A Novel and Divergent Gyrovirus with Unusual Genomic Features Detected in Wild Passerine Birds from a Remote Rainforest in French Guiana
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Processing and Next Generation Sequencing
2.3. Prevalence of the Novel GyV11 in Cloacal, Oral, and Blood Samples
2.4. Genomic Characterization and Phylogeny
3. Results
3.1. Molecular Screening of GyV11
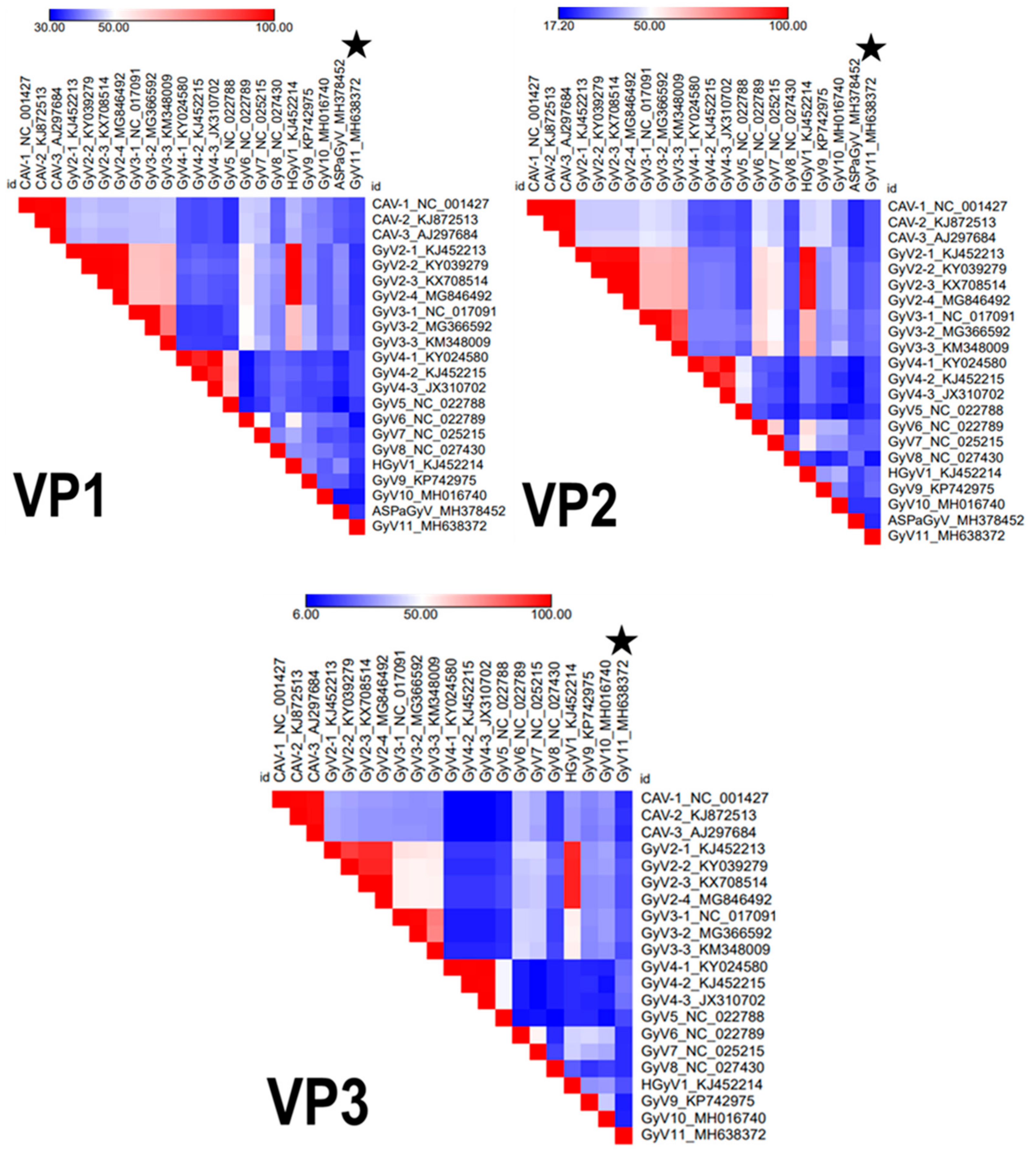
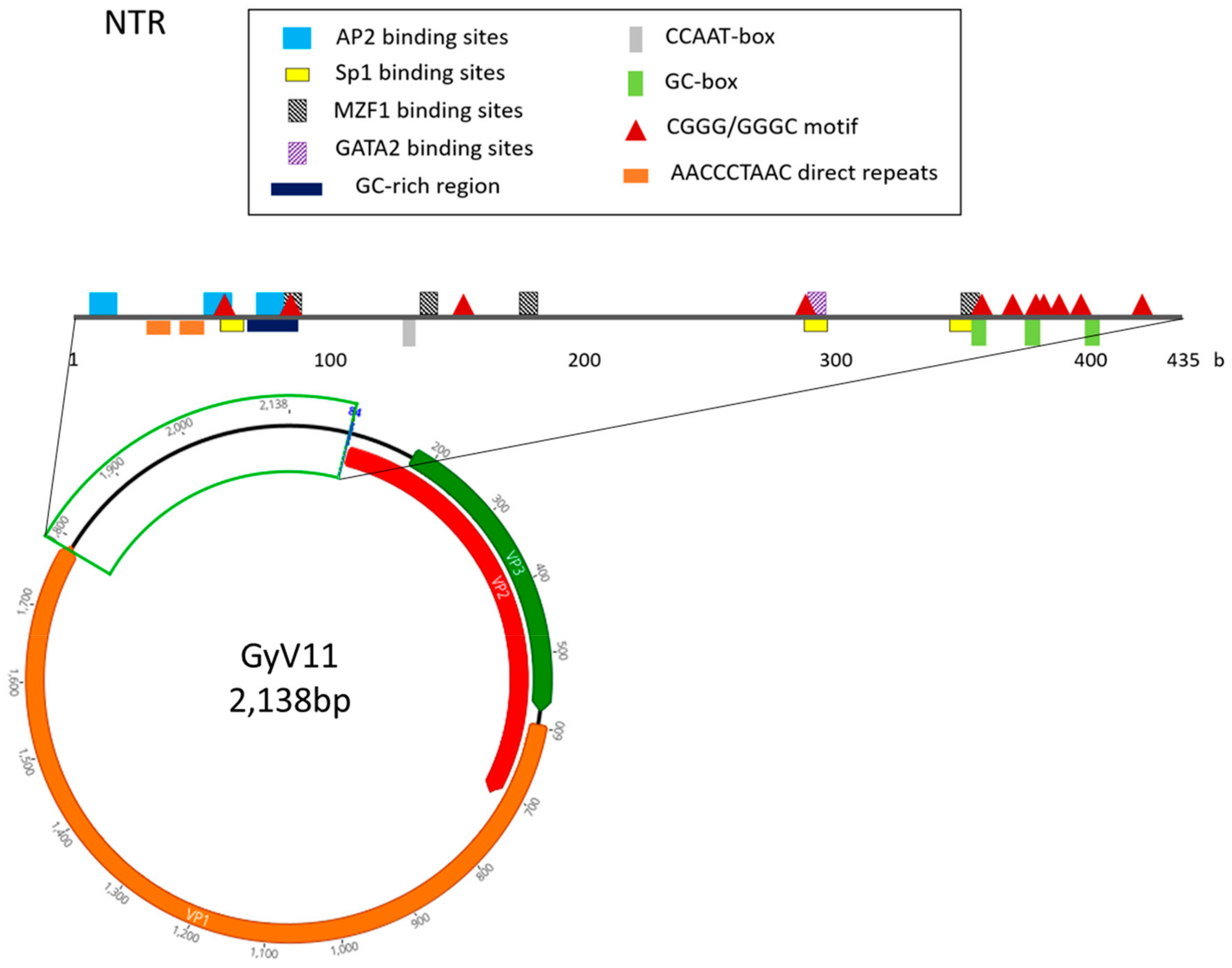
3.2. Genomic Characterization
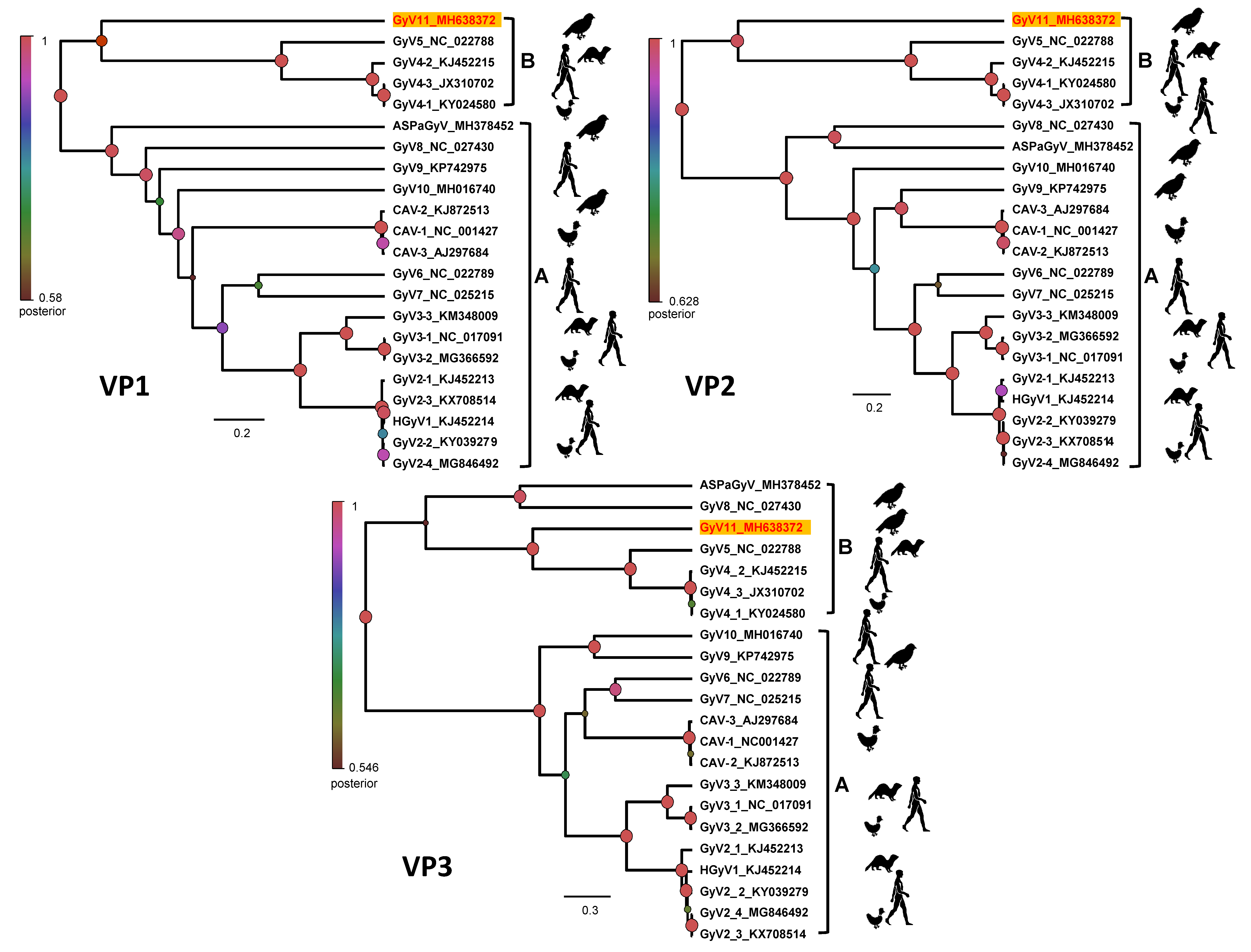
3.3. Phylogenetic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bodewes, R. Novel viruses in birds: Flying through the roof or is a cage needed? Vet. J. Lond. Engl. 1997 2018, 233, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; To, K.K.-W.; Chen, H.; Yuen, K.-Y. Cross-species transmission and emergence of novel viruses from birds. Curr. Opin. Virol. 2015, 10, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.M.; Silva, F.; João, M.; Brito, C.R.; Teixeira, D.S.; Melo, F.L.; Ribeiro, B.M.; Nagata, T.; Campos, F.S. Faecal Virome Analysis of Wild Animals from Brazil. Viruses 2019, 11, 803. [Google Scholar] [CrossRef]
- Kapgate, S.S.; Barbuddhe, S.B.; Kumanan, K. Next generation sequencing technologies: Tool to study avian virus diversity. Acta Virol. 2015, 59, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Rosario, K.; Breitbart, M.; Harrach, B.; Segalés, J.; Delwart, E.; Biagini, P.; Varsani, A. Revisiting the taxonomy of the family Circoviridae: Establishment of the genus Cyclovirus and removal of the genus Gyrovirus. Arch. Virol. 2017, 162, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, N.; Taniguchi, T.; Goda, M.; Shibatani, M.; Imada, T.; Hihara, H. Isolation of chicken anemia agent with MDCC-MSB1 cells from chickens in the field. Natl. Inst. Anim. Health Q. (Tokyo) 1983, 23, 75–77. [Google Scholar] [PubMed]
