High-Throughput MicroRNA Profiles of Permissive Madin-Darby Canine Kidney Cell Line Infected with Influenza B Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Virus Infection
2.2. MicroRNA and RNA Extraction
2.3. Library Preparation and Next-Generation Sequencing
2.4. Reverse Transcription and Quantitative Polymerase Chain Reaction (RT-qPCR)
2.5. Classification of the Target Genes and In Silico Target Site Prediction
2.6. Plasmid Construction
2.7. Overexpression and Inhibition of microRNAs
2.8. Dual Luciferase Assay
2.9. Statistical Analysis
3. Results
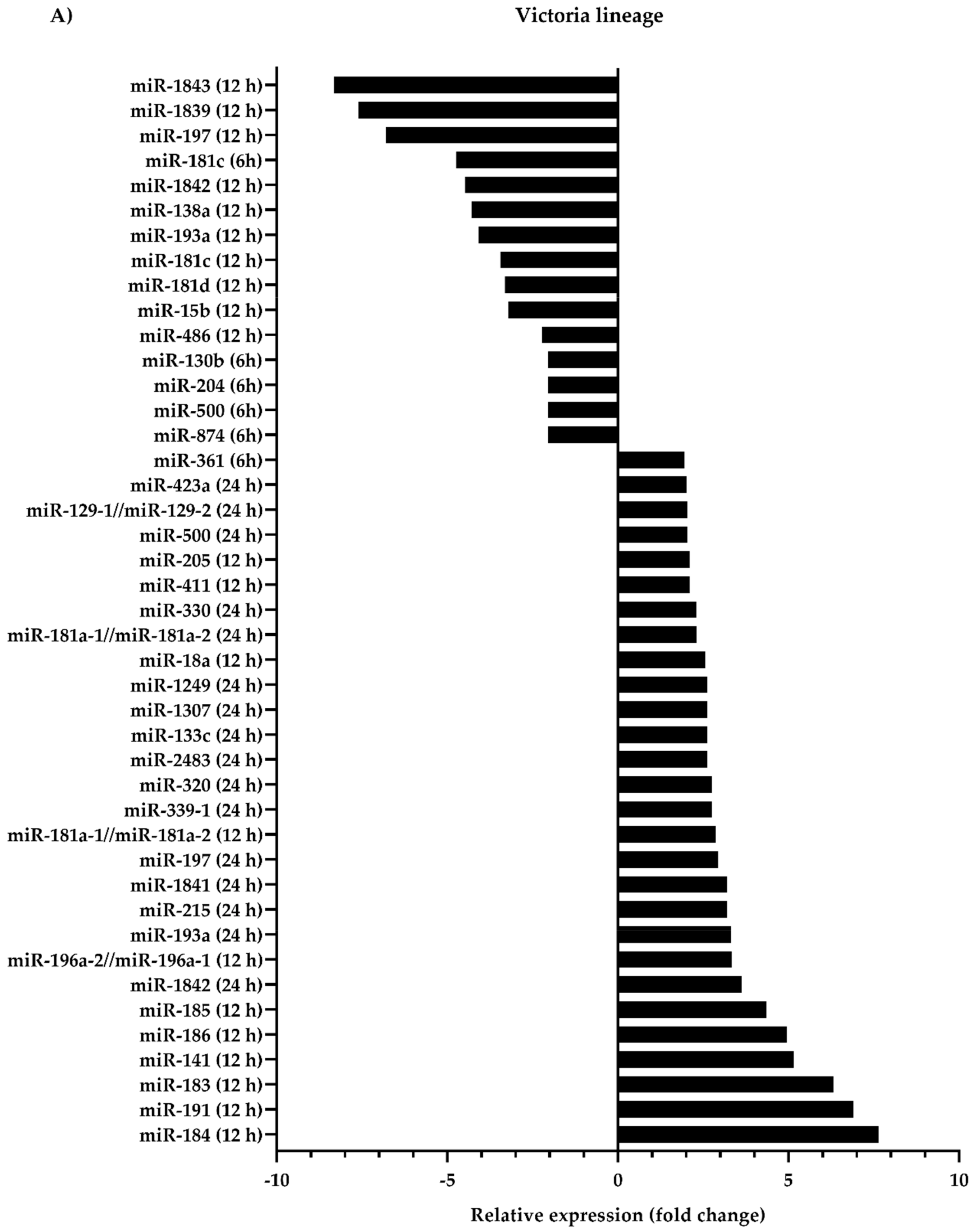
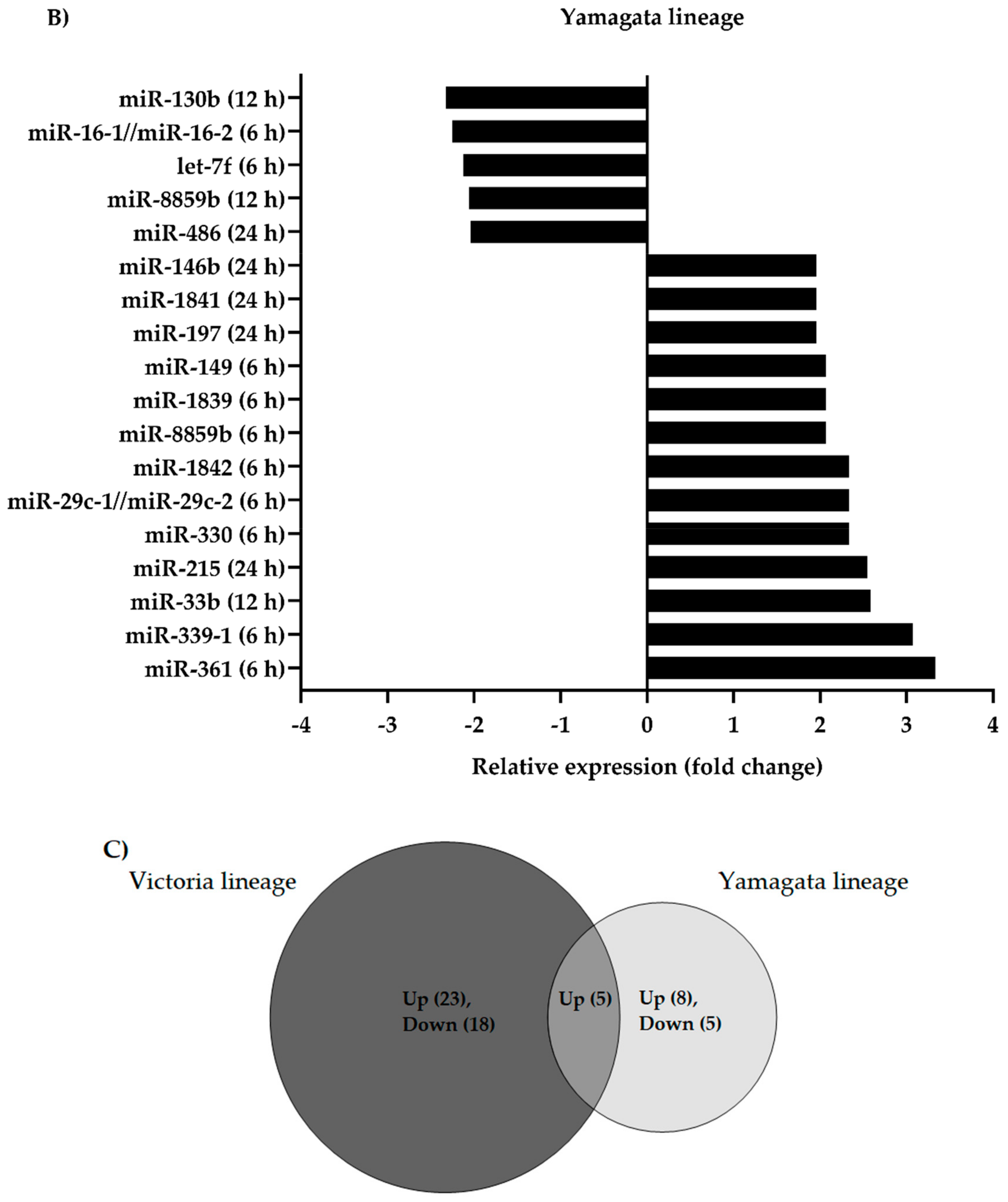
3.1. Differential Expression of Canine microRNAs upon Infection of Influenza B Viruses
3.2. Biological Classification of the Predicted Target Genes
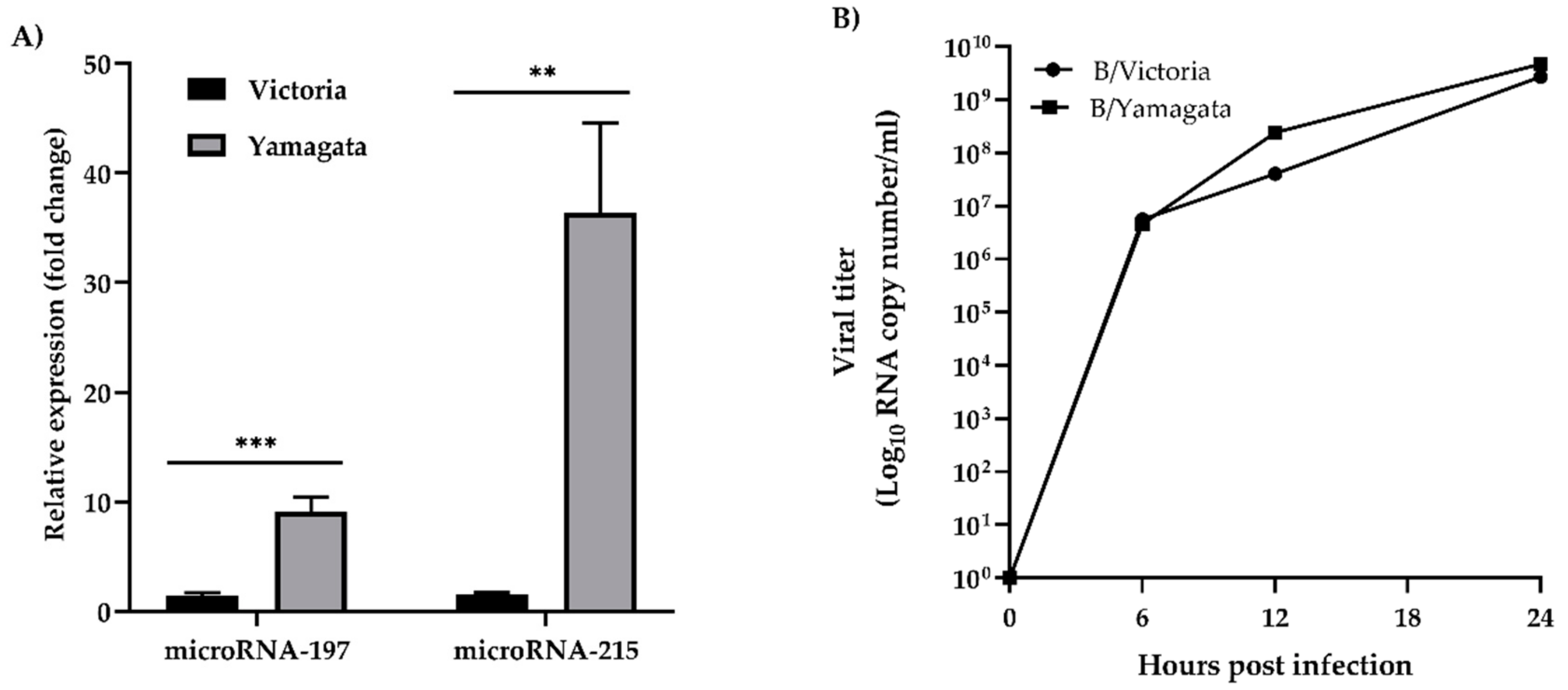
3.3. Validation of cfa-miR-197 and cfa-miR-215
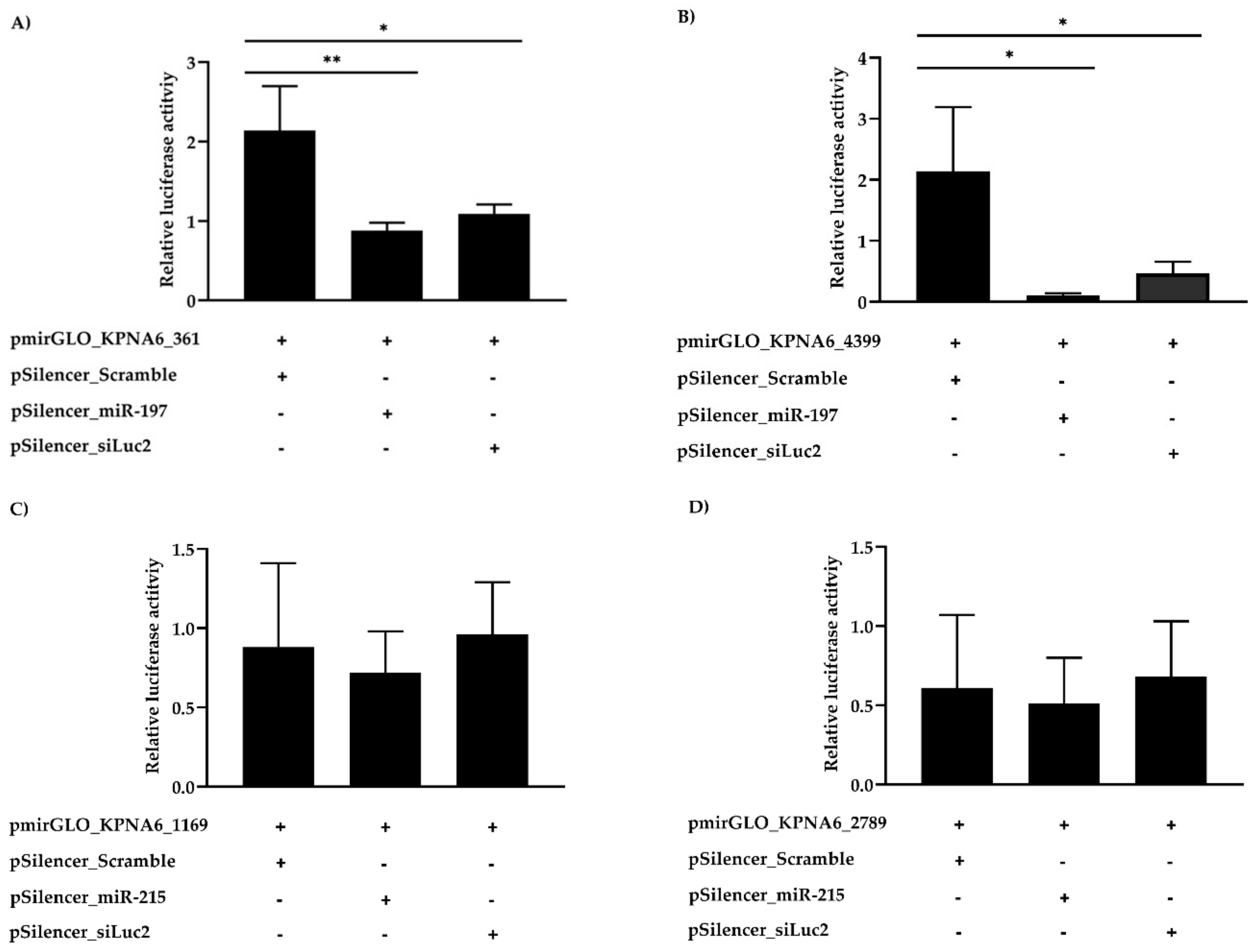
3.4. Karyopherin Alpha 6 (KPNA6) as a Target of cfa-miR-197
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hilleman, M.R. Realities and enigmas of human viral influenza: Pathogenesis, epidemiology and control. Vaccine 2002, 20, 3068–3087. [Google Scholar] [CrossRef]
- Webster, R.G.; Wright, S.M.; Castrucci, M.R.; Bean, W.J.; Kawaoka, Y. Influenza—A model of an emerging virus disease. Intervirology 1993, 35, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Rota, P.A.; Wallis, T.R.; Harmon, M.W.; Rota, J.S.; Kendal, A.P.; Nerome, K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 1990, 175, 59–68. [Google Scholar] [CrossRef]
- Jackson, D.; Elderfield, R.A.; Barclay, W.S. Molecular studies of influenza B virus in the reverse genetics era. J. Gen. Virol. 2011, 92, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Caini, S.; Huang, Q.S.; Ciblak, M.A.; Kusznierz, G.; Owen, R.; Wangchuk, S.; Henriques, C.M.; Njouom, R.; Fasce, R.A.; Yu, H.; et al. Epidemiological and virological characteristics of influenza B: Results of the global influenza B study. Influenza Other Respir. Viruses 2015, 9, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Lapinscki, B.; Pereira, L.A.; Nogueira, M.B.; Vidal, L.R.; Riediger, I.; Debur, M.C.; Presibella, M.; Raboni, S.M. Molecular epidemiology of influenza B virus and implications in immunization strategy, Southern Brazil. Vaccine 2018, 36, 107–113. [Google Scholar] [CrossRef]
