A New Genotype of Feline Morbillivirus Infects Primary Cells of the Lung, Kidney, Brain and Peripheral Blood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Isolation of Primary Feline Cells
2.3. Collection of Samples, Virus Isolation and Virus Infection Assays
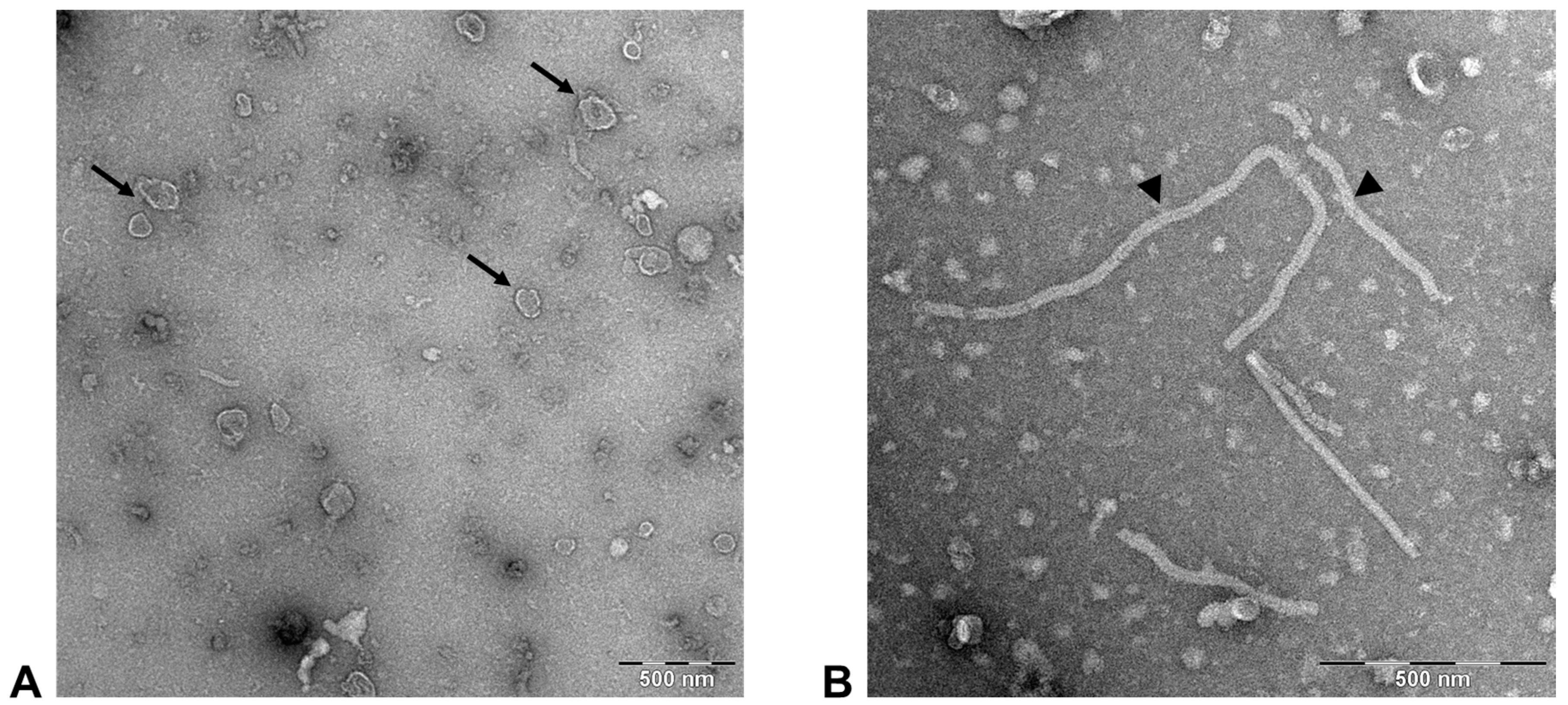
2.4. Electron Microscopy
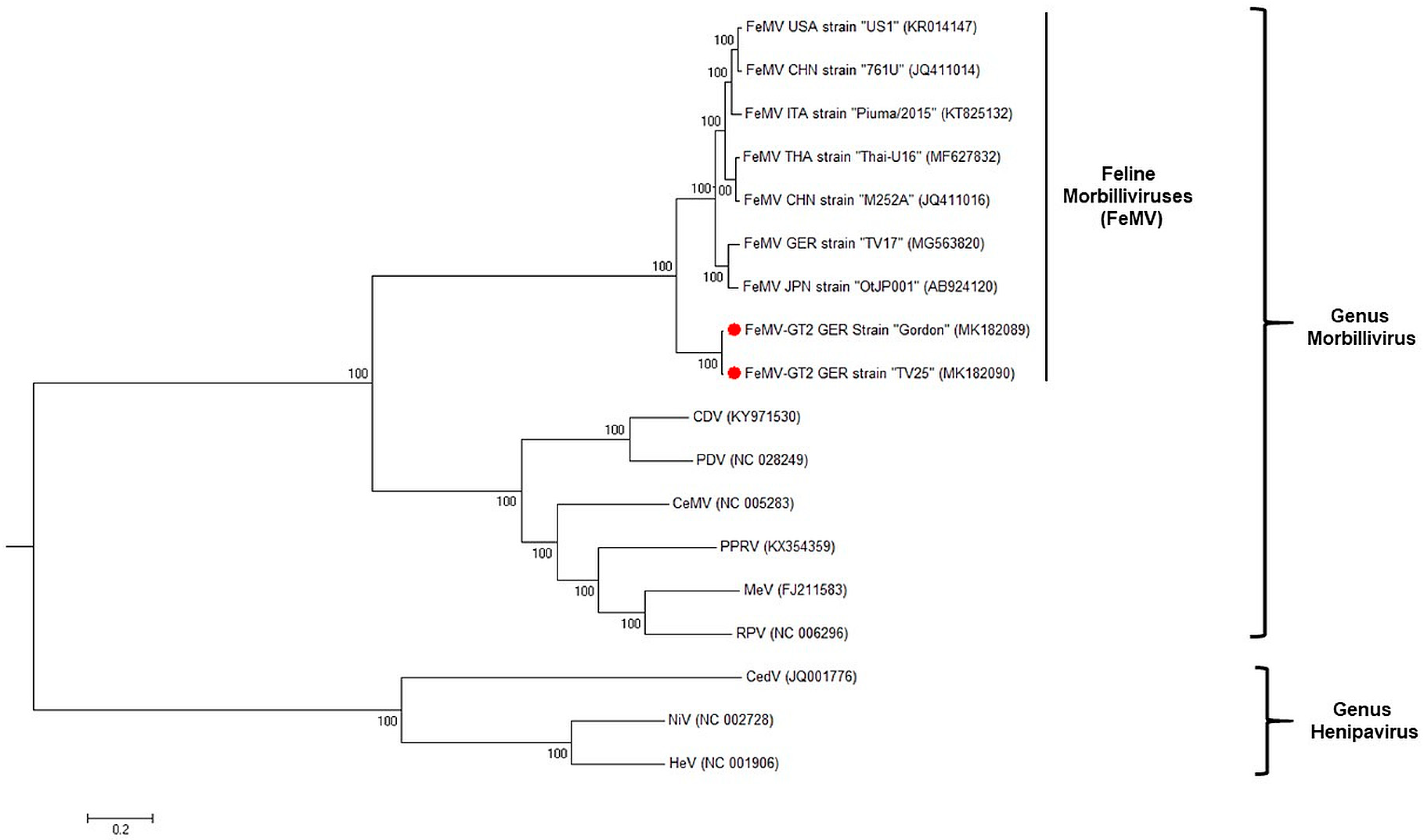
2.5. Detection, Genome Sequencing and Phylogenetic Analysis
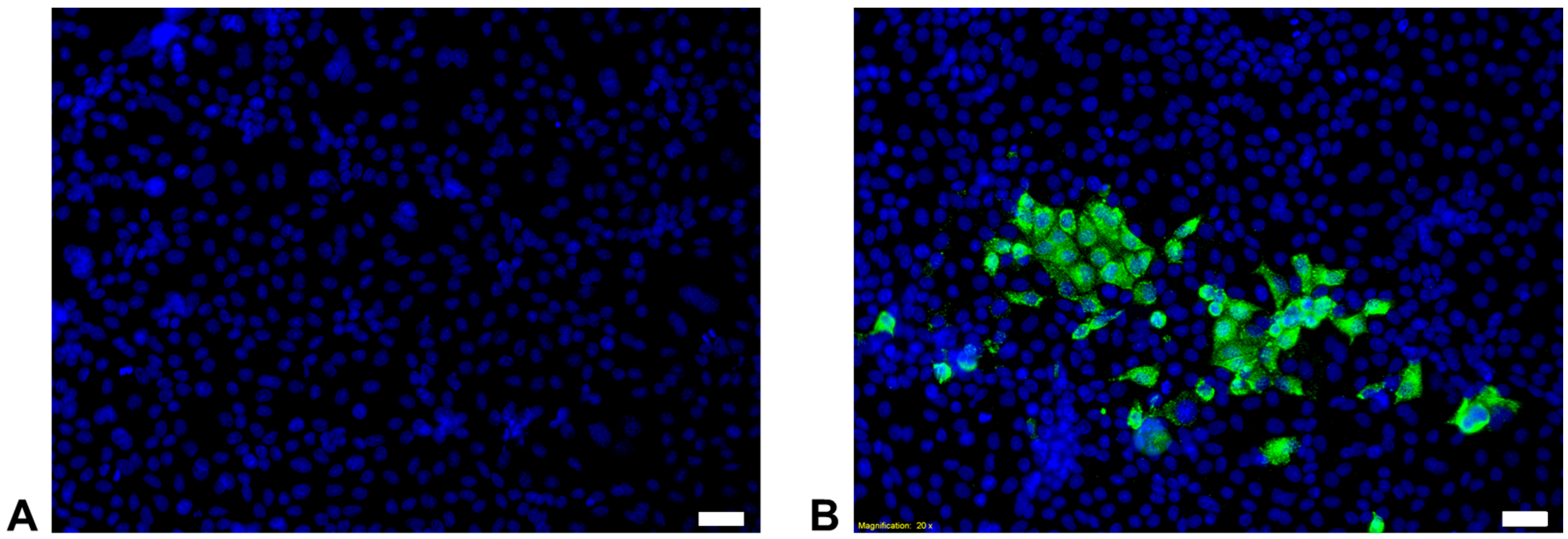
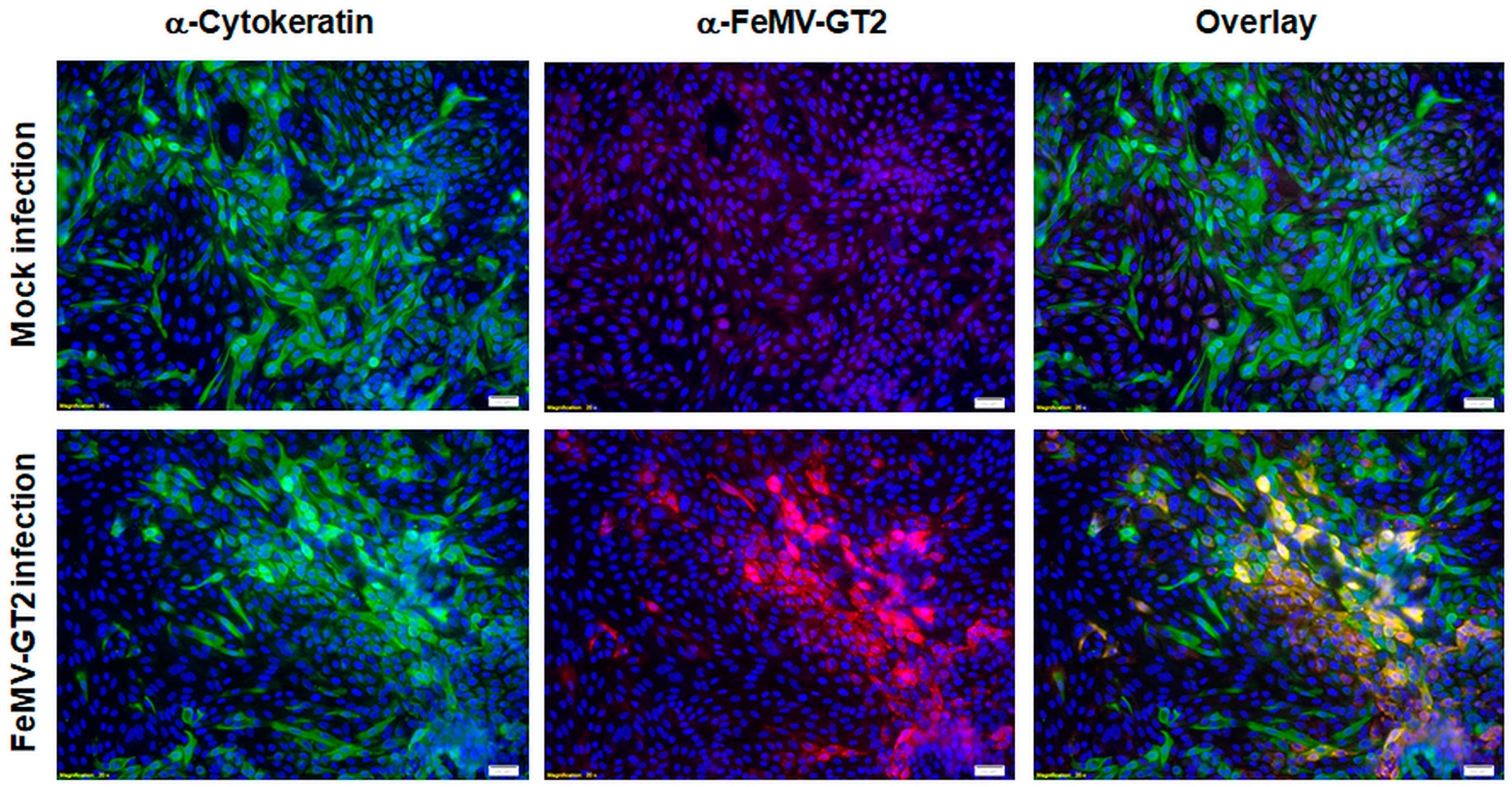
2.6. Cell Infection Assays and Immunofluorescence Staining
2.7. Neutralization Assay
2.8. Infection of Feline PBMC
2.9. Infection of Feline Organotypic Brain Slice Cultures with FeMV-GT2
3. Results
3.1. Detection, Isolation and Culture of FeMV-GT2
3.2. FeMV-GT2 Virions Display a Morphology Typical for Paramyxoviruses
3.3. FeMV-GT2 Replicates in Kidney Cell Lines from Simian, Feline and Rodent Origin
3.4. FeMV-GT2 Differs from Classical Feline Morbillivirus Strains Genetically
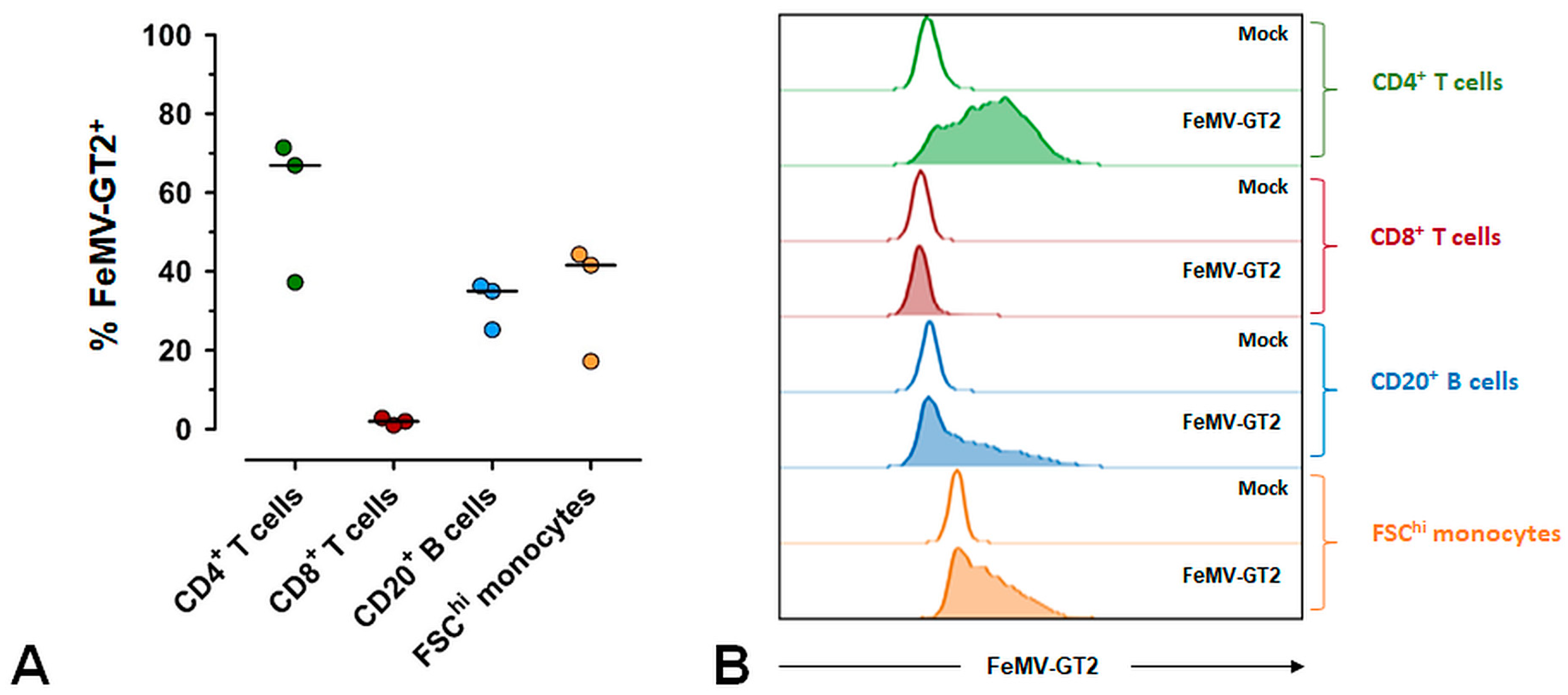
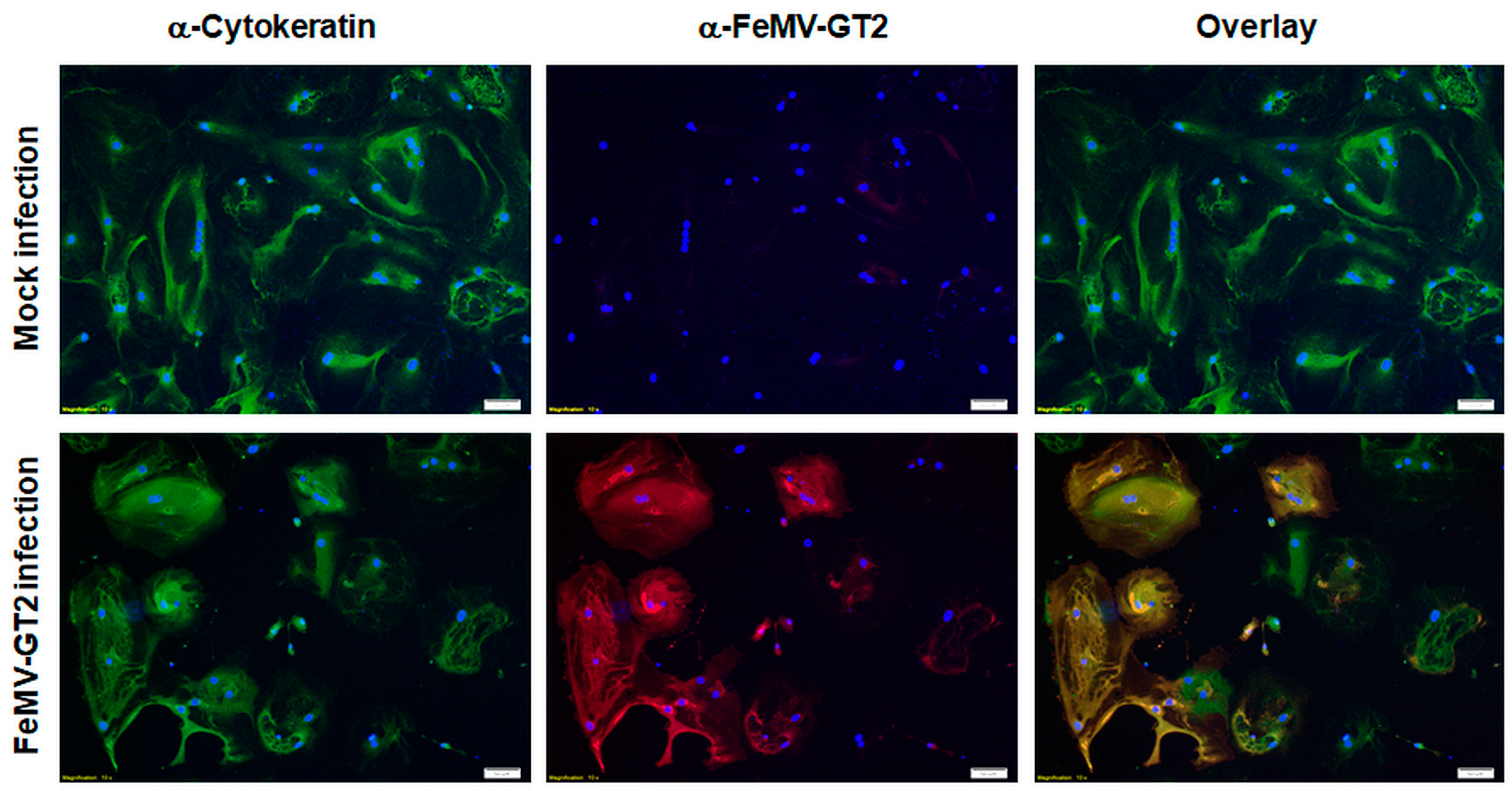
3.5. Primary Epithelial Cells from the Feline Kidney and Lung as Wells as Immune Cells are Susceptible to FeMV-GT2 Infection
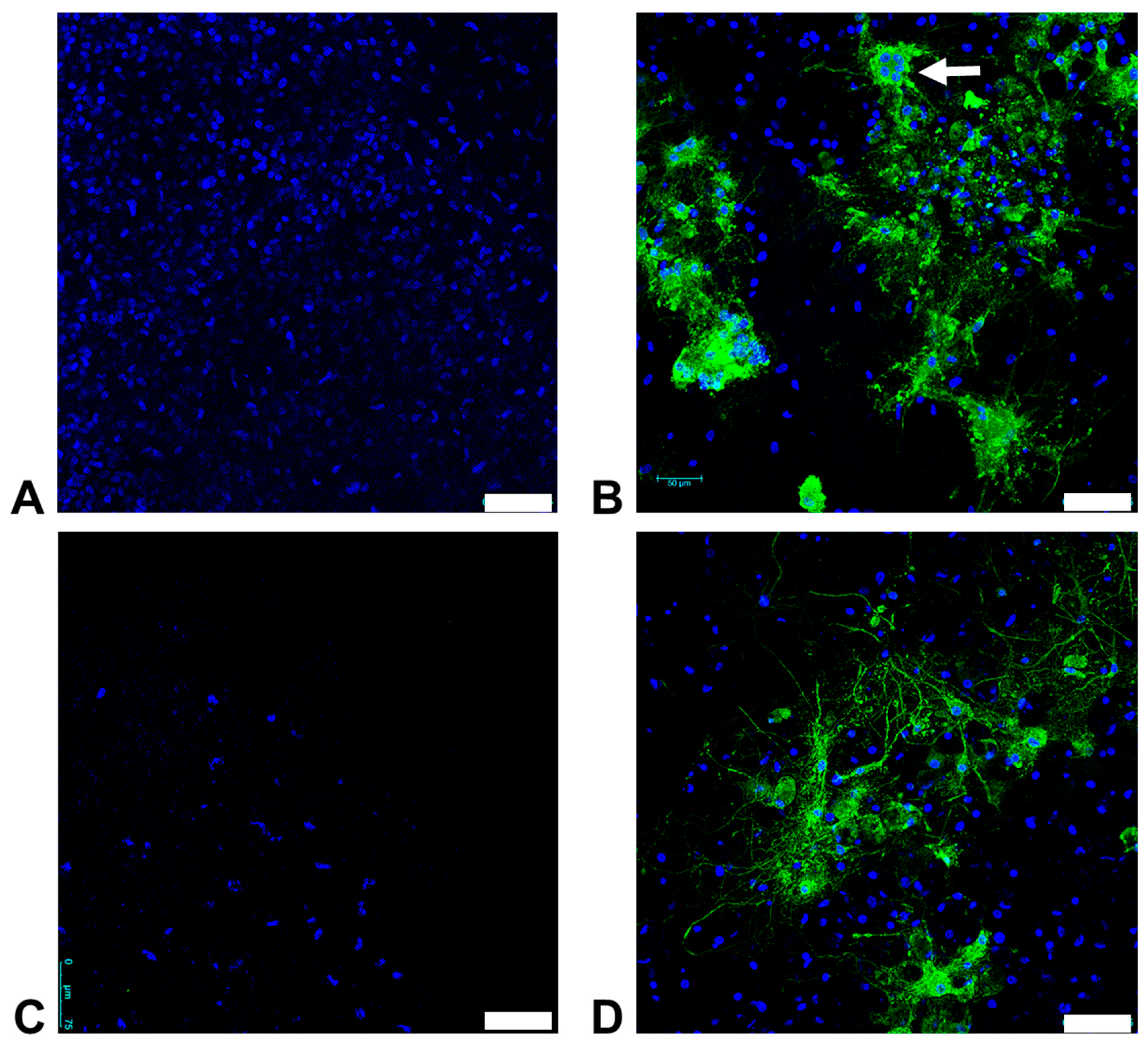
3.6. Organotypic Brain Slice Cultures from the Feline Cerebrum and Cerebellum are Susceptible to FeMV-GT2 Infection In Vitro
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lamb, R.A.; Parks, G.D. Paramyxoviridae: The Viruses and Their Replication. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 1, pp. 1449–1496. ISBN 978-1451105636. [Google Scholar]