- Eltahir, Y.M.; Qian, K.; Jin, W.; Qin, A. Analysis of chicken anemia virus genome: Evidence of intersubtype recombination. Virol. J. 2011, 8, 512. [Google Scholar] [CrossRef]
- Maggi, F.; Macera, L.; Focosi, D.; Vatteroni, M.L.; Boggi, U.; Antonelli, G.; Eloit, M.; Pistello, M. Human gyrovirus DNA in human blood, Italy. Emerg. Infect. Dis. 2012, 18, 956–959. [Google Scholar] [CrossRef]
- Biagini, P.; Bédarida, S.; Touinssi, M.; Galicher, V.; de Micco, P. Human gyrovirus in healthy blood donors, France. Emerg. Infect. Dis. 2013, 19, 1014–1015. [Google Scholar] [CrossRef]
- Rijsewijk, F.A.M.; Dos Santos, H.F.; Teixeira, T.F.; Cibulski, S.P.; Varela, A.P.M.; Dezen, D.; Franco, A.C.; Roehe, P.M. Discovery of a genome of a distant relative of chicken anemia virus reveals a new member of the genus Gyrovirus. Arch. Virol. 2011, 156, 1097–1100. [Google Scholar] [CrossRef]
- Dos Santos, H.F.; Knak, M.B.; de Castro, F.L.; Slongo, J.; Ritterbusch, G.A.; Klein, T.A.P.; Esteves, P.A.; Silva, A.D.; Trevisol, I.M.; Claassen, E.A.W.; et al. Variants of the recently discovered avian gyrovirus 2 are detected in Southern Brazil and The Netherlands. Vet. Microbiol. 2012, 155, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Ji, J.; Chen, F.; Sun, B.; Xue, C.; Ma, J.; Bi, Y.; Xie, Q. Identification of a chicken anemia virus variant-related gyrovirus in stray cats in china, 2012. BioMed. Res. Int. 2014, 2014, 313252. [Google Scholar] [PubMed]
- Chu, D.K.W.; Poon, L.L.M.; Chiu, S.S.S.; Chan, K.H.; Ng, E.M.; Bauer, I.; Cheung, T.K.; Ng, I.H.Y.; Guan, Y.; Wang, D.; et al. Characterization of a novel gyrovirus in human stool and chicken meat. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2012, 55, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Gia Phan, T.; Phung Vo, N.; Sdiri-Loulizi, K.; Aouni, M.; Pothier, P.; Ambert-Balay, K.; Deng, X.; Delwart, E. Divergent gyroviruses in the feces of Tunisian children. Virology 2013, 446, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; da Costa, A.C.; Zhang, W.; Pothier, P.; Ambert-Balay, K.; Deng, X.; Delwart, E. A new gyrovirus in human feces. Virus Genes 2015, 51, 132–135. [Google Scholar] [CrossRef]