- Balish, A.L.; Katz, J.M.; Klimov, A.I. Influenza: Propagation, quantification, and storage. Curr. Protoc. Microbiol. 2013. [Google Scholar] [CrossRef]
- Govorkova, E.A.; Murti, G.; Meignier, B.; de Taisne, C.; Webster, R.G. African green monkey kidney (Vero) cells provide an alternative host cell system for influenza A and B viruses. J. Virol. 1996, 70, 5519–5524. [Google Scholar] [Green Version]
- Aggarwal, K.; Jing, F.; Maranga, L.; Liu, J. Bioprocess optimization for cell culture based influenza vaccine production. Vaccine 2011, 29, 3320–3328. [Google Scholar] [CrossRef]
- Ugiyadi, M.; Tan, M.I.; Giri-Rachman, E.A.; Zuhairi, F.R.; Sumarsono, S.H. The expression of essential components for human influenza virus internalisation in Vero and MDCK cells. Cytotechnology 2014, 66, 515–523. [Google Scholar] [CrossRef]
- Girardi, E.; Lopez, P.; Pfeffer, S. On the importance of host microRNAs during viral infection. Front. Genet. 2018, 9, 439. [Google Scholar] [CrossRef] [PubMed]
- Trobaugh, D.W.; Klimstra, W.B. MicroRNA regulation of RNA virus replication and pathogenesis. Trends Mol. Med. 2017, 23, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. MiRBase: Annotating high confidence microRNAs using deep sequencing data. Nucl. Acids Res. 2014, 42. [Google Scholar] [CrossRef] [PubMed]
- Makkoch, J.; Poomipak, W.; Saengchoowong, S.; Khongnomnan, K.; Praianantathavorn, K.; Jinato, T.; Poovorawan, Y.; Payungporn, S. Human microRNAs profiling in response to influenza A viruses (subtypes pH1N1, H3N2, and H5N1). Exp. Biol. Med. 2016, 241, 409–420. [Google Scholar] [CrossRef]
- Mei, Q.; Li, X.; Meng, Y.G.; Wu, Z.Q.; Guo, M.Z.; Zhao, Y.L.; Fu, X.B.; Han, W.D. A facile and specific assay for quantifying MicroRNA by an optimized RT-qPCR approach. PLoS ONE 2012, 7, e46890. [Google Scholar] [CrossRef]
- Wong, N.; Wang, X. MiRDB: An online resource for microRNA target prediction and functional annotations. Nucl. Acids Res. 2015, 43. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucl. Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4. [Google Scholar] [CrossRef]
