomized clinical trial. JAMA 2014, 312, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Nithichanon, A.; Nobusawa, E.; Moise, L.; Martin, W.D.; Yamamoto, N.; Terahara, K.; Hagiwara, H.; Odagiri, T.; Tashiro, M.; et al. Hhumanized mouse model identifies key amino acids for low immunogenicity of H7N9 vaccines. Sci. Rep. 2017, 7, 1283. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.; Ballesteros, A.; Qiu, Q.; Pow Sang, L.; Shashikumar, S.; Casares, S.; Brumeanu, T.D. Generation and testing anti-influenza human monoclonal antibodies in a new humanized mouse model (DRAGA: HLA-A2. HLA-DR4. Rag1 KO. IL-2Rγc KO. NOD). Hum. Vaccin Immunother. 2018, 14, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, I.I.; Mihaylova, N.M.; Manoylov, I.K.; Makatsori, D.; Lolov, S.; Nikolova, M.H.; Mamalaki, A.; Prechl, J.; Tchorbanov, A.I. Targeting of Influenza Viral Epitopes to Antigen-Presenting Cells by Genetically Engineered Chimeric Molecules in a Humanized NOD SCID Gamma Transfer Model. Hum. Gene Ther. 2018, 29, 1056–1070. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, E.; Momose, H.; Hiradate, Y.; Furuhata, K.; Mizukami, T.; Hamaguchi, I. Development of a preclinical humanized mouse model to evaluate acute toxicity of an influenza vaccine. Oncotarget 2018, 9, 25751. [Google Scholar] [CrossRef] [PubMed]
- Alagarasu, K.; Bachal, R.V.; Bhagat, A.B.; Shah, P.S.; Dayaraj, C. Elevated levels of vitamin D and deficiency of mannose binding lectin in dengue hemorrhagic fever. Virol. J. 2012, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Cecilia, D.; Kakade, M.; Alagarasu, K.; Patil, J.; Salunke, A.; Parashar, D.; Shah, P.S. Development of a multiplex real-time RT-PCR assay for simultaneous detection of dengue and chikungunya viruses. Arch. Virol. 2015, 160, 323–327. [Google Scholar] [CrossRef]
- Zompi, S.; Harris, E. Animal models of dengue virus infection. Viruses 2012, 4, 62–82. [Google Scholar] [CrossRef] [PubMed]
- Falzarano, D.; Bente, D.A. Animal models for viral hemorrhagic fever. Clin. Microbiol. Infect. 2014. [Google Scholar] [CrossRef]
- Van den Broek, M.F.; Muller, U.; Huang, S.; Aguet, M.; Zinkernagel, R.M. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 1995, 69, 4792–4796. [Google Scholar] [PubMed]
- Johnson, A.J.; Roehrig, J.T. New mouse model for dengue virus vaccine testing. J. Virol. 1999, 73, 783–786. [Google Scholar] [PubMed]
- Sarathy, V.V.; Milligan, G.N.; Bourne, N.; Barrett, A.D. Mouse models of dengue virus infection for vaccine testing. Vaccine 2015, 33, 7051–7060. [Google Scholar] [CrossRef] [PubMed]
- Milligan, G.N.; White, M.; Zavala, D.; Pyles, R.B.; Sarathy, V.V.; Barrett, A.D.; Bourne, N. Spectrum of activity testing for therapeutics against all four dengue virus serotypes in AG129 mouse models: Proof-of-concept studies with the adenosine nucleoside inhibitor NITD-008. Antivir. Res. 2018, 154, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Orozco, S.; Schmid, M.A.; Parameswaran, P.; Lachica, R.; Henn, M.R.; Beatty, R.; Harris, E. Characterization of a model of lethal dengue virus 2 infection in C57BL/6 mice deficient in the alpha/beta interferon receptor. J. Gen. Virol. 2012, 93, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.T.; Prestwood, T.R.; Lada, S.M.; Benedict, C.A.; Shresta, S. Cardif-mediated signaling controls the initial innate response to dengue virus in vivo. J. Virol. 2009, 83, 8276–8281. [Google Scholar] [CrossRef]
- Yauch, L.E.; Zellweger, R.M.; Kotturi, M.F.; Qutubuddin, A.; Sidney, J.; Peters, B.; Prestwood, T.R.; Sette, A.; Shresta, S. A protective role for dengue virus-specific CD8+ T cells. J. Immunol. 2009, 182, 4865–4873. [Google Scholar] [CrossRef]
