Recombinant Modified Vaccinia Virus Ankara (MVA) Vaccines Efficiently Protect Cockatiels Against Parrot Bornavirus Infection and Proventricular Dilatation Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Generation of ORFV Constructs Encoding Avian Bornavirus N and P Genes
2.3. Immunization and Experimental Bornavirus Infection of Cockatiels
2.4. Detection of PaBV-2- and PaBV-4-Specific RNA by RT-qPCR Assays
2.5. Detection of Antibodies Directed Against Bornaviruses, NDV and MVA
2.6. Histopathological Analysis
3. Results
3.1. Expression of N and P Protein in Mammalian and Avian Cells Infected with Recombinant ORFV Vector Vaccines
3.2. MVA Vector Vaccines Carrying PaBV-4 N and P Genes Protect Cockatiels Against Heterologous PaBV-2 Challenge Infection
3.3. Immunization of Persistently PaBV-4-Infected Cockatiels Does Not Affect Course of Infection and Disease
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heffels-Redmann, U.; Enderlein, D.; Herzog, S.; Herden, C.; Piepenbring, A.; Neumann, D.; Müller, H.; Capelli, S.; Müller, H.; Oberhauser, K.; et al. Occurrence of avian bornavirus infection in captive psittacines in various European countries and its association with proventricular dilatation disease. Avian Pathol. 2011, 40, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Rubbenstroth, D.; Schmidt, V.; Rinder, M.; Legler, M.; Twietmeyer, S.; Schwemmer, P.; Corman, V.M. Phylogenetic analysis supports horizontal transmission as a driving force of the spread of avian bornaviruses. PLoS ONE 2016, 11, e0160936. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, G.K.; Ayllon, M.A.; Bao, Y.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Taxonomy of the order Mononegavirales: Update 2019. Arch. Virol. 2019, 164, 1967–1980. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Dürrwald, R.; Bao, Y.; Briese, T.; Carbone, K.; Clawson, A.N.; deRisi, J.L.; Garten, W.; Jahrling, P.B.; Kolodziejek, J.; et al. Taxonomic reorganization of the family Bornaviridae. Arch. Virol. 2015, 160, 621–632. [Google Scholar] [CrossRef]
- Heffels-Redmann, U.; Enderlein, D.; Herzog, S.; Piepenbring, A.; Bürkle, M.; Neumann, D.; Herden, C.; Lierz, M. Follow-up investigations on different courses of natural avian bornavirus infections in psittacines. Avian Dis. 2012, 56, 153–159. [Google Scholar] [CrossRef]
- Olbert, M.; Römer-Oberdörfer, A.; Herden, C.; Malberg, S.; Runge, S.; Staeheli, P.; Rubbenstroth, D. Viral vector vaccines expressing nucleoprotein and phosphoprotein genes of avian bornaviruses ameliorate homologous challenge infections in cockatiels and common canaries. Sci. Rep. 2016, 6, 36840. [Google Scholar] [CrossRef]
