Telbivudine Reduces Parvovirus B19-Induced Apoptosis in Circulating Angiogenic Cells
Abstract
1. Introduction
2. Methods
2.1. Cell lines and Isolation of Primary Cells
2.2. Telbivudine Treatment of Cell Cultures
2.3. Virus Stock and Infection
2.4. RNA Isolation and Reverse Transcriptase PCR
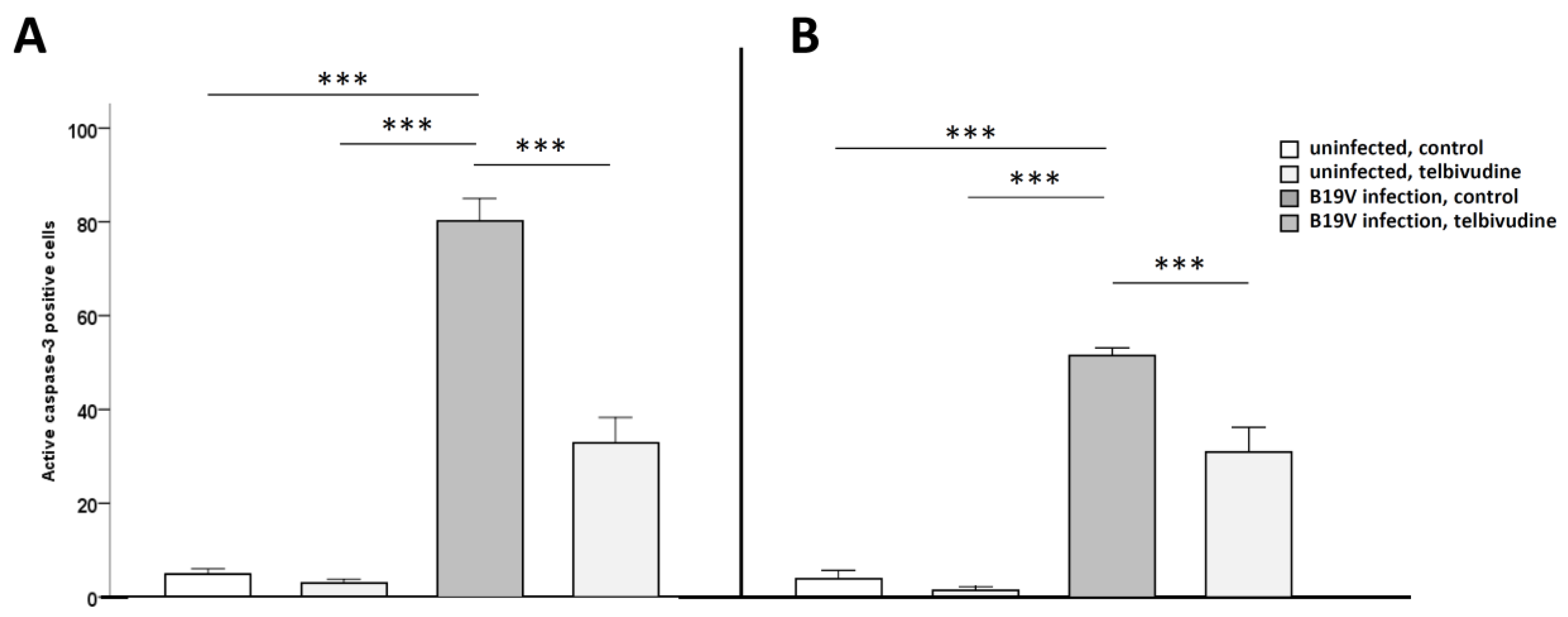
2.5. Fluorescence Associated Cell Sorting (FACS) Analysis for Active Caspase-3
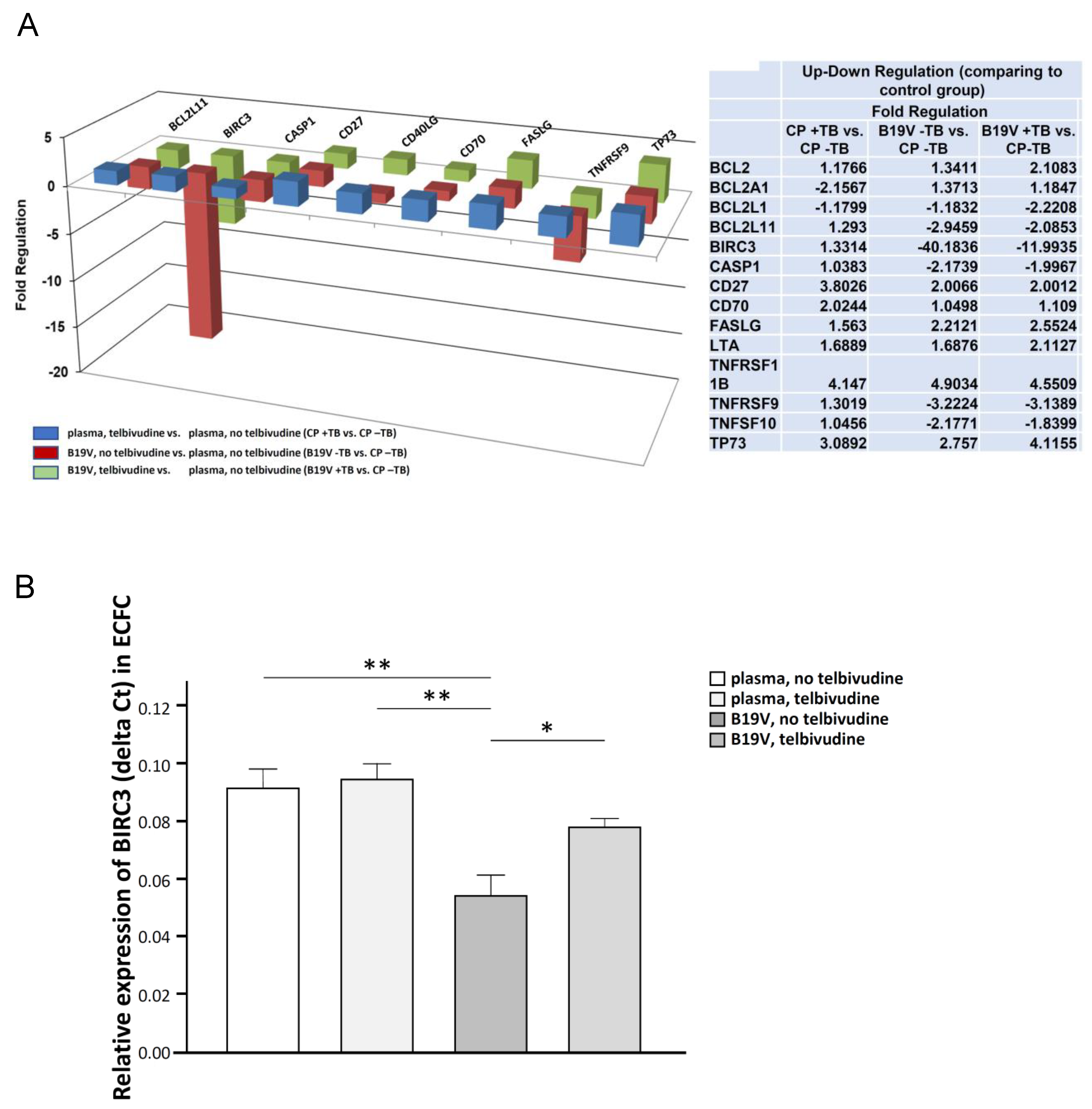
2.6. PCR Array for Human Apoptosis and Angiogenesis
2.7. BIRC3 Single PCR Levels
2.8. Statistical Analysis
3. Results
Telbivudine Inhibits Activation of B19V-Induced Apoptosis through Stabilisation of BIRC3 Levels
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| B19V | human parvovirus B19 |
| BIRC3 | baculoviral inhibitor of apoptosis repeat-containing protein 3 |
| CAC | circulating angiogenic cells |
| cIAP-2 | cellular inhibitor of apoptosis-2 |
| eo-EPC | early outgrowth endothelial progenitor cells |
| ECFC | endothelial colony forming cells |
| FACS | fluorescence associated cell sorting |
| GE | genome equivalents |
| HBV | hepatitis B virus |
| IAP | inhibitor of apoptosis |
| KDR | kinase insert domain receptor |
| MNC | mononuclear cells |
| PBS | phosphate buffered saline |
| RT | reverse transcriptase |
| RIP1 | receptor interacting protein (RIP) kinases 1 |
| TNF | tumor necrosis factor |
| TNFR | TNFα receptor |
| TRAF1 | TNF receptor associated factor 1 |
| TRAF2 | TNF receptor associated factor 2 |
| TRAIL | TNF-related apoptosis-inducing ligand |
References
- Cossart, Y.E.; Cant, B.; Field, A.M.; Widdows, D. Parvovirus-like particles in human sera. Lancet 1975, 305, 72–73. [Google Scholar] [CrossRef]
- Schmidt-Lucke, C.; Zobel, T.; Schrepfer, S.; Kuhl, U.; Wang, D.; Klingel, K.; Becher, P.M.; Fechner, H.; Pozzuto, T.; Van Linthout, S.; et al. Impaired Endothelial Regeneration Through Human Parvovirus B19-Infected Circulating Angiogenic Cells in Patients with Cardiomyopathy. J. Infect. Dis. 2015, 212, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.E.; Anderson, S.; Young, N.S. Erythrocyte P antigen: Cellular receptor for B19 parvovirus. Science 1993, 262, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Lucke, C.; Zobel, T.; Escher, F.; Tschope, C.; Lassner, D.; Kuhl, U.; Gubbe, K.; Volk, H.D.; Schultheiss, H.P. Human Parvovirus B19 (B19V) Up-regulates CXCR4 Surface Expression of Circulating Angiogenic Cells: Implications for Cardiac Ischemia in B19V Cardiomyopathy. J. Infect. Dis. 2018, 217, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Bua, G.; Manaresi, E.; Bonvicini, F.; Gallinella, G. Parvovirus B19 Replication and Expression in Differentiating Erythroid Progenitor Cells. PLoS ONE 2016, 11, e0148547. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Zhi, N.; Filippone, C.; Keyvanfar, K.; Kajigaya, S.; Brown, K.E.; Young, N.S. Ex Vivo-Generated CD36+ Erythroid Progenitors Are Highly Permissive to Human Parvovirus B19 Replication. J. Virol. 2008, 82, 2470–2476. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, S.; Komatsu, N.; Frickhofen, N.; Anderson, S.; Kajigaya, S.; Young, N. First continuous propagation of B19 parvovirus in a cell line. Blood 1992, 79, 18–24. [Google Scholar] [PubMed]
- Kuhl, U.; Lassner, D.; Dorner, A.; Rohde, M.; Escher, F.; Seeberg, B.; Hertel, E.; Tschope, C.; Skurk, C.; Gross, U.M.; et al. A distinct subgroup of cardiomyopathy patients characterized by transcriptionally active cardiotropic erythrovirus and altered cardiac gene expression. Basic Res. Cardiol. 2013, 108, 372. [Google Scholar] [CrossRef] [PubMed]
- Bearzi, C.; Leri, A.; Lo Monaco, F.; Rota, M.; Gonzalez, A.; Hosoda, T.; Pepe, M.; Qanud, K.; Ojaimi, C.; Bardelli, S.; et al. Identification of a coronary vascular progenitor cell in the human heart. Proc. Natl. Acad. Sci. USA 2009, 106, 15885–15890. [Google Scholar] [CrossRef] [PubMed]
- Vasa, M.; Fichtlscherer, S.; Adler, K.; Aicher, A.; Martin, H.; Zeiher, A.; Dimmeler, S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation 2001, 103, 2885–2890. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Pollok, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004, 104, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Yoder, M.C.; Mead, L.E.; Prater, D.; Krier, T.R.; Mroueh, K.N.; Li, F.; Krasich, R.; Temm, C.J.; Prchal, J.T.; Ingram, D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007, 109, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Meneveau, N.; Deschaseaux, F.; Séronde, M.-F.; Chopard, R.; Schiele, F.; Jehl, J.; Tiberghien, P.; Bassand, J.-P.; Kantelip, J.-P.; Davani, S. Presence of endothelial colony-forming cells is associated with reduced microvascular obstruction limiting infarct size and left ventricular remodelling in patients with acute myocardial infarction. Basic Res. Cardiol. 