Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART
Abstract
1. Introduction
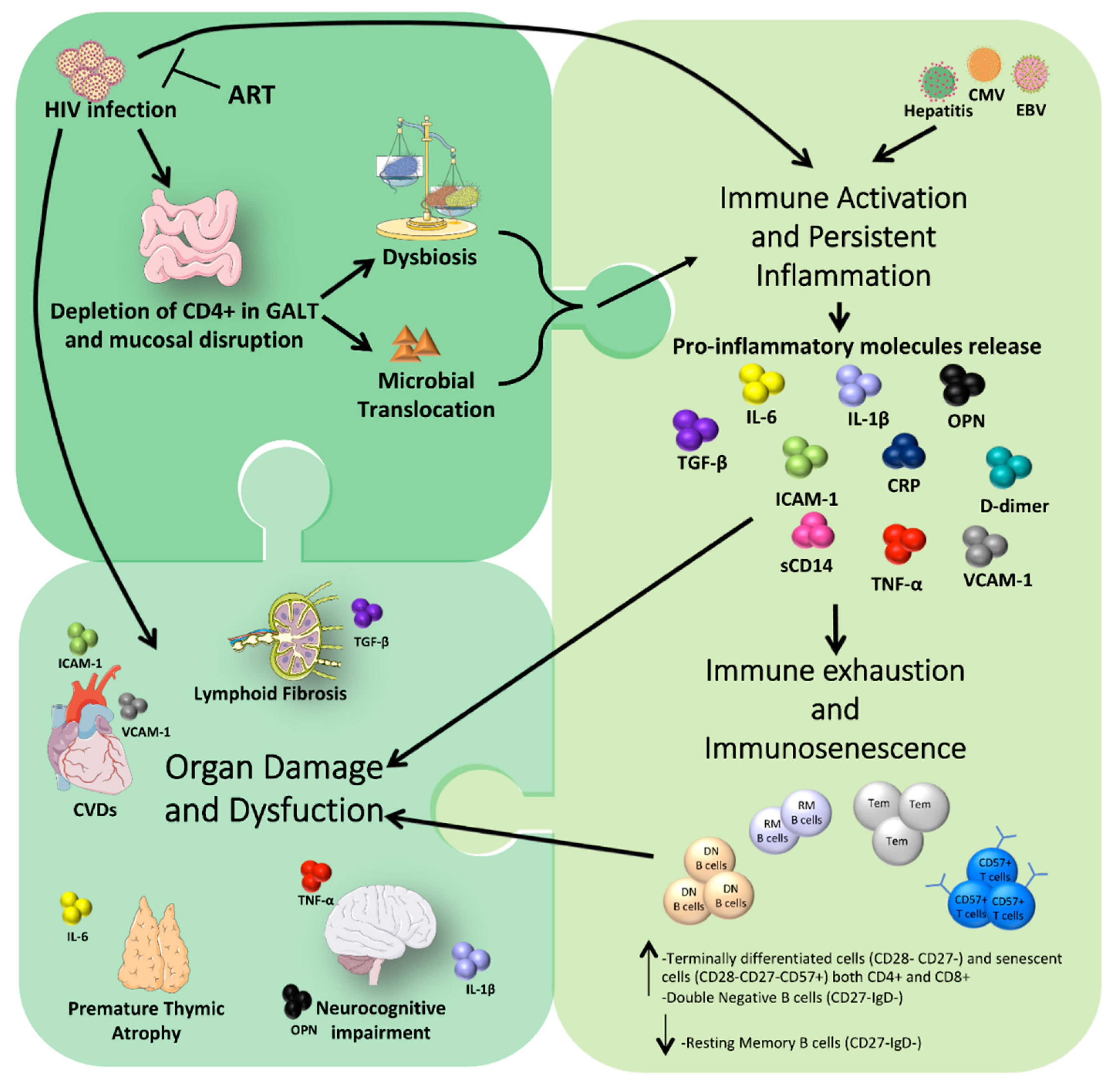
2. How HIV Infection Impacts Microbiota Composition, Chronic Inflammation, Immune Activation, and Premature Aging
3. Initiation and Duration of ART Can Affect Chronic Immune Activation and Inflammation
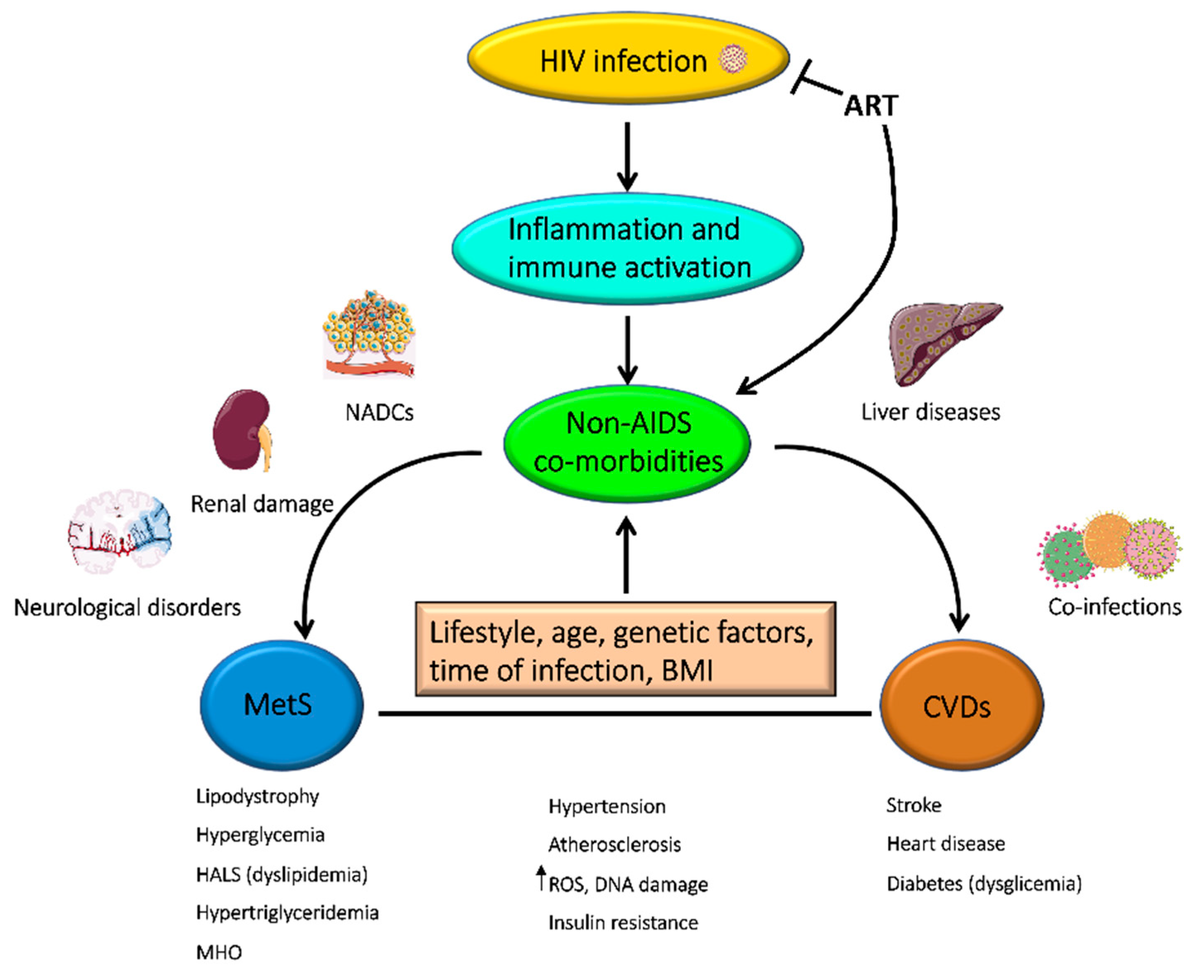
4. Non-AIDS Co-Morbidities in PLWH
5. Effects of HIV and ART Exposure on Inflammation, Immunodysfunction and Premature Aging in Perinatally HIV-Infected Children
6. Novel ART Strategies to Reduce Inflammation: Two-Drug Therapies and Immunomodulators
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. HIV/AIDS. Available online: https://www.who.int/gho/hiv/en/ (accessed on 31 December 2017).