- Rehmsmeier, M.; Steffen, P.; Hochsmann, M.; Giegerich, R. Fast and effective prediction of microRNA/target duplexes. Rna 2004, 10, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.F.; Huang, R.T.; Chien, K.Y.; Huang, J.Y.; Lau, K.S.; Jheng, J.R.; Chiu, C.H.; Wu, T.Y.; Chen, C.Y.; Horng, J.T. Host MicroRNA miR-197 plays a negative regulatory role in the enterovirus 71 infectious cycle by targeting the RAN protein. J. Virol. 2016, 90, 1424–1438. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; He, Z. MiR-215 enhances HCV replication by targeting TRIM22 and inactivating NF-kappaB Signaling. Yonsei Med. J. 2018, 59, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Fu, S.; Wang, J. Hepatitis C virus infection decreases the expression of Toll-like receptors 3 and 7 via upregulation of miR-758. Arch. Virol. 2014, 159, 2997–3003. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Di Bisceglie, A.M.; Ray, R.B. Hepatitis C virus-mediated enhancement of microRNA miR-373 impairs the JAK/STAT signaling pathway. J. Virol. 2015, 89, 3356–3365. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Duan, X.; Li, Y.; Liu, B.; McGilvray, I.; Chen, L. MicroRNA-130a inhibits HCV replication by restoring the innate immune response. J. Viral. Hepat. 2014, 21, 121–128. [Google Scholar] [CrossRef]
- Smith, J.L.; Grey, F.E.; Uhrlaub, J.L.; Nikolich-Zugich, J.; Hirsch, A.J. Induction of the cellular microRNA, Hs_154, by West Nile virus contributes to virus-mediated apoptosis through repression of antiapoptotic factors. J. Virol. 2012, 86, 5278–5287. [Google Scholar] [CrossRef]
- Othumpangat, S.; Walton, C.; Piedimonte, G. MicroRNA-221 modulates RSV replication in human bronchial epithelium by targeting NGF expression. PLoS ONE 2012, 7, e30030. [Google Scholar] [CrossRef]
- Ho, B.C.; Yu, I.S.; Lu, L.F.; Rudensky, A.; Chen, H.Y.; Tsai, C.W.; Chang, Y.L.; Wu, C.T.; Chang, L.Y.; Shih, S.R.; et al. Inhibition of miR-146a prevents enterovirus-induced death by restoring the production of type I interferon. Nat. Commun. 2014, 5, 3344. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; He, L.; Li, Y.; Wang, T.; Feng, L.; Jiang, L.; Zhang, P.; Huang, X. MiR-146a facilitates replication of dengue virus by dampening interferon induction by targeting TRAF6. J. Infect. 2013, 67, 329–341. [Google Scholar] [CrossRef]