- Perry, S.T.; Buck, M.D.; Lada, S.M.; Schindler, C.; Shresta, S. STAT2 mediates innate immunity to Dengue virus in the absence of STAT1 via the type I interferon receptor. PLoS Pathog. 2011, 7, 1001297. [Google Scholar] [CrossRef] [PubMed]
- Christofferson, R.C.; McCracken, M.K.; Johnson, A.M.; Chisenhall, D.M.; Mores, C.N. Development of a transmission model for dengue virus. J. Virol. 2013, 10, 127. [Google Scholar] [CrossRef]
- Carlin, A.F.; Plummer, E.M.; Vizcarra, E.A.; Sheets, N.; Joo, Y.; Tang, W.; Day, J.; Greenbaum, J.; Glass, C.K.; Diamond, M.S.; et al. An IRF-3-, IRF-5-, and IRF-7-independent pathway of dengue viral resistance Utilizes IRF-1 to stimulate type I and II interferon responses. Cell Rep. 2017, 21, 1600–1612. [Google Scholar] [CrossRef]
- Marques, R.E.; Guabiraba, R.; Del Sarto, J.L.; Rocha, R.F.; Queiroz, A.L.; Cisalpino, D.; Marques, P.E.; Pacca, C.C.; Fagundes, C.T.; Menezes, G.B.; et al. Dengue virus requires the CC-chemokine receptor CCR5 for replication and infection development. Immunology 2015, 145, 583–596. [Google Scholar] [CrossRef]
- Jhan, M.K.; HuangFu, W.C.; Chen, Y.F.; Kao, J.C.; Tsai, T.T.; Ho, M.R.; Shen, T.J.; Tseng, P.C.; Wang, Y.T.; Lin, C.F. Anti-TNF-α restricts dengue virus-induced neuropathy. J. Leukoc. Biol. 2018, 104, 961–968. [Google Scholar] [CrossRef]
- Weiskopf, D.; Yauch, L.E.; Angelo, M.A.; John, D.V.; Greenbaum, J.A.; Sidney, J.; Kolla, R.V.; De Silva, A.D.; de Silva, A.M.; Grey, H.; et al. Insights into HLA-restricted T cell responses in a novel mouse model of dengue virus infection point toward new implications for vaccine design. J. Immunol. 2011, 187, 4268. [Google Scholar] [CrossRef]
- Yauch, L.E.; Prestwood, T.R.; May, M.M.; Morar, M.M.; Zellweger, R.M.; Peters, B.; Sette, A.; Shresta, S. CD4+ T cells are not required for the induction of dengue virus-specific CD8+ T cell or antibody responses but contribute to protection after vaccination. J. Immunol. 2010, 1001709. [Google Scholar] [CrossRef]
- Bente, D.A.; Melkus, M.W.; Garcia, J.V.; Rico-Hesse, R. Dengue fever in humanized NOD/SCID mice. J. Virol. 2005, 79, 13797–13799. [Google Scholar] [CrossRef]
- Kuruvilla, J.G.; Troyer, R.M.; Devi, S.; Akkina, R. Dengue virus infection and immune response in humanized RAG2−/− γc−/−(RAG-hu) mice. Virology 2007, 369, 143–152. [Google Scholar] [CrossRef]
- Mota, J.; Rico-Hesse, R. Dengue virus tropism in humanized mice recapitulates human dengue fever. PLoS ONE 2011, 6, 20762. [Google Scholar] [CrossRef]
- Jaiswal, S.; Pazoles, P.; Woda, M.; Shultz, L.D.; Greiner, D.L.; Brehm, M.A.; Mathew, A. Enhanced humoral and HLA-A2-restricted dengue virus-specific T-cell responses in humanized BLT NSG mice. Immunology 2012, 136, 334–343. [Google Scholar] [CrossRef]
- Jaiswal, S.; Smith, K.; Ramirez, A.; Woda, M.; Pazoles, P.; Shultz, L.D.; Greiner, D.L.; Brehm, M.A.; Mathew, A. Dengue virus infection induces broadly cross-reactive human IgM antibodies that recognize intact virions in humanized BLT-NSG mice. Exp. Biol. Med. 2015, 240, 67–78. [Google Scholar] [CrossRef]
- Akkina, R. Humanized mice for studying human immune responses and generating human monoclonal antibodies. Microbiol. Spectr. 2014, 2, AID-0003-2012. [Google Scholar]
- Jangalwe, S.; Shultz, L.D.; Mathew, A.; Brehm, M.A. Improved B cell development in humanized NOD-SCID IL2Rγnull mice transgenically expressing human stem cell factor, granulocyte-macrophage colony-stimulating factor and interleukin-3. Immun. Inflamm. Dis. 2016, 4, 427–440. [Google Scholar] [CrossRef]
- Cui, L.; Hou, J.; Fang, J.; Lee, Y.H.; Costa, V.V.; Wong, L.H.; Chen, Q.; Ooi, E.E.; Tannenbaum, S.R.; Chen, J.; et al. Serum metabolomics investigation of humanized mouse model with dengue infection. J. Virol. 2017, 91, e00386-17. [Google Scholar] [CrossRef]