- Piepenbring, A.K.; Enderlein, D.; Herzog, S.; Al-Ibadi, B.; Heffels-Redmann, U.; Heckmann, J.; Lange-Herbst, H.; Herden, C.; Lierz, M. Parrot Bornavirus (PaBV)-2 isolate causes different disease patterns in cockatiels than PaBV-4. Avian Pathol. 2016, 45, 156–168. [Google Scholar] [CrossRef]
- Piepenbring, A.K.; Enderlein, D.; Herzog, S.; Kaleta, E.F.; Heffels-Redmann, U.; Ressmeyer, S.; Herden, C.; Lierz, M. Pathogenesis of avian bornavirus in experimentally infected cockatiels. Emerg. Infect. Dis. 2012, 18, 234–241. [Google Scholar] [CrossRef]
- Rubbenstroth, D.; Brosinski, K.; Rinder, M.; Olbert, M.; Kaspers, B.; Korbel, R.; Staeheli, P. No contact transmission of avian bornavirus in experimentally infected cockatiels (Nymphicus hollandicus) and domestic canaries (Serinus canaria forma domestica). Vet. Microbiol. 2014, 172, 146–156. [Google Scholar] [CrossRef]
- Runge, S.; Olbert, M.; Herden, C.; Malberg, S.; Römer-Oberdörfer, A.; Staeheli, P.; Rubbenstroth, D. Viral vector vaccines protect cockatiels from inflammatory lesions after heterologous parrot bornavirus 2 challenge infection. Vaccine 2017, 35, 557–563. [Google Scholar] [CrossRef]
- Hameed, S.S.; Guo, J.; Tizard, I.; Shivaprasad, H.L.; Payne, S. Studies on immunity and immunopathogenesis of parrot bornaviral disease in cockatiels. Virology 2018, 515, 81–91. [Google Scholar] [CrossRef]
- Hausmann, J.; Hallensleben, W.; de la Torre, J.C.; Pagenstecher, A.; Zimmermann, C.; Pircher, H.; Staeheli, P. T cell ignorance in mice to Borna disease virus can be overcome by peripheral expression of the viral nucleoprotein. Proc. Natl. Acad. Sci. USA 1999, 96, 9769–9774. [Google Scholar] [CrossRef]
- Hausmann, J.; Pagenstecher, A.; Baur, K.; Richter, K.; Rziha, H.J.; Staeheli, P. CD8 T cells require gamma interferon to clear borna disease virus from the brain and prevent immune system-mediated neuronal damage. J. Virol. 2005, 79, 13509–13518. [Google Scholar] [CrossRef] [PubMed]
- Richt, J.A.; Pfeuffer, I.; Christ, M.; Frese, K.; Bechter, K.; Herzog, S. Borna disease virus infection in animals and humans. Emerg. Infect. Dis. 1997, 3, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Stitz, L.; Bilzer, T.; Planz, O. The immunopathogenesis of Borna disease virus infection. Front. Biosci. 2002, 7, d541–d555. [Google Scholar] [CrossRef] [PubMed]
- Enderlein, D.; Piepenbring, A.; Herzog, S.; Herden, C.; Crosta, L.; Oberhäuser, K.; Müller, H.; Hebel, C.; Hammer, S.; Kaleta, E.F.; et al. The situation of ABV in endangered psittacines like the Spix’s macaw. In Proceedings of the 11th Conference of The European Committee of the Association of Avian Veterinarians (EAAV), Madrid, Spain, 26–30 April 2011; pp. 228–229. [Google Scholar]
- Stagegaard, J.; Bruslund, S.; Lierz, M. Could introducing confiscated parrots to zoological collections jeopardise conservation breeding programs? Bird Conserv. Int. 2018, 28, 493–498. [Google Scholar] [CrossRef]