- Sharma, N.; Verma, R.; Kumawat, K.L.; Basu, A.; Singh, S.K. MiR-146a suppresses cellular immune response during Japanese encephalitis virus JaOArS982 strain infection in human microglial cells. J. Neuroinflamm. 2015, 12, 30. [Google Scholar] [CrossRef]
- Othumpangat, S.; Noti, J.D.; Blachere, F.M.; Beezhold, D.H. Expression of non-structural-1A binding protein in lung epithelial cells is modulated by miRNA-548an on exposure to influenza A virus. Virology 2013, 447, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Othumpangat, S.; Noti, J.D.; Beezhold, D.H. Lung epithelial cells resist influenza A infection by inducing the expression of cytochrome c oxidase VIc which is modulated by miRNA 4276. Virology 2014. [Google Scholar] [CrossRef] [PubMed]
- Ingle, H.; Kumar, S.; Raut, A.A.; Mishra, A.; Kulkarni, D.D.; Kameyama, T.; Takaoka, A.; Akira, S.; Kumar, H. The microRNA miR-485 targets host and influenza virus transcripts to regulate antiviral immunity and restrict viral replication. Sci. Signal 2015, 8, ra126. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, C.M.; Podyminogin, R.L.; Navarro, G.; Zhao, G.W.; Askovich, P.S.; Weiss, M.J.; Aderem, A. MiR-451 regulates dendritic cell cytokine responses to influenza infection. J. Immunol. 2012, 189, 5965–5975. [Google Scholar] [CrossRef]
- Dong, C.; Sun, X.; Guan, Z.; Zhang, M.; Duan, M. Modulation of influenza A virus replication by microRNA-9 through targeting MCPIP1. J. Med. Virol. 2017, 89, 41–48. [Google Scholar] [CrossRef]
- Zheng, Y.; Fu, X.; Wang, L.; Zhang, W.; Zhou, P.; Zhang, X.; Zeng, W.; Chen, J.; Cao, Z.; Jia, K.; et al. Comparative analysis of MicroRNA expression in dog lungs infected with the H3N2 and H5N1 canine influenza viruses. Microb. Pathog. 2018, 121, 252–261. [Google Scholar] [CrossRef]