2011, 106, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Deveraux, Q.L.; Roy, N.; Stennicke, H.R.; Van Arsdale, T.; Zhou, Q.; Srinivasula, S.M.; Alnemri, E.S.; Salvesen, G.S.; Reed, J.C. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998, 17, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Rothe, M.; Pan, M.G.; Henzel, W.J.; Ayres, T.M.; Goeddel, D.V. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 1995, 83, 1243–1252. [Google Scholar] [CrossRef]
- Liston, P.; Roy, N.; Tamai, K.; Lefebvre, C.; Baird, S.; Cherton-Horvat, G.; Farahani, R.; McLean, M.; Ikeda, J.E.; MacKenzie, A.; et al. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature 1996, 379, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Deveraux, Q.L.; Takahashi, R.; Salvesen, G.S.; Reed, J.C. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997, 16, 6914–6925. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lu, G.; Wu, F. Simvastatin suppresses homocysteine-induced apoptosis in endothelial cells: Roles of caspase-3, cIAP-1 and cIAP-2. Hypertens. Res. 2009, 32, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Du, F.; Wang, X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell 2008, 133, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhu, W.G.; Morrison, C.D.; Brena, R.M.; Smiraglia, D.J.; Raval, A.; Wu, Y.Z.; Rush, L.J.; Ross, P.; Molina, J.R.; et al. A comprehensive search for DNA amplification in lung cancer identifies inhibitors of apoptosis cIAP1 and cIAP2 as candidate oncogenes. Hum. Mol. Genet. 2003, 12, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shao, J.; Xiong, W.; Yu, S.; Hu, Y.; Liu, J.; Wang, X.; Xiang, L.; Yuan, Z. Cellular cIAP2 gene expression associated with anti-HBV activity of TNF-alpha in hepatoblastoma cells. J. Interf. Cytokine Res. 2005, 25, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ni, J.; Li, J.; Shi, B.; Xu, Y.; Yuan, Z. Inhibition of hepatitis B virus replication by cIAP2 involves accelerating the ubiquitin-proteasome-mediated destruction of polymerase. J. Virol. 2011, 85, 11457–11467. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.P.; Li, S.; Zhong, B.; Li, Y.; Yan, J.; Li, Q.; Teng, C.; Shu, H.B. Virus-triggered ubiquitination of TRAF3/6 by cIAP1/2 is essential for induction of interferon-beta (IFN-beta) and cellular antiviral response. J. Biol. Chem. 2010, 285, 9470–9476. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Gane, E.; Liaw, Y.F.; Hsu, C.W.; Thongsawat, S.; Wang, Y.; Chen, Y.; Heathcote, E.J.; Rasenack, J.; Bzowej, N.; et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N. Engl. J. Med. 2007, 357, 2576–2588. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, H.L.; McHutchison, J.G. Telbivudine versus lamivudine in patients with chronic hepatitis B. N. Engl. J. Med. 2008, 358, 1517–1518. [Google Scholar] [PubMed]
- Chen, Y.; Li, X.; Ye, B.; Yang, X.; Wu, W.; Chen, B.; Pan, X.; Cao, H.; Li, L. Effect of telbivudine therapy on the cellular immune response in chronic hepatitis B. Antivir. Res. 2011, 91, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Huang, Z.; Liu, H.; Chen, J.; Xie, Z.; Chen, Z.; Peng, J.; Sun, J.; Hou, J.; Zhang, X. Lower Expression of MicroRNA-155 Contributes to Dysfunction of Natural Killer Cells in Patients with Chronic Hepatitis B. Front. Immunol. 2017, 8, 1173. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.G.; Yan, W.M.; Guo, W.; Chen, T.; Zou, Y.; Wang, H.W.; Wang, X.J.; Yang, X.J.; Lu, Y.L.; Luo, X.P.; et al. Telbivudine preserves T-helper 1 cytokine production and downregulates programmed death ligand 1 in a mouse model of viral hepatitis. J. Viral Hepat. 2010, 17 (Suppl. 1), 24–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, D. Telbivudine attenuates UUO-induced renal fibrosis via TGF-beta/Smad and NF-kappaB signaling. Int. Immunopharmacol. 2018, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Losordo, D.; Dimmeler, S. Critical Reevaluation of Endothelial Progenitor Cell Phenotypes for Therapeutic and Diagnostic Use. Circ. Res. 2012, 110, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Lucke, C.; Spillmann, F.; Bock, T.; Kuhl, U.; Van Linthout, S.; Schultheiss, H.P.; Tschope, C. Interferon beta modulates endothelial damage in patients with cardiac persistence of human parvovirus B19V infection. J. Infect. Dis. 2010, 201, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Zhang, E.Y.; Guan, W.; Cheng, F.; Kleiboeker, S.; Yankee, T.M.; Qiu, J. The small 11kDa nonstructural protein of human parvovirus B19 plays a key role in inducing apoptosis during B19 virus infection of primary erythroid progenitor cells. Blood 2010, 115, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, D.J.; Cheung, H.H.; Mrad, R.L.; Plenchette, S.; Simard, C.; Enwere, E.; Arora, V.; Mak, T.W.; Lacasse, E.C.; Waring, J.; et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc. Natl. Acad. Sci. USA 2008, 105, 11778–11783. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Muta, K.; Wickrema, A.; Krantz, S.B.; Nishimura, J.; Nawata, H. Interferon gamma delays apoptosis of mature erythroid progenitor cells in the absence of erythropoietin. Blood 2000, 95, 3742–3749. [Google Scholar] [PubMed]
- Schoemaker, M.H.; Ros, J.E.; Homan, M.; Trautwein, C.; Liston, P.; Poelstra, K.; van Goor, H.; Jansen, P.L.; Moshage, H. Cytokine regulation of pro- and anti-apoptotic genes in rat hepatocytes: NF-kappaB-regulated inhibitor of apoptosis protein 2 (cIAP2) prevents apoptosis. J. Hepatol. 2002, 36, 742–750. [Google Scholar] [CrossRef]
- Guo, S.; Messmer-Blust, A.F.; Wu, J.; Song, X.; Philbrick, M.J.; Shie, J.L.; Rana, J.S.; Li, J. Role of A20 in cIAP-2 protection against tumor necrosis factor alpha (TNF-alpha)-mediated apoptosis in endothelial cells. Int. J. Mol. Sci. 2014, 15, 3816–3833. [Google Scholar] [CrossRef] [PubMed]
- Ebert, G.; Preston, S.; Allison, C.; Cooney, J.; Toe, J.G.; Stutz, M.D.; Ojaimi, S.; Scott, H.W.; Baschuk, N.; Nachbur, U.; et al. Cellular inhibitor of apoptosis proteins prevent clearance of hepatitis B virus. Proc. Natl. Acad. Sci. USA 2015, 112, 5797–5802. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zobel, T.; Bock, C.-T.; Kühl, U.; Rohde, M.; Lassner, D.; Schultheiss, H.-P.; Schmidt-Lucke, C. Telbivudine Reduces Parvovirus B19-Induced Apoptosis in Circulating Angiogenic Cells. Viruses 2019, 11, 227. https://doi.org/10.3390/v11030227
Zobel T, Bock C-T, Kühl U, Rohde M, Lassner D, Schultheiss H-P, Schmidt-Lucke C. Telbivudine Reduces Parvovirus B19-Induced Apoptosis in Circulating Angiogenic Cells. Viruses. 2019; 11(3):227. https://doi.org/10.3390/v11030227
Chicago/Turabian StyleZobel, Thomas, C.-Thomas Bock, Uwe Kühl, Maria Rohde, Dirk Lassner, Heinz-Peter Schultheiss, and Caroline Schmidt-Lucke. 2019. "Telbivudine Reduces Parvovirus B19-Induced Apoptosis in Circulating Angiogenic Cells" Viruses 11, no. 3: 227. https://doi.org/10.3390/v11030227
APA StyleZobel, T., Bock, C.-T., Kühl, U., Rohde, M., Lassner, D., Schultheiss, H.-P., & Schmidt-Lucke, C. (2019). Telbivudine Reduces Parvovirus B19-Induced Apoptosis in Circulating Angiogenic Cells. Viruses, 11(3), 227. https://doi.org/10.3390/v11030227