- Frias-Staheli, N.; Dorner, M.; Marukian, S.; Billerbeck, E.; Labitt, R.N.; Rice, C.M.; Ploss, A. Utility of humanized BLT mice for analysis of dengue virus infection and antiviral drug testing. J. Virol. 2014, 88, 2205–2218. [Google Scholar] [CrossRef]
- Mota, J.; Rico-Hesse, R. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J. Virol. 2009, 83, 8638–8645. [Google Scholar] [CrossRef]
- Cox, J.; Mota, J.; Sukupolvi-Petty, S.; Diamond, M.S.; Rico-Hesse, R. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J. Virol. 2012, 86, 7637–7649. [Google Scholar] [CrossRef]
- Züst, R.; Dong, H.; Li, X.F.; Chang, D.C.; Zhang, B.; Balakrishnan, T.; Toh, Y.X.; Jiang, T.; Li, S.H.; Deng, Y.Q.; et al. Rational design of a live attenuated dengue vaccine: 2′-o-methyltransferase mutants are highly attenuated and immunogenic in mice and macaques. PLoS Pathog. 2013, 9, e1003521. [Google Scholar] [CrossRef]
- Snoy, P.J. Establishing efficacy of human products using animals: The US food and drug administration’s “animal rule”. Vet. Pathol. 2010, 47, 774–778. [Google Scholar] [CrossRef]
- Mairuhu, A.T.; Mac Gillavry, M.R.; Setiati, T.E.; Soemantri, A.; Ten Cate, H.; Brandjes, D.P.; Van Gorp, E.C. Is clinical outcome of dengue-virus infections influenced by coagulation and fibrinolysis? A critical review of the evidence. Lancet Infect. Dis. 2003, 3, 33. [Google Scholar] [CrossRef]
- Lynch, S.F.; Ludlam, C.A. Plasma microparticles and vascular disorders. Br. J. Haematol. 2007, 137, 36. [Google Scholar] [CrossRef]
- Doyle, A.; McGarry, M.P.; Lee, N.A.; Lee, J.J. The construction of transgenic and gene knockout/knockin mouse models of human disease. Transgenic Res. 2012, 21, 327–349. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S. Animal models of tumor immunity, immunotherapy and cancer vaccines. Curr. Opin. Immunol. 2004, 16, 143–150. [Google Scholar] [CrossRef]
- Bouabe, H.; Okkenhaug, K. Gene Targeting in Mice: A Review. Methods Mol. Biol. 2013, 1064, 315–336. [Google Scholar]

| S.No | Virus | Mouse Model | Name of the Knocked out Gene | Research Application | Reference |
|---|---|---|---|---|---|
| 1. | HIV | Samhd1 KO (samhd1−/−) | Samhd1 deletion. | HIV-1 and HIV-2 entry and pathogenesis | [23,24] |
| 2. | Influenza | C57BL/6J (IL-1R1−/−) | Interleukin receptors α &β. | Pathogenesis of influenza. | [75] |
| C57BL/6J (IL-81−/−) | Interleukin 18 | Pathogenesis of influenza. | [76] | ||
| C57BL/6J (cryp−/− and casp1−/−) | Cryopyrin and caspase deficient. | Innate immunity and moderating lung pathology in influenza pneumonia | [77] | ||
| C57BL/6J (FABP5−/−) | Deletion of FABP5. | Anti inflammatory response against H1N1 influenza A | [79] | ||
| 3. | Dengue | 129Sv(ev) (AG129 KO) | Type I& II- IFN receptors deficient. | Vaccines research and anti-viral drug design. | [105,106,107] |
| STAT1−/− 129/Sv/Ev | Deletion in the DNA binding domain of the STAT1 gene. | STAT1-independent host defense mechanism against viruses | [109,110,111] | ||
| C57BL/6J (Irf3−/−x Irf5−/−x Irf7−/− triple knockout) | Deletion of interfereon regulators factors (IRF) 3, 5 and 7 | Antiviral role of IRF-1 by indcuing IFN responses against DENV infection | [113] |
| S.No | Virus | Mouse Model | Transgene | Application | Reference |
|---|---|---|---|---|---|
| 1. | HIV | C57BL/6. hu CD4/CCR5 Tg | Human CD4 and chemokine receptor genes | To observe pathological phenotypes of HIV | [31] |
| C57BL/6 × C3HF2. CD4C/rtTA × TRE/HIVNef) or (CD4C/rtTA2S-M2 × TRE/HIVNef) double-Tg mice upon doxycycline (DOX) | HIV Nef gene | Cellular and molecular pathways of Nef in HIV pathogenesis | [37,38] | ||