- Zhou, P.; Tu, L.; Lin, X.; Hao, X.; Zheng, Q.; Zeng, W.; Zhang, X.; Zheng, Y.; Wang, L.; Li, S. Cfa-miR-143 promotes apoptosis via the p53 pathway in canine influenza virus H3N2-infected cells. Viruses 2017, 9. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, C.; Sun, X.; Li, Z.; Zhang, M.; Guan, Z.; Duan, M. Induction of the cellular miR-29c by influenza virus inhibits the innate immune response through protection of A20 mRNA. Biochem. Biophys. Res. Commun. 2014, 450, 755–761. [Google Scholar] [CrossRef]
- Khongnomnan, K.; Poomipak, W.; Praianantathavorn, K.; Saengchoowong, S.; Pisitkun, T.; Poovorawan, Y.; Payungporn, S. Human MicroRNAs expression profiles in influenza B virus-infected cells based on Illumina MiSeq platform. Microrna 2018, 7, 204–214. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, C. MiR1973pinduced downregulation of lysine 63 deubiquitinase promotes cell proliferation and inhibits cell apoptosis in lung adenocarcinoma cell lines. Mol. Med. Rep. 2018, 17, 3921–3927. [Google Scholar] [CrossRef]
- Ge, G.Q.; Zhang, W.; Niu, L.G.; Yan, Y.; Ren, Y.; Zou, Y.L. MiR-215 functions as a tumor suppressor in epithelial ovarian cancer through regulation of the X-chromosome-linked inhibitor of apoptosis. Oncol. Rep. 2016, 35, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.X.; Zhang, J.; Zhang, J.; Miao, Q.; Yao, L.B.; Zhang, J. Curcumin promotes apoptosis by activating the p53-miR-1925–p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015, 357, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, R.; Yang, S.; Ma, Z.; Lin, S.; Nan, Y.; Li, Q.; Tang, Q.; Zhang, Y.J. Karyopherin alpha 6 is required for replication of porcine reproductive and respiratory syndrome virus and zika virus. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.; Feldmann, F.; Reimer, R.; Thiele, S.; Fischer, M.; Hartmann, E.; Bader, M.; Ebihara, H.; Hoenen, T.; Feldmann, H. Importin-alpha7 is involved in the formation of ebola virus inclusion bodies but is not essential for pathogenicity in mice. J. Infect. Dis 2015, 212. [Google Scholar] [CrossRef]
- Schwarz, T.M.; Edwards, M.R.; Diederichs, A.; Alinger, J.B.; Leung, D.W.; Amarasinghe, G.K.; Basler, C.F. VP24-Karyopherin alpha binding affinities differ between ebolavirus species, influencing interferon inhibition and vp24 stability. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Resa-Infante, P.; Gabriel, G. The nuclear import machinery is a determinant of influenza virus host adaptation. Bioessays 2013, 35, 23–27. [Google Scholar] [CrossRef]