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of aids: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef]
- Brites-Alves, C.; Luz, E.; Netto, E.M.; Ferreira, T.; Diaz, R.S.; Pedroso, C.; Page, K.; Brites, C. Immune activation, proinflammatory cytokines, and conventional risks for cardiovascular disease in HIV patients: A case-control study in bahia, brazil. Front. Immunol. 2018, 9, 1469. [Google Scholar] [CrossRef] [PubMed]
- Manjati, T.; Nkambule, B.; Ipp, H. Immune activation is associated with decreased thymic function in asymptomatic, untreated HIV-infected individuals. South. Afr. J. HIV Med. 2016, 17, 445. [Google Scholar] [CrossRef] [PubMed]
- Zevin, A.S.; McKinnon, L.; Burgener, A.; Klatt, N.R. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr. Opin. HIV AIDS 2016, 11, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Sokoya, T.; Steel, H.C.; Nieuwoudt, M.; Rossouw, T.M. HIV as a cause of immune activation and immunosenescence. Mediators Inflamm. 2017, 2017, 6825493. [Google Scholar] [CrossRef] [PubMed]
- Bosho, D.D.; Dube, L.; Mega, T.A.; Adare, D.A.; Tesfaye, M.G.; Eshetie, T.C. Prevalence and predictors of metabolic syndrome among people living with human immunodeficiency virus (PLWHIV). Diabetol. Metab. Syndr. 2018, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.A.; Armiliato, G.N.A.; Guimaraes, N.S.; Caram, C.A.; Silveira, R.D.S.; Tupinambas, U. Metabolic disorders and cardiovascular risk in people living with HIV/AIDS without the use of antiretroviral therapy. Rev. Soc. Bras. Med. Trop. 2017, 50, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.M. Metabolic syndrome and cardiovascular risk. J. Fam. Community Med. 2010, 17, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, F.C.; Avershina, E.; Wilson, R.; Rudi, K. Gut microbiota in HIV infection: Implication for disease progression and management. Gastroenterol. Res.Pract. 2014, 2014, 803185. [Google Scholar] [CrossRef] [PubMed]
- Nazli, A.; Chan, O.; Dobson-Belaire, W.N.; Ouellet, M.; Tremblay, M.J.; Gray-Owen, S.D.; Arsenault, A.L.; Kaushic, C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010, 6, e1000852. [Google Scholar] [CrossRef] [PubMed]
- Gootenberg, D.B.; Paer, J.M.; Luevano, J.M.; Kwon, D.S. HIV-associated changes in the enteric microbial community: Potential role in loss of homeostasis and development of systemic inflammation. Curr. Opin. Infect. Dis. 2017, 30, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Abad-Fernandez, M.; Vallejo, A.; Hernandez-Novoa, B.; Diaz, L.; Gutierrez, C.; Madrid, N.; Munoz, M.A.; Moreno, S. Correlation between different methods to measure microbial translocation and its association with immune activation in long-term suppressed HIV-1-infected individuals. J. Acquir. Immune Defic. Syndr. 2013, 64, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Castellanos, J.F.; Serrano-Villar, S.; Latorre, A.; Artacho, A.; Ferrus, M.L.; Madrid, N.; Vallejo, A.; Sainz, T.; Martinez-Botas, J.; Ferrando-Martinez, S.; et al. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal. Immunol. 2015, 8, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.A.; Keshavarzian, A.; Losurdo, J.; Swanson, G.; Siewe, B.; Forsyth, C.; French, A.; Demarais, P.; Sun, Y.; Koenig, L.; et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014, 10, e1003829. [Google Scholar] [CrossRef] [PubMed]
- Dinh, D.M.; Volpe, G.E.; Duffalo, C.; Bhalchandra, S.; Tai, A.K.; Kane, A.V.; Wanke, C.A.; Ward, H.D. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J. Infect. Dis. 2015, 211, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic-Cvijin, I.; Dunham, R.M.; Iwai, S.; Maher, M.C.; Albright, R.G.; Broadhurst, M.J.; Hernandez, R.D.; Lederman, M.M.; Huang, Y.; Somsouk, M.; et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci. Transl. Med. 2013, 5, 193ra191. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Meng, D.; Weng, M.; Zhu, W.; Wu, W.; Kasper, D.; Walker, W.A. The symbiotic bacterial surface factor polysaccharide a on bacteroides fragilis inhibits il-1beta-induced inflammation in human fetal enterocytes via toll receptors 2 and 4. PLoS ONE 2017, 12, e0172738. [Google Scholar]
- Paquin-Proulx, D.; Ching, C.; Vujkovic-Cvijin, I.; Fadrosh, D.; Loh, L.; Huang, Y.; Somsouk, M.; Lynch, S.V.; Hunt, P.W.; Nixon, D.F.; et al. Bacteroides are associated with galt inkt cell function and reduction of microbial translocation in HIV-1 infection. Mucosal. Immunol. 2017, 10, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, G.; Tincati, C.; Silvestri, G. Microbial translocation in the pathogenesis of HIV infection and aids. Clin. Microbiol. Rev. 2013, 26, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Burdo, T.H.; Lentz, M.R.; Autissier, P.; Krishnan, A.; Halpern, E.; Letendre, S.; Rosenberg, E.S.; Ellis, R.J.; Williams, K.C. Soluble cd163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J. Infect. Dis. 2011, 204, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, T.B.; Ertner, G.; Petersen, J.; Moller, H.J.; Moestrup, S.K.; Eugen-Olsen, J.; Kronborg, G.; Benfield, T. Plasma soluble cd163 level independently predicts all-cause mortality in HIV-1-infected individuals. J. Infect. Dis. 2016, 214, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by tlr4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Lagares, G.; Romero-Sanchez, M.C.; Ruiz-Mateos, E.; Genebat, M.; Ferrando-Martinez, S.; Munoz-Fernandez, M.A.; Pacheco, Y.M.; Leal, M. Long-term suppressive combined antiretroviral treatment does not normalize the serum level of soluble cd14. J. Infect. Dis. 2013, 207, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, J.A.; Dunne, E.; Gurwith, M.; Lambert, J.S.; Sheehan, G.J.; Feeney, E.R.; Pozniak, A.; Reiss, P.; Kenny, D.; Mallon, P. The effect of initiation of antiretroviral therapy on monocyte, endothelial and platelet function in HIV-1 