- Woo, P.C.; Lau, S.K.; Wong, B.H.; Wong, A.Y.; Poon, R.W.; Yuen, K.Y. Complete genome sequence of a novel paramyxovirus, Tailam virus, discovered in Sikkim rats. J. Virol. 2011, 85, 13473–13474. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, L.S.; Yu, M.; Anderson, D.E.; Crameri, G.; Eaton, B.T.; Wang, L.F. Complete genome sequence of Nariva virus, a rodent paramyxovirus. Arch. Virol. 2009, 154, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Alkhovsky, S.; Butenko, A.; Eremyan, A.; Shchetinin, A. Genetic characterization of bank vole virus (BaVV), a new paramyxovirus isolated from kidneys of bank voles in Russia. Arch. Virol. 2017. [Epub ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.F.; Corman, V.M.; Müller, M.A.; Maganga, G.D.; Vallo, P.; Binger, T.; Gloza-Rausch, F.; Cottontail, V.M.; Rasche, A.; Yordanov, S.; et al. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012, 3, 796. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Lau, S.K.; Wong, B.H.; Fan, R.Y.; Wong, A.Y.; Zhang, A.J.; Wu, Y.; Choi, G.K.; Li, K.S.; Hui, J.; et al. Feline morbillivirus, a previously undescribed paramyxovirus associated with tubulointerstitial nephritis in domestic cats. Proc. Natl. Acad. Sci. USA 2012, 109, 5435–5440. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Sassa, Y.; Omatsu, T.; Nagai, M.; Fukushima, R.; Shibutani, M.; Yamaguchi, T.; Uematsu, Y.; Shirota, K.; Mizutani, T. Existence of feline morbillivirus infection in Japanese cat populations. Arch. Virol. 2014, 159, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Sharp, C.R.; Nambulli, S.; Acciardo, A.S.; Rennick, L.J.; Drexler, J.F.; Rima, B.K.; Williams, T.; Duprex, W.P. Chronic Infection of Domestic Cats with Feline Morbillivirus, United States. Emerg. Infect. Dis. 2016, 22, 760–762. [Google Scholar] [CrossRef]
- Yilmaz, H.; Tekelioglu, B.K.; Gurel, A.; Bamac, O.E.; Ozturk, G.Y.; Cizmecigil, U.Y.; Altan, E.; Aydin, O.; Yilmaz, A.; Berriatua, E.; et al. Frequency, clinicopathological features and phylogenetic analysis of feline morbillivirus in cats in Istanbul, Turkey. J. Feline. Med. Surg. 2017, 19, 1206–1214. [Google Scholar] [CrossRef]