- Fehér, E.; Pazár, P.; Kovács, E.; Farkas, S.L.; Lengyel, G.; Jakab, F.; Martella, V.; Bányai, K. Molecular detection and characterization of human gyroviruses identified in the ferret fecal virome. Arch. Virol. 2014, 159, 3401–3406. [Google Scholar] [CrossRef]
- Li, L.; Pesavento, P.A.; Gaynor, A.M.; Duerr, R.S.; Phan, T.G.; Zhang, W.; Deng, X.; Delwart, E. A gyrovirus infecting a sea bird. Arch. Virol. 2015, 160, 2105–2109. [Google Scholar] [CrossRef]
- Goldberg, T.L.; Clyde, V.L.; Gendron-Fitzpatrick, A.; Sibley, S.D.; Wallace, R. Severe neurologic disease and chick mortality in crested screamers (Chauna torquata) infected with a novel Gyrovirus. Virology 2018, 520, 111–115. [Google Scholar] [CrossRef]
- Waits, K.; Bradley, R.W.; Warzybok, P.; Kraberger, S.; Fontenele, R.S.; Varsani, A. Genome Sequence of a Gyrovirus Associated with Ashy Storm-Petrel. Microbiol. Resour. Announc. 2018, 7, e00958-18. [Google Scholar] [CrossRef]
- Yao, S.; Gao, X.; Tuo, T.; Han, C.; Gao, Y.; Qi, X.; Zhang, Y.; Liu, C.; Gao, H.; Wang, Y.; et al. Novel characteristics of the avian gyrovirus 2 genome. Sci. Rep. 2017, 7, 41068. [Google Scholar] [CrossRef]
- Fernández-Correa, I.; Truchado, D.A.; Gomez-Lucia, E.; Doménech, A.; Pérez-Tris, J.; Schmidt-Chanasit, J.; Cadar, D.; Benítez, L. A novel group of avian astroviruses from Neotropical passerine birds broaden the diversity and host range of Astroviridae. Sci. Rep. 2019, 9, 9513. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Beier, S.; Flade, I.; Górska, A.; El-Hadidi, M.; Mitra, S.; Ruscheweyh, H.-J.; Tappu, R. MEGAN Community Edition—Interactive Exploration and Analysis of Large-Scale Microbiome Sequencing Data. PLoS Comput. Biol. 2016, 12, e1004957. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, S.; Hasebe, M.; Tomita, M.; Yanagawa, H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. USA 2009, 106, 10171–10176. [Google Scholar] [CrossRef] [PubMed]