- Rziha, H.J.; Rohde, J.; Amann, R. Generation and Selection of Orf Virus (ORFV) Recombinants. Methods Mol. Biol. 2016, 1349, 177–200. [Google Scholar] [CrossRef]
- Rohde, J.; Schirrmeier, H.; Granzow, H.; Rziha, H.J. A new recombinant Orf virus (ORFV, Parapoxvirus) protects rabbits against lethal infection with rabbit hemorrhagic disease virus (RHDV). Vaccine 2011, 29, 9256–9264. [Google Scholar] [CrossRef]
- Martins, M.; Joshi, L.R.; Rodrigues, F.S.; Anziliero, D.; Frandoloso, R.; Kutish, G.F.; Rock, D.L.; Weiblen, R.; Flores, E.F.; Diel, D.G. Immunogenicity of ORFV-based vectors expressing the rabies virus glycoprotein in livestock species. Virology 2017, 511, 229–239. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Liu, F.; Luo, S. Orf virus: A promising new therapeutic agent. Rev. Med. Virol. 2019, 29, e2013. [Google Scholar] [CrossRef]
- Henkel, M.; Planz, O.; Fischer, T.; Stitz, L.; Rziha, H.J. Prevention of virus persistence and protection against immunopathology after Borna disease virus infection of the brain by a novel Orf virus recombinant. J. Virol. 2005, 79, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Rubbenstroth, D.; Rinder, M.; Kaspers, B.; Staeheli, P. Efficient isolation of avian bornaviruses (ABV) from naturally infected psittacine birds and identification of a new ABV genotype from a salmon-crested cockatoo (Cacatua moluccensis). Vet. Microbiol. 2012, 161, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kremer, M.; Volz, A.; Kreijtz, J.H.; Fux, R.; Lehmann, M.H.; Sutter, G. Easy and efficient protocols for working with recombinant vaccinia virus MVA. Methods Mol. Biol. 2012, 890, 59–92. [Google Scholar] [CrossRef]
- Römer-Oberdörfer, A.; Mundt, E.; Mebatsion, T.; Buchholz, U.J.; Mettenleiter, T.C. Generation of recombinant lentogenic Newcastle disease virus from cDNA. J. Gen. Virol. 1999, 80 (Pt 11), 2987–2995. [Google Scholar] [CrossRef]
- Rubbenstroth, D.; Rinder, M.; Stein, M.; Höper, D.; Kaspers, B.; Brosinski, K.; Horie, M.; Schmidt, V.; Legler, M.; Korbel, R.; et al. Avian bornaviruses are widely distributed in canary birds (Serinus canaria f. domestica). Vet. Microbiol. 2013, 165, 287–295. [Google Scholar] [CrossRef]
- Fischer, T.; Planz, O.; Stitz, L.; Rziha, H.J. Novel recombinant parapoxvirus vectors induce protective humoral and cellular immunity against lethal herpesvirus challenge infection in mice. J. Virol. 2003, 77, 9312–9323. [Google Scholar] [CrossRef]
- Rziha, H.; Henkel, M.; Cottone, R.; Bauer, B.; Auge, U.; Gotz, F.; Pfaff, E.; Rottgen, M.; Dehio, C.; Buttner, M. Generation of recombinant parapoxviruses: Non-essential genes suitable for insertion and expression of foreign genes. J. Biotechnol. 2000, 83, 137–145. [Google Scholar] [CrossRef]