- Darold, G.M.; Alfieri, A.A.; Muraro, L.S.; Amude, A.M.; Zanatta, R.; Yamauchi, K.C.; Alfieri, A.F.; Lunardi, M. First report of feline morbillivirus in South America. Arch. Virol. 2017, 162, 469–475. [Google Scholar] [CrossRef]
- Chaiyasak, S.; Techangamsuwan, S. First evidence of Feline morbillivirus detected in sheltered cats in Thailand. Thai. J. Vet. Med. Suppl. 2017, 47, 127–128. [Google Scholar]
- Lorusso, A.; Di Tommaso, M.; Di Felice, E.; Zaccaria, G.; Luciani, A.; Marcacci, M.; Aste, G.; Boari, A.; Savini, G. First report of feline morbillivirus in Europe. Vet. Ital. 2015, 51, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Sieg, M.; Heenemann, K.; Rückner, A.; Burgener, I.; Oechtering, G.; Vahlenkamp, T.W. Discovery of new feline paramyxoviruses in domestic cats with chronic kidney disease. Virus Genes 2015, 51, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Nakagawa, S.; Yoshikawa, R.; Kuwahara, C.; Hagiwara, H.; Asai, K.I.; Kawakami, K.; Yamamoto, Y.; Ogawa, M.; Miyazawa, T. Genetic diversity of feline morbilliviruses isolated in Japan. J. Gen. Virol. 2014, 95, 1464–1468. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Suzuki, M.; Kimura, M.; Mizutani, H.; Saito, R.; Kubota, N.; Hasuike, Y.; Okajima, J.; Kasai, H.; Sato, Y.; et al. Epidemiological and pathological study of feline morbillivirus infection in domestic cats in Japan. BMC Vet. Res. 2016, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, K.; Wachi, A.; Imura, Y.; Sutummaporn, K.; Kai, C.; Park, E.S.; Morikawa, S.; Uematsu, Y.; Yamaguchi, T.; Furuya, T. Development of an ELISA for serological detection of feline morbillivirus infection. Arch. Virol. 2017, 162, 2421–2425. [Google Scholar] [CrossRef] [PubMed]