- Pumroy, R.A.; Ke, S.; Hart, D.J.; Zachariae, U.; Cingolani, G. Molecular determinants for nuclear import of influenza A PB2 by importin alpha isoforms 3 and 7. Structure 2015, 23, 374–384. [Google Scholar] [CrossRef]
- Gabriel, G.; Herwig, A.; Klenk, H.D. Interaction of polymerase subunit PB2 and NP with importin alpha1 is a determinant of host range of influenza A virus. PLoS Pathog. 2008, 4, e11. [Google Scholar] [CrossRef]
- Resa-Infante, P.; Thieme, R.; Ernst, T.; Arck, P.C.; Ittrich, H.; Reimer, R.; Gabriel, G. Importin-alpha7 is required for enhanced influenza A virus replication in the alveolar epithelium and severe lung damage in mice. J. Virol. 2014, 88, 8166–8179. [Google Scholar] [CrossRef]
- Bertram, S.; Thiele, S.; Dreier, C.; Resa-Infante, P.; Preuss, A.; van Riel, D.; Mok, C.K.P.; Schwalm, F.; Peiris, J.S.M.; Klenk, H.D.; et al. H7N9 influenza a virus exhibits importin-alpha7-mediated replication in the mammalian respiratory tract. Am. J. Pathol. 2017, 187, 831–840. [Google Scholar] [CrossRef]
- Hudjetz, B.; Gabriel, G. Human-like PB2 627K influenza virus polymerase activity is regulated by importin-alpha1 and -alpha7. PLoS Pathog. 2012, 8, e1002488. [Google Scholar] [CrossRef] [PubMed]
- Resa-Infante, P.; Paterson, D.; Bonet, J.; Otte, A.; Oliva, B.; Fodor, E.; Gabriel, G. Targeting importin-alpha7 as a therapeutic approach against pandemic influenza viruses. J. Virol. 2015, 89, 9010–9020. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Sankhala, R.S.; Florio, T.J.; Zhou, L.; Nguyen, N.L.T.; Lokareddy, R.K.; Cingolani, G.; Pante, N. Synergy of two low-affinity NLSs determines the high avidity of influenza A virus nucleoprotein NP for human importin alpha isoforms. Sci. Rep. 2017, 7, 11381. [Google Scholar] [CrossRef] [PubMed]
- Labaronne, A.; Milles, S.; Donchet, A.; Jensen, M.R.; Blackledge, M.; Bourhis, J.M.; Ruigrok, R.W.H.; Crepin, T. Structural analysis of the complex between influenza B nucleoprotein and human importin-alpha. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, T.; Zhao, F.; Lau, A.; Birch, C.M.; Zhang, D.D. KPNA6 (Importin {alpha}7)-mediated nuclear import of Keap1 represses the Nrf2-dependent antioxidant response. Mol. Cell Biol. 2011, 31, 1800–1811. [Google Scholar] [CrossRef]