infection. HIV Med. 2015, 16, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Longenecker, C.T.; Jiang, Y.; Orringer, C.E.; Gilkeson, R.C.; Debanne, S.; Funderburg, N.T.; Lederman, M.M.; Storer, N.; Labbato, D.E.; McComsey, G.A. Soluble cd14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014, 28, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Hanna, D.B.; Lin, J.; Post, W.S.; Hodis, H.N.; Xue, X.; Anastos, K.; Cohen, M.H.; Gange, S.J.; Haberlen, S.A.; Heath, S.L.; et al. Association of macrophage inflammation biomarkers with progression of subclinical carotid artery atherosclerosis in HIV-infected women and men. J. Infect. Dis. 2017, 215, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Sandler, N.G.; Wand, H.; Roque, A.; Law, M.; Nason, M.C.; Nixon, D.E.; Pedersen, C.; Ruxrungtham, K.; Lewin, S.R.; Emery, S.; et al. Plasma levels of soluble cd14 independently predict mortality in HIV infection. J. Infect. Dis. 2011, 203, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Kuller, L.H.; Tracy, R.; Belloso, W.; De Wit, S.; Drummond, F.; Lane, H.C.; Ledergerber, B.; Lundgren, J.; Neuhaus, J.; Nixon, D.; et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008, 5, e203. [Google Scholar] [CrossRef] [PubMed]
- Rodger, A.J.; Fox, Z.; Lundgren, J.D.; Kuller, L.H.; Boesecke, C.; Gey, D.; Skoutelis, A.; Goetz, M.B.; Phillips, A.N.; INSIGHT Strategies for Management of Antiretroviral Therapy (SMART) Study Group. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J. Infect. Dis. 2009, 200, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Smith, A.J.; Wietgrefe, S.W.; Southern, P.J.; Schacker, T.W.; Reilly, C.S.; Estes, J.D.; Burton, G.F.; Silvestri, G.; Lifson, J.D.; et al. Cumulative mechanisms of lymphoid tissue fibrosis and t cell depletion in HIV-1 and SIV infections. J. Clin. Investig. 2011, 121, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Schacker, T.W.; Nguyen, P.L.; Beilman, G.J.; Wolinsky, S.; Larson, M.; Reilly, C.; Haase, A.T. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J. Clin. Investig. 2002, 110, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Moysi, E.; Pallikkuth, S.; de Armas, L.R.; Gonzalez, L.E.; Ambrozak, D.; George, V.; Huddleston, D.; Pahwa, R.; Koup, R.A.; Petrovas, C.; et al. Altered immune cell follicular dynamics in HIV infection following influenza vaccination. J. Clin. Investig. 2018, 128, 3171–3185. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.L.; Hunt, P.W.; Reilly, C.S.; Hatano, H.; Beilman, G.J.; Khoruts, A.; Jasurda, J.S.; Somsouk, M.; Thorkelson, A.; Russ, S.; et al. Lymphoid fibrosis occurs in long-term nonprogressors and persists with antiretroviral therapy but may be reversible with curative interventions. J. Infect. Dis. 2015, 211, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Grund, B.; Baker, J.V.; Deeks, S.G.; Wolfson, J.; Wentworth, D.; Cozzi-Lepri, A.; Cohen, C.J.; Phillips, A.; Lundgren, J.D.; Neaton, J.D.; et al. Relevance of interleukin-6 and d-dimer for serious non-aids morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS ONE 2016, 11, e0155100. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.M.; Rajwans, N.; Jaoko, W.; Estambale, B.B.; McClelland, R.S.; Overbaugh, J.; Liles, W.C. Endothelial activation biomarkers increase after HIV-1 acquisition: Plasma vascular cell adhesion molecule-1 predicts disease progression. AIDS 2013, 27, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Fourie, C.M.; Schutte, A.E.; Smith, W.; Kruger, A.; van Rooyen, J.M. Endothelial activation and cardiometabolic profiles of treated and never-treated HIV infected africans. Atherosclerosis 2015, 240, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Mosepele, M.; Mohammed, T.; Mupfumi, L.; Moyo, S.; Bennett, K.; Lockman, S.; Hemphill, L.C.; Triant, V.A. HIV disease is associated with increased biomarkers of endothelial dysfunction despite viral suppression on long-term antiretroviral therapy in botswana. Cardiovasc. J. Afr. 2018, 29, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.; Hope-Lucas, C.; White, T.; Hairston, T.K.; Rameau, T.; Brown, A. Cortical neurons are a prominent source of the proinflammatory cytokine osteopontin in HIV-associated neurocognitive disorders. J. Neurovirol. 2015, 21, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Bandera, A.; Ferrario, G.; Saresella, M.; Marventano, I.; Soria, A.; Zanini, F.; Sabbatini, F.; Airoldi, M.; Marchetti, G.; Franzetti, F.; et al. Cd4+ t cell depletion, immune activation and increased production of regulatory t cells in the thymus of HIV-infected individuals. PLoS ONE 2010, 5, e10788. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.L.; Shive, C.L.; Nguyen, T.P.; Younes, S.A.; Panigrahi, S.; Lederman, M.M. Cytokines and t-cell homeostasis in HIV infection. J. Infect. Dis. 2016, 214 (Suppl. 2), S51–S57. [Google Scholar] [CrossRef]
- De Voeght, A.; Martens, H.; Renard, C.; Vaira, D.; Debruche, M.; Simonet, J.; Geenen, V.; Moutschen, M.; Darcis, G. Exploring the link between innate immune activation and thymic function by measuring scd14 and trecs in hiv patients living in belgium. PLoS ONE 2017, 12, e0185761. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, C.; Hao, Z.; Rajewsky, K.; Potocnik, A.J.; Stockinger, B. Ablation of thymic export causes accelerated decay of naive cd4 t cells in the periphery because of activation by environmental antigen. Proc. Natl. Acad. Sci. USA 2008, 105, 8691–8696. [Google Scholar] [CrossRef] [PubMed]
- Marquez, M.; Romero-Cores, P.; Montes-Oca, M.; Martin-Aspas, A.; Soto-Cardenas, M.J.; Guerrero, F.; Fernandez-Gutierrez, C.; Giron-Gonzalez, J.A. Immune activation response in chronic HIV-infected patients: Influence of hepatitis c virus coinfection. PLoS ONE 2015, 10, e0119568. [Google Scholar] [CrossRef] [PubMed]