- Jepson, R.E. Current understanding of the pathogenesis of progressive chronic kidney disease in cats. Vet. Clin. North Am. Small Anim. Pract. 2016, 46, 1015–1048. [Google Scholar] [CrossRef] [PubMed]
- Taub, M. Primary Kidney Proximal Tubule Cells. Methods Mol. Biol. 1997, 75, 153–161. [Google Scholar]
- Tong, S.; Chern, S.W.W.; Li, Y.; Pallansch, M.A.; Anderson, L.J. Sensitive and broadly reactive reverse transcription-PCR assays to detect novel paramyxoviruses. J. Clin. Microbiol. 2008, 46, 2652–2658. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Reed, L.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Toth, T.E.; Smith, B.; Pyle, H. Simultaneous separation and purification of mononuclear and polymorphonuclear cells from the peripheral blood of cats. J. Virol. Methods 1992, 36, 185–195. [Google Scholar] [CrossRef]
- Townsend, W.M.; Jacobi, S.; Tai, S.H.; Kiupel, M.; Wise, A.G.; Maes, R.K. Ocular and neural distribution of feline herpesvirus-1 during active and latent experimental infection in cats. BMC Vet. Res. 2013, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Papetti, A.; Sabelli, C.; Rosti, E.; Moreno, A.; Boniotti, M. Detection of coronaviruses in bats of various species in Italy. Viruses 2013, 5, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Abd-Eldaim, M.M.; Wilkes, R.P.; Thomas, K.V.; Kennedy, M.A. Development and validation of a TaqMan real-time reverse transcription-PCR for rapid detection of feline calicivirus. Arch. Virol. 2009, 154, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Frölich, K.; Streich, W.J.; Fickel, J.; Jung, S.; Truyen, U.; Hentschke, J.; Dedek, J.; Prager, D.; Latz, N. Epizootiologic investigations of parvovirus infections in free-ranging carnivores from Germany. J. Wildl. Dis. 2005, 41, 231–235. [Google Scholar] [CrossRef]