- Medvedev, R.; Ploen, D.; Spengler, C.; Elgner, F.; Ren, H.; Bunten, S.; Hildt, E. HCV-induced oxidative stress by inhibition of Nrf2 triggers autophagy and favors release of viral particles. Free Radic. Biol. Med. 2017, 110, 300–315. [Google Scholar] [CrossRef]
- Shen, J.; Wang, G.; Zuo, J. Caffeic acid inhibits HCV replication via induction of IFNalpha antiviral response through p62-mediated Keap1/Nrf2 signaling pathway. Antiviral Res. 2018, 154, 166–173. [Google Scholar] [CrossRef]

| Primers | Nucleotide Sequences (5′–3′) | PCR Conditions (40 Cycles) |
|---|---|---|
| Cfa-miR-361_F | TCAGAATCTCCAGGGGTAC | 95 °C 15 s, 58 °C 30 s, 62 °C 30 s |
| Cfa-miR-197_F | ACCACCTTCTCCACCCAG | 95 °C 15 s, 58 °C 30 s, 62 °C 30 s |
| Cfa-miR-215_F | TGACCTACGAATTGATAGACA | 95 °C 15 s, 55 °C 30 s, 62 °C 30 s |
| Cfa- RNU6-2 _F | CTCGCTTCGGCAGCACA | 95 °C 15 s, 55 °C 30 s, 62 °C 30 s |
| Cfa-miRNA-qPCR_R | TGCGGATAACAATTTCACACA | - |
| Cfa-KPNA6_F775 | CCAAAGAGCCTAGTCCTCCA | 95 °C 15 s, 64 °C 20 s, 80 °C 20 s |
| Cfa-KPNA6_R926 | CTGCTGAGAGGTTCCAGAGG | |
| Cfa-GAPDH_F85 | GTGAAGGTCGGAGTCAACGG | 95 °C 15 s, 60 °C 20 s, 72 °C 20 s |
| Cfa-GAPDH_R191 | TCAATGAAGGGGTCATTGATGG | |
| Flu B_PB1_F269 | AGGCTTTGGATAGAATGGATGA | 95 °C 15 s, 57 °C 20 s, 72 °C 30 s |
| Flu B_PB1_R385 | AAGTCTGTCTCCCCTGGGTT |
| Oligonucleotides 1 | Nucleotide Sequences (5′–3′) | Plasmids |
|---|---|---|
| Cfa-miR-197_TS | GATCCGCGGGTAGAGAGGGCAGTGGGAGGTAAGAGCTCTT CACCCTTCACCACCTTCTCCACCCAGCTTTTTTGGAAA | pSilencer 3.0-H1 |
| Cfa-miR-197_BS | AGCTTTTCCAAAAAAGCTGGGTGGAGAAGGTGGTGAAGGG TGAAGAGCTCTTACCTCCCACTGCCCTCTCTACCCGCG | |
| Cfa-miR-215_TS | GATCCATGACCTACGAATTGATAGACAATTTGGCTAAGTTT GTCTGTCATTTTTGTAGGCCATTTTTTGGAAA | pSilencer 3.0-H1 |
| Cfa-miR-215_BS | AGCTTTTCCAAAAAATGGCCTACAAAAATGACAGACAAAC TTAGCCAAATTGTCTATCAATTCGTAGGTCATG | |
| Cfa-miR-Scramble_TS | GATCCGCAGGTCTTTCATCTAGAACGATGCGGGTTCAAGAG ACCCGCATCGTTCTAGATGAAAGACCTGTTTTTTGGAAA | pSilencer 3.0-H1 |
| Cfa-miR-Scramble_BS | AGCTTTTCCAAAAAACAGGTCTTTCATCTAGAACGATGCGG GTCTCTTGAACCCGCATCGTTCTAGATGAAAGACCTGCG | |