- Honkavuori, K.S.; Shivaprasad, H.L.; Williams, B.L.; Quan, P.L.; Hornig, M.; Street, C.; Palacios, G.; Hutchison, S.K.; Franca, M.; Egholm, M.; et al. Novel borna virus in psittacine birds with proventricular dilatation disease. Emerg. Infect. Dis. 2008, 14, 1883–1886. [Google Scholar] [CrossRef]
- Zimmermann, V.; Rinder, M.; Kaspers, B.; Staeheli, P.; Rubbenstroth, D. Impact of antigenic diversity on laboratory diagnosis of Avian bornavirus infections in birds. J. Vet. Diagn. Investig. 2014, 26, 769–777. [Google Scholar] [CrossRef]
- Richt, J.A.; Schmeel, A.; Frese, K.; Carbone, K.M.; Narayan, O.; Rott, R. Borna disease virus-specific T cells protect against or cause immunopathological Borna disease. J. Exp. Med. 1994, 179, 1467–1473. [Google Scholar] [CrossRef]
- Kim, T.J.; Jin, H.T.; Hur, S.Y.; Yang, H.G.; Seo, Y.B.; Hong, S.R.; Lee, C.W.; Kim, S.; Woo, J.W.; Park, K.S.; et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat. Commun. 2014, 5, 5317. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.J.; Senne, D.A. Newcastle Disease, other avian paramyxoviruses, and pneumovirus infections. In Diseases of Poultry, 12th ed.; Saif, Y.M., Fadly, A.M., Glisson, J.R., McDougald, L.R., Nolan, L.K., Swayne, D.E., Eds.; Iowa State University Press: Ames, IA, USA, 2008; pp. 75–116. [Google Scholar]
- Leal de Araujo, J.; Rech, R.R.; Heatley, J.J.; Guo, J.; Giaretta, P.R.; Tizard, I.; Rodrigues-Hoffmann, A. From nerves to brain to gastrointestinal tract: A time-based study of parrot bornavirus 2 (PaBV-2) pathogenesis in cockatiels (Nymphicus hollandicus). PLoS ONE 2017, 12, e0187797. [Google Scholar] [CrossRef] [PubMed]
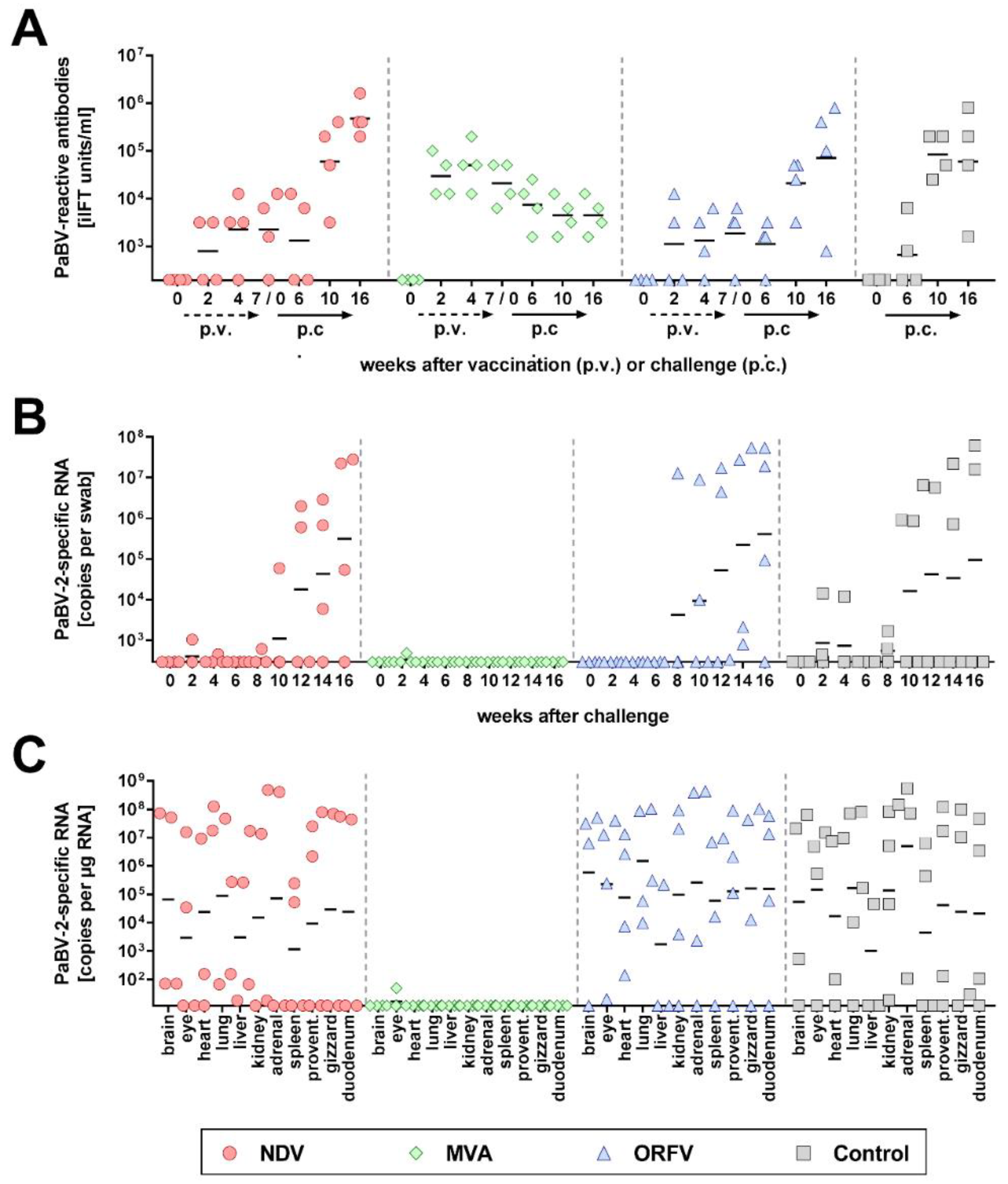
| Group | Anti-PaBV Titre at Challenge (Log iIFT units/mL) | PaBV-2 Shedding a | Number of PaBV-2-pos. Organs b | Clinical Disease c (Weeks p.c. d) | Proven-tricular Dilatation | Mononuclear Infiltration Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bird | Cere-Brum | Adrenal Gland | Heart | Crop | Proven-triculus | Gizzard | Duo-denum | |||||
| NDV | ||||||||||||
| A1 | 4.1 | + | 11 | - | - | + | n.a. e | +++ | +++ | - | +++ | - |
| A2 | <2.6 | - | 4 | - | - | - | + | + | - | - | + | - |
| A3 | 3.2 | + | 11 | - | - | + | +++ | +++ | ++ | ++ | - | +++ |
| A4 | 3.8 | + | 11 | - | - | - | +++ | +++ | + | ++ | +++ | ++ |
| MVA | ||||||||||||
| B1 | 4.7 | - | 1 | - | - | - | - | - | - | - | - | - |
| B2 | 4.7 | - | - | - | - | - | - | - | - | - | - | - |
| B3 | 3.8 | - | - | - | - | - | n.a. | - | - | - | - | - |
| B4 | 4.1 | - | - | - | - | - | - | - | - | + | - | - |
| ORFV | ||||||||||||
| C1 | <2.6 | + | 11 | - | - | - | +++ | +++ | + | - | - | - |
| C2 | 3.5 | + | 10 | 15–16 | + | +++ | n.a. | ++ | ++ | ++ | - | ++ |