- Takata, K.; Matsuzaki, T.; Tajika, Y.; Ablimit, A.; Hasegawa, T. Localization and trafficking of aquaporin 2 in the kidney. Histochem. Cell. Biol. 2008, 130, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Timurkaan, S.; Tarakci, B.G. Immunohistochemical determination of calbindin-d28k in the kidney of postnatal rats. Veterinarni. Medicina. 2012, 49, 334–338. [Google Scholar] [CrossRef]
- Verpooten, G.F.; Nouwen, E.J.; Hoylaerts, M.F.; Hendrix, P.G.; de Broe, M.E. Segment-specific localization of intestinal-type alkaline phosphatase in human kidney. Kidney Int. 1989, 36, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, P.; Wang, L.F. Problems of classification in the family Paramyxoviridae. Arch. Virol. 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Van Bressem, M.F.; Duignan, P.J.; Banyard, A.; Barbieri, M.; Colegrove, K.M.; De Guise, S.; et al. Cetacean morbillivirus: current knowledge and future directions. Viruses 2014, 6, 5145–5181. [Google Scholar] [CrossRef] [PubMed]
- Koide, R.; Sakaguchi, S.; Miyazawa, T. Basic biological characterization of feline morbillivirus. J. Vet. Med. Sci. 2015, 77, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Appel, M.J. Pathogenesis of canine distemper. Am. J. Vet. Res. 1969, 30, 1167–1182. [Google Scholar] [PubMed]
- Chen, R.T.; Markowitz, L.E.; Albrecht, P.; Stewart, J.A.; Mofenson, L.M.; Preblud, S.R.; Orenstein, W.A. Measles antibody: reevaluation of protective titers. J. Infect. Dis. 1990, 162, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, P.C.; Van Buren, J.W.; Neterer, M.; Zhou, C. Disease surveillance and referral bias in the veterinary medical database. Prev. Vet. Med. 2010, 94, 264–271. [Google Scholar] [CrossRef]
- Marino, C.L.; Lascelles, B.D.X.; Vaden, S.L.; Gruen, M.E.; Marks, S.L. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J. Feline Med. Surg. 2014, 16, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Da Fontoura Budaszewski, R.; von Messling, V. Morbillivirus experimental animal models: Measles virus pathogenesis insights from canine distemper virus. Viruses 2016, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Van der Hauwaert, C.; Savary, G.; Gnemmi, V.; Glowacki, F.; Pottier, N.; Bouillez, A.; Maboudou, P.; Zini, L.; Leroy, X.; Cauffiez, C.; et al. Isolation and characterization of a primary proximal tubular epithelial cell model from human kidney by CD10/CD13 double labeling. PLoS One 2013, 8, e66750. [Google Scholar] [CrossRef]
- Southgate, J.; Hutton, K.A.; Thomas, D.F.; Trejdosiewicz, L.K. Normal human urothelial cells in vitro: Proliferation and induction of stratification. Lab Invest. 1994, 71, 583–594. [Google Scholar]
- De Luca, E.; Crisi, P.E.; Di Domenico, M.; Malatesta, D.; Vincifori, G.; Di Tommaso, M.; Di Guardo, G.; Di Francesco, G.; Petrini, A.; Savini, G.; et al. A real-time RT-PCR assay for molecular identification and quantitation of feline morbillivirus RNA from biological specimens. J. Virol. Methods 2018, 258, 24–28. [Google Scholar] [CrossRef]
- Von Messling, V.; Milosevic, D.; Cattaneo, R. Tropism illuminated: lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc. Natl. Acad. Sci. USA 2004, 101, 14216–14221. [Google Scholar] [CrossRef]
- De Swart, R.L.; Ludlow, M.; De Witte, L.; Yanagi, Y.; Van Amerongen, G.; McQuaid, S.; Yüksel, S.; Geijtenbeek, T.B.; Duprex, W.P.; Osterhaus, A.D. Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 2007, 3, e178. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Yoneda, M.; Honda, T.; Kai, C. Morbillivirus receptors and tropism: multiple pathways for infection. Front. Microbiol. 2012, 3, 75. [Google Scholar] [CrossRef] [PubMed]

| Animal ID | Sex | Age (in Years) | Clinical Diagnosis | Duration of FeMV-GT2 Shedding in Urine | nAb-titer Against FeMV-GT2 |
|---|---|---|---|---|---|
| 90604 | male | 18 | CKD (IRIS stage 2, normotensive), pancreatitis | n.a.1 | n.a.2 |