- Maidji, E.; Somsouk, M.; Rivera, J.M.; Hunt, P.W.; Stoddart, C.A. Replication of cmv in the gut of HIV-infected individuals and epithelial barrier dysfunction. PLoS Pathog. 2017, 13, e1006202. [Google Scholar] [CrossRef] [PubMed]
- Gianella, S.; Anderson, C.M.; Var, S.R.; Oliveira, M.F.; Lada, S.M.; Vargas, M.V.; Massanella, M.; Little, S.J.; Richman, D.D.; Strain, M.C.; et al. Replication of human herpesviruses is associated with higher HIV DNA levels during antiretroviral therapy started at early phases of HIV infection. J. Virol. 2016, 90, 3944–3952. [Google Scholar] [CrossRef] [PubMed]
- Gianella, S.; Massanella, M.; Richman, D.D.; Little, S.J.; Spina, C.A.; Vargas, M.V.; Lada, S.M.; Daar, E.S.; Dube, M.P.; Haubrich, R.H.; et al. Cytomegalovirus replication in semen is associated with higher levels of proviral HIV DNA and cd4+ t cell activation during antiretroviral treatment. J. Virol. 2014, 88, 7818–7827. [Google Scholar] [CrossRef] [PubMed]
- Henrich, T.J.; Hobbs, K.S.; Hanhauser, E.; Scully, E.; Hogan, L.E.; Robles, Y.P.; Leadabrand, K.S.; Marty, F.M.; Palmer, C.D.; Jost, S.; et al. Human immunodeficiency virus type 1 persistence following systemic chemotherapy for malignancy. J. Infect. Dis. 2017, 216, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Christensen-Quick, A.; Vanpouille, C.; Lisco, A.; Gianella, S. Cytomegalovirus and HIV persistence: Pouring gas on the fire. AIDS Res. Hum. Retroviruses 2017, 33, S23–S30. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Leung, J.M.; Ngan, D.A.; Nashta, N.F.; Guillemi, S.; Harris, M.; Lima, V.D.; Um, S.J.; Li, Y.; Tam, S.; et al. Absolute leukocyte telomere length in HIV-infected and uninfected individuals: Evidence of accelerated cell senescence in HIV-associated chronic obstructive pulmonary disease. PLoS ONE 2015, 10, e0124426. [Google Scholar] [CrossRef] [PubMed]
- Appay, V.; Almeida, J.R.; Sauce, D.; Autran, B.; Papagno, L. Accelerated immune senescence and HIV-1 infection. Exp. Gerontol. 2007, 42, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Dock, J.N.; Effros, R.B. Role of cd8 T cell replicative senescence in human aging and in HIV-mediated immunosenescence. Aging Dis. 2011, 2, 382–397. [Google Scholar] [PubMed]
- Cohen, J.; Torres, C. HIV-associated cellular senescence: A contributor to accelerated aging. Ageing Res. Rev. 2017, 36, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Wing, E.J. HIV and aging. Int. J. Infect. Dis. 2016, 53, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Landay, A. Early immune senescence in HIV disease. Curr. HIV/AIDS Rep. 2010, 7, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.; French, M.A.; Price, P. Immunosenescent cd57+cd4+ T-cells accumulate and contribute to interferon-gamma responses in HIV patients responding stably to art. Dis. Mark. 2011, 31, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Gianesin, K.; Noguera-Julian, A.; Zanchetta, M.; Del Bianco, P.; Petrara, M.R.; Freguja, R.; Rampon, O.; Fortuny, C.; Camos, M.; Mozzo, E.; et al. Premature aging and immune senescence in HIV-infected children. AIDS 2016, 30, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.; Mendez-Lagares, G.; Correa-Rocha, R.; Pacheco, Y.M.; Ferrando-Martinez, S.; Ruiz-Mateos, E.; del Mar del Pozo-Balado, M.; Leon, J.A.; Gurbindo, M.D.; Isabel de Jose, M.; et al. Detectable viral load aggravates immunosenescence features of cd8 T-cell subsets in vertically HIV-infected children. J. Acquir. Immune Defic. Syndr. 2012, 60, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Appay, V.; Sauce, D. Assessing immune aging in HIV-infected patients. Virulence 2017, 8, 529–538. [Google Scholar] [CrossRef] [PubMed]
- George, V.K.; Pallikkuth, S.; Parmigiani, A.; Alcaide, M.; Fischl, M.; Arheart, K.L.; Pahwa, S. HIV infection worsens age-associated defects in antibody responses to influenza vaccine. J. Infect. Dis. 2015, 211, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.F.; Mellors, J.W. Changes in HIV reservoirs during long-term antiretroviral therapy. Curr. Opin. HIV AIDS 2015, 10, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Deeks, S.G. The benefits of early antiretroviral therapy for HIV infection: How early is early enough? EBio Med. 2016, 11, 7–8. [Google Scholar] [CrossRef] [PubMed]
- De Paula, H.H.S.; Ferreira, A.C.G.; Caetano, D.G.; Delatorre, E.; Teixeira, S.L.M.; Coelho, L.E.; Joao, E.G.; de Andrade, M.M.; Cardoso, S.W.; Grinsztejn, B.; et al. Reduction of inflammation and t cell activation after 6 months of cart initiation during acute, but not in early chronic HIV-1 infection. Retrovirology 2018, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Allers, K.; Puyskens, A.; Epple, H.J.; Schurmann, D.; Hofmann, J.; Moos, V.; Schneider, T. The effect of timing of antiretroviral therapy on cd4+ t-cell reconstitution in the intestine of HIV-infected patients. Mucosal. Immunol. 2016, 9, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Ghislain, M.; Bastard, J.P.; Meyer, L.; Capeau, J.; Fellahi, S.; Gerard, L.; May, T.; Simon, A.; Vigouroux, C.; Goujard, C.; et al. Late antiretroviral therapy (art) initiation is associated with long-term persistence of systemic inflammation and metabolic abnormalities. PLoS ONE 2015, 10, e0144317. [Google Scholar] [CrossRef] [PubMed]