- la Cour, T.; Kiemer, L.; Mølgaard, A.; Gupta, R.; Skriver, K.; Brunak, S. Analysis and prediction of leucine-rich nuclear export signals. Protein Eng. Des. Sel. PEDS 2004, 17, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, T.; Takagi, T. Estimating transcription factor bindability on DNA. Bioinforma. Oxf. Engl. 1999, 15, 622–630. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef]
- Carver, T.; Thomson, N.; Bleasby, A.; Berriman, M.; Parkhill, J. DNAPlotter: Circular and linear interactive genome visualization. Bioinformatics 2009, 25, 119–120. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Schat, K.A. Chicken anemia virus. Curr. Top. Microbiol. Immunol. 2009, 331, 151–183. [Google Scholar] [PubMed]
- Abolnik, C.; Wandrag, D.B.R. Avian gyrovirus 2 and avirulent Newcastle disease virus coinfection in a chicken flock with neurologic symptoms and high mortalities. Avian Dis. 2014, 58, 90–94. [Google Scholar] [CrossRef]
- Miles, A.M.; Reddy, S.M.; Morgan, R.W. Coinfection of specific-pathogen-free chickens with Marek’s disease virus (MDV) and chicken infectious anemia virus: Effect of MDV pathotype. Avian Dis. 2001, 45, 9–18. [Google Scholar] [CrossRef]
- Lima, D.A.; Cibulski, S.P.; Tochetto, C.; Varela, A.P.M.; Finkler, F.; Teixeira, T.F.; Loiko, M.R.; Cerva, C.; Junqueira, D.M.; Mayer, F.Q.; et al. The intestinal virome of malabsorption syndrome-affected and unaffected broilers through shotgun metagenomics. Virus Res. 2019, 261, 9–20. [Google Scholar] [CrossRef]
- Roussan, D.A.R. Serological Survey on the Prevalence of Chicken Infectious Anemia Virus in Commercial Broiler Chicken Flocks in Northern Jordan. Int. J. Poult. Sci. 2006, 5, 544–546. [Google Scholar]
- Snoeck, C.J.; Komoyo, G.F.; Mbee, B.P.; Nakouné, E.; Le Faou, A.; Okwen, M.P.; Muller, C.P. Epidemiology of chicken anemia virus in Central African Republic and Cameroon. Virol. J. 2012, 9, 189. [Google Scholar] [CrossRef]