| 93436 | female | 15 | Hypertension, renal cyst, lipiduria | n.a.1 | n.a.2 |
| 98450 (‘Gordon’) | male | 13 | CKD (IRIS stage 2, normotensive, non-proteinuric) | >6 months | >256 |
| TV25 | male | 6 | non-obstructive FLUTD, proteinuria, lipiduria | >2 years | >256 |
| 118649 | female | 6 | diabetic ketoacidosis, cholangitis, pancreatitis, azotemia | n.a. | n.a.2 |
| 118650 | female | 13 | CKD, feline triaditis, sepsis, consumption coagulopathy | n.a.1 | n.a.2 |
| Cell line | Tissue | Species | Growth of FeMV-GT2 * |
|---|---|---|---|
| Fcwf-4 | Whole fetus, macrophage | Felis catus | + + + |
| CrFK | Kidney, epithelial | Felis catus | + + |
| KE-R | Embryo, fibroblastic | Felis catus | - |
| LLC-MK2 | Kidney, epithelial | Macaca mulatta | + + + |
| Vero (CCL81) | Kidney, epithelial | Chlorocebus pygerythrus | + |
| MA-104 | Kidney, epithelial | Macaca mulatta | - |
| MARC-145 | Kidney, epithelial | Macaca mulatta | + |
| BHK-21 | Kidney, fibroblastic | Mesocricetus auratus | + |
| A9 | Adipose tissue, fibroblastic | Mus musculus | - |
| HEK-293 | Kidney, epithelial | Homo sapiens | - |
| MDBK | Kidney, epithelial | Bos taurus | - |
| MDCK-II | Kidney, epithelial | Canis lupus familiaris | - |
| FMN-R | Kidney, epithelial | Microtus arvalis | - |
| MGN-R | Kidney, fibroblastic | Myodes glareolus | - |
| RAN-2-R | Kidney, epithelial | Rousettus aegyptiacus | - |
| FLN-R | Kidney, epithelial | Eptesicus serotinus | - |
| Genom Region | Nucleotide Position | Nucleotide Homology to FeMV ‘M252A’ | Amino Acid Identity to FeMV ‘M252A’ |
|---|---|---|---|
| 3‘-untranslated region | 1–107 | 84.1% | not applicable |
| Nucleocapsid-protein | 108–1667 | 81.1% | 89.8% |
| Intergenic region | 1668–1780 | 39.8% | not applicable |
| Phospho-protein | 1781–3256 | 80.5% | 75.1% |
| Intergenic region | 3257–3388 | 44.7% | not applicable |