| C57BL/6 HIVgp120Tg | HIV gp120 | To reveal the role of HIV glycoportein gp120 in binidng to the corecptor CXCR4 in absence of CCR5. CCR5 depletion protects Tg mice against deficits in spatial learning and memory | [41] | ||
| HIV-1 Tg26 transgenic mice | Truncated HIV-1 NL4-3 genome with a 3.1-kb deletion in the Gag and Pol regions | HIV-associated nephropathy | [42] | ||
| 2. | Influenza | B6.SJL Tg Mice | MX1, MX2, FAM3B and TMPRSS2 genes | Zoonotic transmission of influenza A viruses | [83,84] |
| BALB/c. Tg Influenza A HA | Sh-RNAcodes for the knockdown of heamagglutinin | Prevention and control of a viral zoonosis of influenza | [84,85] | ||
| 3. | Dengue | C57BL/6J Tg HLA-A*02:01 and B10. Tg. HLA-DR3 | Geness coding for interspecies hybrid MHC class I molecule of the human HLA-A*0201 allele and the cytoplasmic and transmembrane domains of the mouse H-2Dd class I molecule Genes coding for MHC Class II gene comprising HLA- DR α genomic fragment and a DRB1*030113 | To study the CD8+ T cell response to H7N3 influneza A vaccine. Identification of CD4+T cell epitopes for vaccine development | [85,86] |
| S.No | Virus | Mouse Model | Humanization | Application | Reference |
|---|---|---|---|---|---|
| 1. | HIV | Hu-PBL-SCID mice | SCID mice populated with human peripheral blood leukocytes | HIV infection, replication and pathogenesis | [16] |
| HSCs-BLT mice, NOD-SCID BLT and NSG-BLT. | HSCs-mice engrafted and bone liver/thymus | Human disease pathogenesis, retroviral spread and restored CD4+ and CD8+ T cell numbers on ART treatment | [43,44,45,46,47,48] | ||
| C57BL/6 Rag2−/− γc −/−CD47−/− triple knockout (TKO)-BLT mouse. | Xenotransplantation with human immune system | HIV-latency | [50] | ||
| NOD/ SCID (NS), NOD/SCID IL2rgc−/− (NSG) or NOD/Rag1−/− IL2rgc−/− (NRG) | Reconstitution of different types of human tissues | Treatment of systemic HIV infection with ART and HIV latency | [45,46,47,48] | ||
| 2. | Influenza | C57BL/10SgAiRag2−/−γc−/− mice. | Humanized with huPBMCs | Vaccine based studies and therapeutics for human pathogens | [93] |
| DRAGA mouse; HLA-A2. HLADR4. Rag1KO. IL-2Rgc KO. NOD. | Humanized with functional human immune system | Anti-influenza monoclonal antibodies | [97] | ||
| NOD/Shi-SCID-IL2rγnull (NOG). | Humanized with huPBMCs. | Evaluating vaccine safety | [99] | ||
| 3. | Dengue | RAG2−/−γc−/− mice | Xenografted with human CD34+ hematopoietic stem cells | Antibody responses against DENV | [119] |
| NOD-SCID IL2rγ null | Transplanation of purified cord blood CD34+ cells | To demonstrate differences in the virulence of different DENV-2 strains | [120] | ||
| HIS BLT-NOD/SCID mice | Human immune system | Preclinical testing of antiviral drugs against dengue | [127] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnakumar, V.; Durairajan, S.S.K.; Alagarasu, K.; Li, M.; Dash, A.P. Recent Updates on Mouse Models for Human Immunodeficiency, Influenza, and Dengue Viral Infections. Viruses 2019, 11, 252. https://doi.org/10.3390/v11030252
Krishnakumar V, Durairajan SSK, Alagarasu K, Li M, Dash AP. Recent Updates on Mouse Models for Human Immunodeficiency, Influenza, and Dengue Viral Infections. Viruses. 2019; 11(3):252. https://doi.org/10.3390/v11030252
Chicago/Turabian StyleKrishnakumar, Vinodhini, Siva Sundara Kumar Durairajan, Kalichamy Alagarasu, Min Li, and Aditya Prasad Dash. 2019. "Recent Updates on Mouse Models for Human Immunodeficiency, Influenza, and Dengue Viral Infections" Viruses 11, no. 3: 252. https://doi.org/10.3390/v11030252
APA StyleKrishnakumar, V., Durairajan, S. S. K., Alagarasu, K., Li, M., & Dash, A. P. (2019). Recent Updates on Mouse Models for Human Immunodeficiency, Influenza, and Dengue Viral Infections. Viruses, 11(3), 252. https://doi.org/10.3390/v11030252