- Amu, S.; Lantto Graham, R.; Bekele, Y.; Nasi, A.; Bengtsson, C.; Rethi, B.; Sorial, S.; Meini, G.; Zazzi, M.; Hejdeman, B.; et al. Dysfunctional phenotypes of cd4+ and cd8+ t cells are comparable in patients initiating art during early or chronic HIV-1 infection. Medicine 2016, 95, e3738. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; de Spiegelaere, W.; Cozzi-Lepri, A.; Kiselinova, M.; Pollakis, G.; Beloukas, A.; Vandekerckhove, L.; Strain, M.; Richman, D.; Phillips, A.; et al. During stably suppressive antiretroviral therapy integrated HIV-1 DNA load in peripheral blood is associated with the frequency of cd8 cells expressing hla-dr/dp/dq. EBio Med. 2015, 2, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Cozzi-Lepri, A.; Beloukas, A.; Richman, D.; Khoo, S.; Phillips, A.; Geretti, A.M.; Group, E.S. Factors associated with persistence of plasma HIV-1 RNA during long-term continuously suppressive firstline antiretroviral therapy. Open Forum Infect. Dis. 2018, 5, ofy032. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.; Zangari, P.; Alteri, C.; Tchidjou, H.K.; Manno, E.C.; Liuzzi, G.; Perno, C.F.; Rossi, P.; Bertoli, A.; Bernardi, S. Early antiretroviral treatment (eart) limits viral diversity over time in a long-term HIV viral suppressed perinatally infected child. BMC Infect. Dis. 2016, 16, 742. [Google Scholar] [CrossRef] [PubMed]
- Cotugno, N.; Douagi, I.; Rossi, P.; Palma, P. Suboptimal immune reconstitution in vertically hiv infected children: A view on how HIV replication and timing of haart initiation can impact on t and b-cell compartment. Clin. Dev. Immunol. 2012, 2012, 805151. [Google Scholar] [CrossRef] [PubMed]
- Tagarro, A.; Chan, M.; Zangari, P.; Ferns, B.; Foster, C.; De Rossi, A.; Nastouli, E.; Munoz-Fernandez, M.A.; Gibb, D.; Rossi, P.; et al. Early and highly suppressive antiretroviral therapy are main factors associated with low viral reservoir in european perinatally HIV-infected children. J. Acquir. Immune Defic. Syndr. 2018, 79, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.; Palma, P.; Luzuriaga, K.; Pahwa, S.; Nastouli, E.; Gibb, D.M.; Rojo, P.; Borkowsky, W.; Bernardi, S.; Zangari, P.; et al. Early antiretroviral therapy in children perinatally infected with HIV: A unique opportunity to implement immunotherapeutic approaches to prolong viral remission. Lancet. Infect. Dis. 2015, 15, 1108–1114. [Google Scholar] [CrossRef]
- Schuetz, A.; Deleage, C.; Sereti, I.; Rerknimitr, R.; Phanuphak, N.; Phuang-Ngern, Y.; Estes, J.D.; Sandler, N.G.; Sukhumvittaya, S.; Marovich, M.; et al. Initiation of art during early acute HIV infection preserves mucosal th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014, 10, e1004543. [Google Scholar] [CrossRef] [PubMed]
- Ryom, L.; Boesecke, C.; Bracchi, M.; Ambrosioni, J.; Pozniak, A.; Arribas, J.; Behrens, G.; Mallon, P.; Puoti, M.; Rauch, A.; et al. Highlights of the 2017 european aids clinical society (eacs) guidelines for the treatment of adult HIV-positive persons version 9.0. HIV Med. 2018, 19, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Bamford, A.; Turkova, A.; Lyall, H.; Foster, C.; Klein, N.; Bastiaans, D.; Burger, D.; Bernadi, S.; Butler, K.; Chiappini, E.; et al. Paediatric european network for treatment of AIDS (penta) guidelines for treatment of paediatric HIV-1 infection 2015: Optimizing health in preparation for adult life. HIV Med. 2018, 19, e1–e42. [Google Scholar] [CrossRef] [PubMed]
- Bourgi, K.; Wanjalla, C.; Koethe, J.R. Inflammation and metabolic complications in HIV. Curr. HIV/AIDS Rep. 2018, 15, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Swami, A. Metabolic syndrome and HIV infection. J. HIV Retrovirus 2016, 2, 1. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative stress during HIV infection: Mechanisms and consequences. Oxid. Med. Cell. Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Lagathu, C.; Cossarizza, A.; Bereziat, V.; Nasi, M.; Capeau, J.; Pinti, M. Basic science and pathogenesis of ageing with HIV: Potential mechanisms and biomarkers. AIDS 2017, 31 (Suppl. 2), S105–S119. [Google Scholar] [CrossRef]
- Schoeman, J.C.; Moutloatse, G.P.; Harms, A.C.; Vreeken, R.J.; Scherpbier, H.J.; van Leeuwen, L.; Kuijpers, T.W.; Reinecke, C.J.; Berger, R.; Hankemeier, T.; et al. Fetal metabolic stress disrupts immune homeostasis and induces proinflammatory responses in human immunodeficiency virus type 1- and combination antiretroviral therapy-exposed infants. J. Infect. Dis. 2017, 216, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Theengh, D.P.; Yadav, P.; Jain, A.K.; Nandy, P. Assessment of metabolic syndrome in HIV-infected individuals. Indian J. Sex. Transm. Dis. AIDS 2017, 38, 152–156. [Google Scholar] [PubMed]
- Nguyen, K.A.; Peer, N.; Mills, E.J.; Kengne, A.P. A meta-analysis of the metabolic syndrome prevalence in the global HIV-infected population. PLoS ONE 2016, 11, e0150970. [Google Scholar] [CrossRef] [PubMed]
- Rogalska-Plonska, M.; Grzeszczuk, A.; Rogalski, P.; Lucejko, M.; Flisiak, R. Metabolic syndrome in HIV infected adults in poland. Kardiol. Pol. 2018, 76, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Bedimo, R.; Abodunde, O. Metabolic and cardiovascular complications in HIV/HCV-co-infected patients. Curr. HIV/AIDS Rep. 2016, 13, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.; Naidu, G.; Davies, M.A.; Bohlius, J. HIV-associated malignancies in children. Curr. Opin. HIV AIDS 2017, 12, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ivy, W.; Nesheim, S.R.; Paul, S.M.; Ibrahim, A.R.; Chan, M.; Niu, X.; Lampe, M.A. Cancer among children with perinatal exposure to HIV and antiretroviral medications--new jersey, 1995–2010. J. Acquir. Immune Defic. Syndr. 2015, 70, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.H.; Dubrow, R.; Silverberg, M.J. Factors contributing to risk for cancer among HIV-infected individuals, and evidence that earlier combination antiretroviral therapy will alter this risk. Curr. Opin. HIV AIDS 2014, 9, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Caccuri, F.; Rueckert, C.; Giagulli, C.; Schulze, K.; Basta, D.; Zicari, S.; Marsico, S.; Cervi, E.; Fiorentini, S.; Slevin, M.; et al. HIV-1 matrix protein p17 promotes lymphangiogenesis and activates the endothelin-1/endothelin b receptor axis. Arterioscler. Thromb. Vasc. Biol 2014, 34, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Aids-Defining Conditions. Available online: https://www.cdc.gov/MMWR/PREVIEW/MMWRHTML/rr5710a2.htm (accessed on 5 December 2008).