| Matrix-protein | 3389–4402 | 83.3% | 91.7% |
| Intergenic region | 4403–4949 | 48.6% | not applicable |
| Fusion-protein | 4950–6581 | 80.9% | 88.7% |
| Intergenic region | 6582–6958 | 56.0% | not applicable |
| Hemagglutinin-protein | 6959–8746 | 80.5% | 85.9% |
| Intergenic region | 8747–8887 | 54.6% | not applicable |
| Polymerase-protein | 8888–15496 | 82.5% | 90.9% |
| 5‘-untranslated region | 15497–16047 | 52.4% | not applicable |
| Whole genome | 1–16047 | 78.2% | not applicable |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sieg, M.; Busch, J.; Eschke, M.; Böttcher, D.; Heenemann, K.; Vahlenkamp, A.; Reinert, A.; Seeger, J.; Heilmann, R.; Scheffler, K.; et al. A New Genotype of Feline Morbillivirus Infects Primary Cells of the Lung, Kidney, Brain and Peripheral Blood. Viruses 2019, 11, 146. https://doi.org/10.3390/v11020146
Sieg M, Busch J, Eschke M, Böttcher D, Heenemann K, Vahlenkamp A, Reinert A, Seeger J, Heilmann R, Scheffler K, et al. A New Genotype of Feline Morbillivirus Infects Primary Cells of the Lung, Kidney, Brain and Peripheral Blood. Viruses. 2019; 11(2):146. https://doi.org/10.3390/v11020146
Chicago/Turabian StyleSieg, Michael, Johannes Busch, Maria Eschke, Denny Böttcher, Kristin Heenemann, Annett Vahlenkamp, Anja Reinert, Johannes Seeger, Romy Heilmann, Kira Scheffler, and et al. 2019. "A New Genotype of Feline Morbillivirus Infects Primary Cells of the Lung, Kidney, Brain and Peripheral Blood" Viruses 11, no. 2: 146. https://doi.org/10.3390/v11020146
APA StyleSieg, M., Busch, J., Eschke, M., Böttcher, D., Heenemann, K., Vahlenkamp, A., Reinert, A., Seeger, J., Heilmann, R., Scheffler, K., & Vahlenkamp, T. W. (2019). A New Genotype of Feline Morbillivirus Infects Primary Cells of the Lung, Kidney, Brain and Peripheral Blood. Viruses, 11(2), 146. https://doi.org/10.3390/v11020146