| C3 | 3.8 | (+) f | 3 | - | - | - | - | - | - | - | - | - |
| C4 | 3.5 | + | 11 | - | - | - | n.a. | - | - | - | - | + |
| Control | ||||||||||||
| D1 | <2.6 | + | 11 | - | - | - | - | - | ++ | + | - | - |
| D2 | <2.6 | - | 8 | - | - | - | n.a. | ++ | ++ | - | - | - |
| D3 | <2.6 | + | 11 | - | - | - | n.a. | ++ | +++ | +++ | +++ | +++ |
| D4 | <2.6 | - | 3 | - | - | - | n.a. | - | - | - | - | - |
| Group | Clinical Disease a (Weeks p.i. b) | Death (Week p.i.) | Mononuclear Infiltration Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bird | Before First Immunization | After First Immunization c | Cere-brum | Cere-bellum | Adrenal Gland | Heart | Pan-creas | Crop | Proven-triculus | Gizzard | |
| Immunized | |||||||||||
| #1 | - | - | - | ++ | + | +++ | ++ | ++ | - | - | - |
| #2 | - | - | - | - | - | n.a. d | ++ | ++ | - | - | - |
| #4 | - | - | - | - | - | - | ++ | ++ | ++ | +++ | + |
| #5 | 6–8 | 16–18 | 18 | + | + | ++ | ++ | n.a. | +++ | ++ | ++ |
| #6 | 5 | 17–18 | - | - | - | n.a. | ++ | - | + | + | + |
| #12 | 9 | - | - | - | - | +++ | ++ | + | ++ | + | - |
| Control | |||||||||||
| #3 | 5 | - | - | +++ | +++ | +++ | ++ | +++ | +++ | +++ | +++ |
| #7 | 5–8, 12 | 13–22 | - | - | - | ++ | +++ | +++ | ++ | ++ | + |
| #8 | - | - | - | - | - | n.a. | ++ | + | + | +++ | - |
| #9 | - | - | - | + | - | + | + | +++ | + | ++ | + |
| #10 | - | 18 | - | + | - | ++ | +++ | ++ | +++ | +++ | + |
| #11 | - | - | - | - | - | - | - | - | - | + | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rall, I.; Amann, R.; Malberg, S.; Herden, C.; Rubbenstroth, D. Recombinant Modified Vaccinia Virus Ankara (MVA) Vaccines Efficiently Protect Cockatiels Against Parrot Bornavirus Infection and Proventricular Dilatation Disease. Viruses 2019, 11, 1130. https://doi.org/10.3390/v11121130
Rall I, Amann R, Malberg S, Herden C, Rubbenstroth D. Recombinant Modified Vaccinia Virus Ankara (MVA) Vaccines Efficiently Protect Cockatiels Against Parrot Bornavirus Infection and Proventricular Dilatation Disease. Viruses. 2019; 11(12):1130. https://doi.org/10.3390/v11121130
Chicago/Turabian StyleRall, Isabell, Ralf Amann, Sara Malberg, Christiane Herden, and Dennis Rubbenstroth. 2019. "Recombinant Modified Vaccinia Virus Ankara (MVA) Vaccines Efficiently Protect Cockatiels Against Parrot Bornavirus Infection and Proventricular Dilatation Disease" Viruses 11, no. 12: 1130. https://doi.org/10.3390/v11121130
APA StyleRall, I., Amann, R., Malberg, S., Herden, C., & Rubbenstroth, D. (2019). Recombinant Modified Vaccinia Virus Ankara (MVA) Vaccines Efficiently Protect Cockatiels Against Parrot Bornavirus Infection and Proventricular Dilatation Disease. Viruses, 11(12), 1130. https://doi.org/10.3390/v11121130