- Vaccher, E.; Serraino, D.; Carbone, A.; de Paoli, P. The evolving scenario of non-aids-defining cancers: Challenges and opportunities of care. Oncologist 2014, 19, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Silverberg, M.J.; Abrams, D.I. Non-AIDS-defining malignancies in the HIV-infected population. Curr. Infect. Dis. Rep. 2014, 16, 406. [Google Scholar] [CrossRef] [PubMed]
- Zangari, P.; Santilli, V.; Cotugno, N.; Manno, E.; Palumbo, G.; Lombardi, A.; de Vito, R.; Tchidjou, H.; Baldassari, S.; Ariganello, P.; et al. Raising awareness of non-hodgkin lymphoma in HIV-infected adolescents: Report of 2 cases in the haart era. J. Pediatr. Hematol. Oncol. 2013, 35, e134–e137. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, M.B.; Sterling, R.K. Mechanisms of liver disease in patients infected with HIV. BMJ Open Gastroenterol. 2017, 4, e000166. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, S.; Fitch, K.V.; Wong, K.; O’Malley, T.K.; Maehler, P.; Branch, K.L.; Looby, S.E.; Burdo, T.H.; Martinez-Salazar, E.L.; Torriani, M.; et al. Randomized, placebo-controlled trial to evaluate effects of eplerenone on metabolic and inflammatory indices in hiv. J. Clin. Endocrinol. Metab. 2018, 103, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Perazzo, H.; Cardoso, S.W.; Yanavich, C.; Nunes, E.P.; Morata, M.; Gorni, N.; da Silva, P.S.; Cardoso, C.; Almeida, C.; Luz, P.; et al. Predictive factors associated with liver fibrosis and steatosis by transient elastography in patients with HIV mono-infection under long-term combined antiretroviral therapy. J. Int. AIDS Soc. 2018, 21, e25201. [Google Scholar] [CrossRef] [PubMed]
- Phalane, E.; Fourie, C.M.T.; Schutte, A.E. The metabolic syndrome and renal function in an african cohort infected with human immunodeficiency virus. South. Afr. J. HIV Med. 2018, 19, 813. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, G.; Srdic, D.; Al Musalhi, K.; Soldatovic, I.; Kusic, J.; Jevtovic, D.; Nair, D. Higher levels of cystatin c in HIV/AIDS patients with metabolic syndrome. Basic Clin. Pharmacol. Toxicol. 2018, 122, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Husain, N.E.; Noor, S.K.; Elmadhoun, W.M.; Almobarak, A.O.; Awadalla, H.; Woodward, C.L.; Mital, D.; Ahmed, M.H. Diabetes, metabolic syndrome and dyslipidemia in people living with HIV in Africa: Re-emerging challenges not to be forgotten. HIV/AIDS 2017, 9, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Pedro, M.N.; Rocha, G.Z.; Guadagnini, D.; Santos, A.; Magro, D.O.; Assalin, H.B.; Oliveira, A.G.; Pedro, R.J.; Saad, M.J.A. Insulin resistance in HIV-patients: Causes and consequences. Front. Endocrinol. 2018, 9, 514. [Google Scholar] [CrossRef] [PubMed]
- Hadigan, C.; Corcoran, C.; Stanley, T.; Piecuch, S.; Klibanski, A.; Grinspoon, S. Fasting hyperinsulinemia in human immunodeficiency virus-infected men: Relationship to body composition, gonadal function, and protease inhibitor use. J. Clin. Endocrinol. Metab. 2000, 85, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Fahme, S.A.; Bloomfield, G.S.; Peck, R. Hypertension in HIV-infected adults: Novel pathophysiologic mechanisms. Hypertension 2018, 72, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, X.; Wang, K. Global prevalence of hypertension among people living with HIV: A systematic review and meta-analysis. J. Am. Soc. Hypertens. 2017, 11, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Idiculla, J.; Swaroop, N.; Shastri, S.; George, N.; Rewari, B.B.; Shet, A. Metabolic syndrome and cardiovascular disease risk assessment among human immunodeficiency virus-infected individuals on antiretroviral therapy. Indian J. Sex. Transm. Dis. AIDS 2018, 39, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Dube, M.P.; Lipshultz, S.E.; Fichtenbaum, C.J.; Greenberg, R.; Schecter, A.D.; Fisher, S.D.; Working, G. Effects of HIV infection and antiretroviral therapy on the heart and vasculature. Circulation 2008, 118, e36–e40. [Google Scholar] [CrossRef] [PubMed]
- Chow, F.C.; Price, R.W.; Hsue, P.Y.; Kim, A.S. Greater risk of stroke of undetermined etiology in a contemporary HIV-infected cohort compared with uninfected individuals. J. Stroke Erebrovasc Dis. 2017, 26, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Scutari, R.; Alteri, C.; Perno, C.F.; Svicher, V.; Aquaro, S. The role of HIV infection in neurologic injury. Brain Sci. 2017, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Obirikorang, C.; Quaye, L.; Osei-Yeboah, J.; Odame, E.A.; Asare, I. Prevalence of metabolic syndrome among hiv-infected patients in ghana: A cross-sectional study. Niger. Med. J. 2016, 57, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.L.; Gala, P.; Rochford, R.; Glesby, M.J.; Mehta, S. HIV/AIDS and lipodystrophy: Implications for clinical management in resource-limited settings. J. Int. AIDS Soc. 2015, 18, 19033. [Google Scholar] [CrossRef] [PubMed]
- Sacilotto, L.B.; Pereira, P.C.M.; Manechini, J.P.V.; Papini, S.J. Body composition and metabolic syndrome components on lipodystrophy different subtypes associated with HIV. J. Nutr. Metab. 2017, 2017, 8260867. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.; Carvalho, D.; Santos, A.C.; Matos, M.J.; Madureira, A.J.; Marques, R.; Martinez, E.; Sarmento, A.; Medina, J.L. Prevalence of obesity and its relationship to clinical lipodystrophy in HIV-infected adults on anti-retroviral therapy. J. Endocrinol. Invest. 2012, 35, 964–970. [Google Scholar] [PubMed]
- Koethe, J.R.; Grome, H.; Jenkins, C.A.; Kalams, S.A.; Sterling, T.R. The metabolic and cardiovascular consequences of obesity in persons with HIV on long-term antiretroviral therapy. AIDS 2016, 30, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.E. The fat of the matter: Obesity and visceral adiposity in treated HIV infection. Curr. HIV/AIDS Rep. 2017, 14, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Antonello, V.S.; Antonello, I.C.; Grossmann, T.K.; Tovo, C.V.; Pupo, B.B.; Winckler Lde, Q. Hypertension--an emerging cardiovascular risk factor in HIV infection. J. Am. Soc. of Hypertens. 2015, 9, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Dimala, C.A.; Atashili, J.; Mbuagbaw, J.C.; Wilfred, A.; Monekosso, G.L. Prevalence of hypertension in HIV/AIDS patients on highly active antiretroviral therapy (haart) compared with haart-naive patients at the limbe regional hospital, cameroon. PLoS ONE 2016, 11, e0148100. [Google Scholar] [CrossRef] [PubMed]
- Cibrian-Ponce, A.; Sanchez-Aleman, M.A.; Garcia-Jimenez, S.; Perez-Martinez, E.; Bernal-Fernandez, G.; Castanon-Mayo, M.; Avila-Jimenez, L.; Toledano-Jaimes, C.D. Changes in cardiovascular risk and clinical outcomes in a HIV/AIDS cohort study over a 1-year period at a specialized clinic in mexico. Ther. Clin. Risk Manag. 2018, 14, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.; Sabin, C.A.; Mallon, P.W.G.; Winston, A.; Tariq, S. Cardiovascular disease in women living with HIV: A narrative review. Maturitas 2018, 108, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.A.; Peer, N.; de Villiers, A.; Mukasa, B.; Matsha, T.E.; Mills, E.J.; Kengne, A.P. Metabolic syndrome in people living with human immunodeficiency virus: An assessment of the prevalence and the agreement between diagnostic criteria. Int. J. Endocrinol. 2017, 2017, 1613657. [Google Scholar] [CrossRef] [PubMed]