- Farkas, T.; Maeda, K.; Sugiura, H.; Kai, K.; Hirai, K.; Otsuki, K.; Hayashi, T. A serological survey of chickens, Japanese quail, pigeons, ducks and crows for antibodies to chicken anaemia virus (CAV) in Japan. Avian Pathol. J. WVPA 1998, 27, 316–320. [Google Scholar] [CrossRef]
- Moens, M.A.J.; Pérez-Tris, J.; Cortey, M.; Benítez, L. Identification of two novel CRESS DNA viruses associated with an Avipoxvirus lesion of a blue-and-gray Tanager (Thraupis episcopus). Infect. Genet. Evol. 2018, 60, 89–96. [Google Scholar] [CrossRef]
- Varela, A.P.M.; Dos Santos, H.F.; Cibulski, S.P.; Scheffer, C.M.; Schmidt, C.; Sales Lima, F.E.; Silva, A.D.; Esteves, P.A.; Franco, A.C.; Roehe, P.M. Chicken anemia virus and avian gyrovirus 2 as contaminants in poultry vaccines. Biol. J. Int. Assoc. Biol. Stand. 2014, 42, 346–350. [Google Scholar] [CrossRef]
- Noteborn, M.H.; Koch, G. Chicken anaemia virus infection: Molecular basis of pathogenicity. Avian Pathol. J. WVPA 1995, 24, 11–31. [Google Scholar] [CrossRef]
- De Villiers, E.-M.; Borkosky, S.S.; Kimmel, R.; Gunst, K.; Fei, J.-W. The diversity of torque teno viruses: In vitro replication leads to the formation of additional replication-competent subviral molecules. J. Virol. 2011, 85, 7284–7295. [Google Scholar] [CrossRef]
- Hromas, R.; Davis, B.; Rauscher, F.J.; Klemsz, M.; Tenen, D.; Hoffman, S.; Xu, D.; Morris, J.F. Hematopoietic transcriptional regulation by the myeloid zinc finger gene, MZF-1. Curr. Top. Microbiol. Immunol. 1996, 211, 159–164. [Google Scholar]
- Tsai, F.Y.; Orkin, S.H. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 1997, 89, 3636–3643. [Google Scholar] [CrossRef]
- Peters, M.A.; Jackson, D.C.; Crabb, B.S.; Browning, G.F. Chicken anemia virus VP2 is a novel dual specificity protein phosphatase. J. Biol. Chem. 2002, 277, 39566–39573. [Google Scholar] [CrossRef]