- Arrive, E.; Viard, J.P.; Salanave, B.; Dollfus, C.; Matheron, S.; Reliquet, V.; Arezes, E.; Nailler, L.; Vigouroux, C.; Warszawski, J.; et al. Metabolic risk factors in young adults infected with HIV since childhood compared with the general population. PLoS ONE 2018, 13, e0206745. [Google Scholar] [CrossRef] [PubMed]
- Tarr, P.E.; Telenti, A. Genetic screening for metabolic and age-related complications in HIV-infected persons. F1000 Med. Rep. 2010, 2, 83. [Google Scholar] [PubMed]
- Haas, D.W.; Tarr, P.E. Perspectives on pharmacogenomics of antiretroviral medications and HIV-associated comorbidities. Curr. Opin. HIV AIDS 2015, 10, 116–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patel, K.; Hernan, M.A.; Williams, P.L.; Seeger, J.D.; McIntosh, K.; Dyke, R.B.; Seage, G.R., 3rd; Pediatric AIDS Clinical Trials Group 219/219C Study Team. Long-term effects of highly active antiretroviral therapy on cd4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin. Infect. Dis. 2008, 46, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.S.; Doerholt, K.; Sharland, M.; Gibb, D.M.; Collaborative HIV Paediatric Study (CHIPS) Steering Committee. Response to highly active antiretroviral therapy varies with age: The UK and ireland collaborative HIV paediatric study. AIDS 2004, 18, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.J.; Klenerman, P.; Goulder, P.J. The impact of differential antiviral immunity in children and adults. Nature Rev. Immunol. 2012, 12, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Roider, J.M.; Muenchhoff, M.; Goulder, P.J. Immune activation and paediatric HIV-1 disease outcome. Curr. Opin. HIV AIDS 2016, 11, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.; Foster, C.; Rojo, P.; Zangari, P.; Yates, A.; Cotugno, N.; Klein, N.; Luzuriaga, K.; Pahwa, S.; Nastouli, E.; et al. The epiical project: An emerging global collaboration to investigate immunotherapeutic strategies in HIV-infected children. J. Virus Erad. 2015, 1, 134–139. [Google Scholar] [PubMed]
- Chiappini, E.; Bianconi, M.; Dalzini, A.; Petrara, M.R.; Galli, L.; Giaquinto, C.; de Rossi, A. Accelerated aging in perinatally HIV-infected children: Clinical manifestations and pathogenetic mechanisms. Aging 2018, 10, 3610–3625. [Google Scholar] [CrossRef] [PubMed]
- Koay, W.L.A.; Lindsey, J.C.; Uprety, P.; Bwakura-Dangarembizi, M.; Weinberg, A.; Levin, M.J.; Persaud, D. Intestinal integrity biomarkers in early antiretroviral-treated perinatally HIV-1-infected infants. J. Infect. Dis. 2018, 218, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Sessa, L.; Reddel, S.; Manno, E.; Quagliariello, A.; Cotugno, N.; Del Chierico, F.; Amodio, D.; Capponi, C.; Leone, F.; Bernardi, S.; et al. Distinct gut microbiota profile in art-treated perinatally HIV-infected patients associated with cardiac and inflammatory biomarkers. AIDS 2019. [Google Scholar] [CrossRef] [PubMed]
- Hazra, R.; Siberry, G.K.; Mofenson, L.M. Growing up with HIV: Children, adolescents, and young adults with perinatally acquired HIV infection. Annu. Rev. Med. 2010, 61, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.T.; Oleske, J.M.; Williams, P.L.; Elgie, C.; Mofenson, L.M.; Dankner, W.M.; van Dyke, R.B.; Pediatric AIDS Clinical Trials Group219/219C Team. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the haart era. J. Acquir. Immune Defic. Syndr. 2010, 53, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Augustemak de Lima, L.R.; Petroski, E.L.; Moreno, Y.M.F.; Silva, D.A.S.; Trindade, E.; Carvalho, A.P.; Back, I.C. Dyslipidemia, chronic inflammation, and subclinical atherosclerosis in children and adolescents infected with HIV: The posithive health study. PLoS ONE 2018, 13, e0190785. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, P.; Mwamzuka, M.; Marshed, F.; Kravietz, A.; Ilmet, T.; Ahmed, A.; Borkowsky, W.; Khaitan, A. Immune activation despite preserved cd4 t cells in perinatally HIV-infected children and adolescents. PLoS ONE 2017, 12, e0190332. [Google Scholar] [CrossRef] [PubMed]
- Ikomey, G.; Assoumou, M.C.; Atashili, J.; Mesembe, M.; Mukwele, B.; Lyonga, E.; Eyoh, A.; Kafando, A.; Ndumbe, P.M. The potentials of fas receptors and ligands in monitoring HIV-1 disease in children in yaounde, cameroon. J. Int. Asso. Prov. AIDS Care 2016, 15, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, L.; Janbazian, L.; Chomont, N.; Said, E.A.; Gimmig, S.; Bessette, B.; Boulassel, M.R.; Delwart, E.; Sepulveda, H.; Balderas, R.S.; et al. Upregulation of pd-1 expression on HIV-specific cd8+ t cells leads to reversible immune dysfunction. Nat. Med. 2006, 12, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, N.; Abel, B.; Scriba, T.J.; Hughes, J.; de Kock, M.; Tameris, M.; Mlenjeni, S.; Denation, L.; Little, F.; Gelderbloem, S.; et al. Significantly skewed memory cd8+ t cell subsets in HIV-1 infected infants during the first year of life. Clin. Immunol. 2009, 130, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Pallikkuth, S.; George, V.K.; de Armas, L.R.; Pahwa, R.; Sanchez, C.M.; Pallin, M.F.; Pan, L.; Cotugno, N.; Dickinson, G.; et al. Paradoxical aging in HIV: Immune senescence of b cells is most prominent in young age. Aging 2017, 9, 1307–1325. [Google Scholar] [CrossRef] [PubMed]
- Cagigi, A.; Rinaldi, S.; di Martino, A.; Manno, E.C.; Zangari, P.; Aquilani, A.; Cotugno, N.; Nicolosi, L.; Villani, A.; Bernardi, S.; et al. Premature immune senescence during HIV-1 vertical infection relates with response to influenza vaccination. J. Allergy Clin. Immunol. 2014, 133, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.; Rinaldi, S.; Cotugno, N.; Santilli, V.; Pahwa, S.; Rossi, P.; Cagigi, A. Premature b-cell senescence as a consequence of chronic immune activation. Hum. Vaccin. Immunother. 2014, 10, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Cagigi, A.; Rinaldi, S.; Santilli, V.; Mora, N.; Manno, E.C.; Cotugno, N.; Zangari, P.; Aquilani, A.; Guzzo, I.; Dello Strologo, L.; et al. Premature ageing of the immune system relates to increased anti-lymphocyte antibodies (ala) after an immunization in HIV-1-infected and kidney-transplanted patients. Clin. Exp. Immunol. 2013, 174, 274–280. [Google Scholar] [PubMed]
- Cotugno, N.; de Armas, L.; Pallikkuth, S.; Rinaldi, S.; Issac, B.; Cagigi, A.; Rossi, P.; Palma, P.; Pahwa, S. Perturbation of b cell gene expression persists in HIV-infected children despite effective antiretroviral therapy and predicts h1n1 response. Front. Immunol. 2017, 8, 1083. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, E.; Berti, E.; Gianesin, K.; Petrara, M.R.; Galli, L.; Giaquinto, C.; de Martino, M.; de Rossi, A. Pediatric human immunodeficiency virus infection and cancer in the highly active antiretroviral treatment (haart) era. Cancer let. 2014, 347, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.I.; Sued, O.G.; Gun, A.M.; Belloso, W.H.; Cecchini, D.M.; Lopardo, G.; Pryluka, D.; Rolon, M.J.; Fink, V.I.; Lloret, S.P.; Cahn, P. Drv/r/3tc fdc for HIV-1 treatment naive patients: Week 48 results of the andes study. In Proceedings of the Conference on Retroviruses and Oppurtunistic Infections (CROI), Boston, MA, USA, 4–7 March 2018. [Google Scholar]
- Cahn, P.; Madero, J.S.; Arribas, J.R.; Antinori, A.; Ortiz, R.; Clarke, A.E.; Hung, C.C.; Rockstroh, J.K.; Girard, P.M.; Sievers, J.; et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (gemini-1 and gemini-2): Week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019, 393, 143–155. [Google Scholar] [PubMed]
- Llibre, J.M.; Hung, C.C.; Brinson, C.; Castelli, F.; Girard, P.M.; Kahl, L.P.; Blair, E.A.; Angelis, K.; Wynne, B.; Vandermeulen, K.; et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: Phase 3, randomised, non-inferiority sword-1 and sword-2 studies. Lancet 2018, 391, 839–849. [Google Scholar] [CrossRef]
- Ruzagira, E.; Baisley, K.; Kamali, A.; Biraro, S.; Grosskurth, H.; Working Group on Linkage to HIV Care. Linkage to HIV care after home-based HIV counselling and testing in sub-saharan africa: A systematic review. Trop. Med. Int. Health 2017, 22, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Quiros-Roldan, E.; Magro, P.; Raffetti, E.; Izzo, I.; Borghetti, A.; Lombardi, F.; Saracino, A.; Maggiolo, F.; Castelli, F.; Cohort, M. Biochemical and inflammatory modifications after switching to dual antiretroviral therapy in HIV-infected patients in Italy: A multicenter retrospective cohort study from 2007 to 2015. BMC Infect. Dis. 2018, 18, 285. [Google Scholar] [CrossRef] [PubMed]
- Pontrelli, G.; Cotugno, N.; Amodio, D.; Zangari, P.; Tchidjou, H.K.; Baldassari, S.; Palma, P.; Bernardi, S. Renal function in HIV-infected children and adolescents treated with tenofovir disoproxil fumarate and protease inhibitors. BMC Infect. Dis. 2012, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Bedimo, R.; Maalouf, N.M.; Zhang, S.; Drechsler, H.; Tebas, P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS 2012, 26, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Paton, N.I.; Stohr, W.; Oddershede, L.; Arenas-Pinto, A.; Walker, S.; Sculpher, M.; Dunn, D.T. The protease inhibitor monotherapy versus ongoing triple therapy (pivot) trial: A randomised controlled trial of a protease inhibitor monotherapy strategy for long-term management of human immunodeficiency virus infection. Health Technol. Assess. 2016, 20, 1–158. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, C.; Mouzaki, A. Immunosuppressive drugs in HIV disease. Curr. Top. Med. Chem. 2006, 6, 1769–1789. [Google Scholar] [CrossRef] [PubMed]
- Bandera, A.; Colella, E.; Rizzardini, G.; Gori, A.; Clerici, M. Strategies to limit immune-activation in HIV patients. Exp. Rev. Anti-Infect. Ther. 2017, 15, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Soare, A.Y.; Durham, N.D.; Gopal, R.; Tweel, B.; Hoffman, K.W.; Brown, J.A.; O’Brien, M.; Bhardwaj, N.; Lim, J.K.; Chen, B.K.; et al. P2x antagonists inhibit hiv-1 productive infection and inflammatory cytokines interleukin-10 (il-10) and il-1beta in a human tonsil explant model. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Chastain, D.B.; Stover, K.R.; Riche, D.M. Evidence-based review of statin use in patients with HIV on antiretroviral therapy. J. Clin. Transl. Endocrinol. 2017, 8, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Nakanjako, D.; Ssinabulya, I.; Nabatanzi, R.; Bayigga, L.; Kiragga, A.; Joloba, M.; Kaleebu, P.; Kambugu, A.D.; Kamya, M.R.; Sekaly, R.; et al. Atorvastatin reduces t-cell activation and exhaustion among HIV-infected cart-treated suboptimal immune responders in Uganda: A randomised crossover placebo-controlled trial. Trop. Med. Int. Health 2015, 20, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.M.; Fitch, K.V.; Grinspoon, S.K. HIV-related cardiovascular disease, statins, and the reprieve trial. Top. Antiviral Med. 2015, 23, 146–149. [Google Scholar]
- Hsue, P.; Deeks, S.G.; Ishai, A.E.; Hur, S.; Li, D.; Sterman, F.; Lalezari, J.; Rupert, A.; Ganz, P.; Tawakol, A. Il-1β inhibition significantly reduces atherosclerotic inflammation in treated HIV. In Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, WA, USA, 13–16 February 2017. [Google Scholar]
- Navarro-Gonzalez, J.F.; Mora-Fernandez, C.; Muros de Fuentes, M.; Donate-Correa, J.; Cazana-Perez, V.; Garcia-Perez, J. Effect of phosphate binders on serum inflammatory profile, soluble cd14, and endotoxin levels in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2272–2279. [Google Scholar] [CrossRef] [PubMed]
- Sandler, N.G.; Zhang, X.; Bosch, R.J.; Funderburg, N.T.; Choi, A.I.; Robinson, J.K.; Fine, D.M.; Coombs, R.W.; Jacobson, J.M.; Landay, A.L.; et al. Sevelamer does not decrease lipopolysaccharide or soluble cd14 levels but decreases soluble tissue factor, low-density lipoprotein (ldl) cholesterol, and oxidized ldl cholesterol levels in individuals with untreated HIV infection. J. Infect. Dis. 2014, 210, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Piconi, S.; Parisotto, S.; Rizzardini, G.; Passerini, S.; Terzi, R.; Argenteri, B.; Meraviglia, P.; Capetti, A.; Biasin, M.; Trabattoni, D.; et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood 2011, 118, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Routy, J.P.; Angel, J.B.; Patel, M.; Kanagaratham, C.; Radzioch, D.; Kema, I.; Gilmore, N.; Ancuta, P.; Singer, J.; Jenabian, M.A. Assessment of chloroquine as a modulator of immune activation to improve cd4 recovery in immune nonresponding HIV-infected patients receiving antiretroviral therapy. HIV Med. 2015, 16, 48–56. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 11, 200. https://doi.org/10.3390/v11030200
Zicari S, Sessa L, Cotugno N, Ruggiero A, Morrocchi E, Concato C, Rocca S, Zangari P, Manno EC, Palma P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses. 2019; 11(3):200. https://doi.org/10.3390/v11030200
Chicago/Turabian StyleZicari, Sonia, Libera Sessa, Nicola Cotugno, Alessandra Ruggiero, Elena Morrocchi, Carlo Concato, Salvatore Rocca, Paola Zangari, Emma C. Manno, and Paolo Palma. 2019. "Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART" Viruses 11, no. 3: 200. https://doi.org/10.3390/v11030200
APA StyleZicari, S., Sessa, L., Cotugno, N., Ruggiero, A., Morrocchi, E., Concato, C., Rocca, S., Zangari, P., Manno, E. C., & Palma, P. (2019). Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses, 11(3), 200. https://doi.org/10.3390/v11030200