- Danen-Van Oorschot, A.A.A.M.; Zhang, Y.-H.; Leliveld, S.R.; Rohn, J.L.; Seelen, M.C.M.J.; Bolk, M.W.; Van Zon, A.; Erkeland, S.J.; Abrahams, J.-P.; Mumberg, D.; et al. Importance of nuclear localization of apoptin for tumor-specific induction of apoptosis. J. Biol. Chem. 2003, 278, 27729–27736. [Google Scholar] [CrossRef]
- Noteborn, M.H.; Todd, D.; Verschueren, C.A.; de Gauw, H.W.; Curran, W.L.; Veldkamp, S.; Douglas, A.J.; McNulty, M.S.; van der EB, A.J.; Koch, G. A single chicken anemia virus protein induces apoptosis. J. Virol. 1994, 68, 346–351. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truchado, D.A.; Diaz-Piqueras, J.M.; Gomez-Lucia, E.; Doménech, A.; Milá, B.; Pérez-Tris, J.; Schmidt-Chanasit, J.; Cadar, D.; Benítez, L. A Novel and Divergent Gyrovirus with Unusual Genomic Features Detected in Wild Passerine Birds from a Remote Rainforest in French Guiana. Viruses 2019, 11, 1148. https://doi.org/10.3390/v11121148
Truchado DA, Diaz-Piqueras JM, Gomez-Lucia E, Doménech A, Milá B, Pérez-Tris J, Schmidt-Chanasit J, Cadar D, Benítez L. A Novel and Divergent Gyrovirus with Unusual Genomic Features Detected in Wild Passerine Birds from a Remote Rainforest in French Guiana. Viruses. 2019; 11(12):1148. https://doi.org/10.3390/v11121148
Chicago/Turabian StyleTruchado, Daniel A., José Manuel Diaz-Piqueras, Esperanza Gomez-Lucia, Ana Doménech, Borja Milá, Javier Pérez-Tris, Jonas Schmidt-Chanasit, Daniel Cadar, and Laura Benítez. 2019. "A Novel and Divergent Gyrovirus with Unusual Genomic Features Detected in Wild Passerine Birds from a Remote Rainforest in French Guiana" Viruses 11, no. 12: 1148. https://doi.org/10.3390/v11121148
APA StyleTruchado, D. A., Diaz-Piqueras, J. M., Gomez-Lucia, E., Doménech, A., Milá, B., Pérez-Tris, J., Schmidt-Chanasit, J., Cadar, D., & Benítez, L. (2019). A Novel and Divergent Gyrovirus with Unusual Genomic Features Detected in Wild Passerine Birds from a Remote Rainforest in French Guiana. Viruses, 11(12), 1148. https://doi.org/10.3390/v11121148