| siLuc/Luc2_TS | GATCCCACCCCAACATCTTCGACGTTCAAGAGACGTCGAAG ATGTTGGGGTGTTTTTTGGAAA | pSilencer 3.0-H1 |
| siLuc/Luc2_BS | AGCTTTTCCAAAAAACACCCCAACATCTTCGACGTCTCTTGA ACGTCGAAGATGTTGGGGTGG | |
| KPNA6_361_TS | CTAGTTATTTTTTTCTTTAGTGGTGACT | pmirGLO |
| KPNA6_361_BS | TCGAAGTCACCACTAAAGAAAAAAATAA | |
| KPNA6_4399_TS | CTAGGCTGTGCCGTGGGGCTGGTGAAGA | pmirGLO |
| KPNA6_4399_BS | TCGATCTTCACCAGCCCCACGGCACAGC | |
| KPNA6_1169_TS | CTAGATTCTATATATTAGGTAGGTCAAT | pmirGLO |
| KPNA6_1169_BS | TCGAATTGACCTACCTAATATATAGAAT | |
| KPNA6_2784_TS | CTAGACCCTGGCTTCGATGAGGTCAAAG | pmirGLO |
| KPNA6_2784_BS | TCGACTTTGACCTCATCGAAGCCAGGGT |
| Biological Processes 1 | The Number and the Percentage of microRNA Target Genes 2 | ||||
|---|---|---|---|---|---|
| Cfa-miR-197 | Cfa-miR-215 | Cfa-miR-361 | Cfa-miR-1841 | Cfa-miR-1842 | |
| BA | 3 (1.95%) | 4 (4.08%) | 3 (1.58%) | 10 (1.72%) | 5 (1.49%) |
| BP | 0 (0%) | 0 (0%) | 1 (0.53%) | 0 (0%) | 0 (0%) |
| BR | 33 (21.43%) | 20 (20.41%) | 38 (20.00%) | 111 (19.07%) | 56 (16.67%) |
| Pro | 1 (0.65%) | 0 (0%) | 2 (1.05%) | 2 (0.34%) | 1 (0.30%) |
| CC | 2 (1.30%) | 0 (0%) | 0 (0%) | 5 (0.86%) | 4 (1.19%) |
| CP | 46 (29.87%) | 31 (31.63%) | 56 (29.47%) | 186 (31.96%) | 94 (27.98%) |
| DP | 3 (1.95%) | 2 (2.04%) | 3 (1.58%) | 15 (2.58%) | 12 (3.57%) |
| IS | 2 (1.30%) | 1 (1.02%) | 2 (1.05%) | 5 (0.86%) | 6 (1.79%) |
| Lo | 20 (12.99%) | 12 (12.24%) | 21 (11.05%) | 62 (10.65%) | 35 (10.42%) |
| MP | 33 (21.43%) | 17 (17.35%) | 47 (24.74%) | 156 (26.80%) | 84 (25.00%) |
| MO | 5 (3.25%) | 6 (6.12%) | 14 (7.37%) | 29 (4.98%) | 23 (6.85%) |
| Pi | 0 (0%) | 0 (0%) | 1 (0.53%) | 0 (0%) | 0 (0%) |
| Re | 0 (0%) | 0 (0%) | 5 (2.63%) | 3 (0.52%) | 5 (1.49%) |
| RS | 5 (3.25%) | 3 (3.06%) | 7 (3.68%) | 18 (3.09%) | 11 (3.27%) |
| RP | 1 (0.65%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Si | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.30%) |
| Total genes | 154 | 98 | 190 | 582 | 336 |
| MicroRNAs | Position on 3′ UTR | Predicted Consequential Pairing between Target Region (top) and microRNA (bottom) | MFE (kcal/mol) |
|---|---|---|---|
| Cfa-miR-197 | 361–367 |  | −21.7 |
| 4399–4405 |  | −27.3 | |
| Cfa-miR-215 | 1169–1176 | 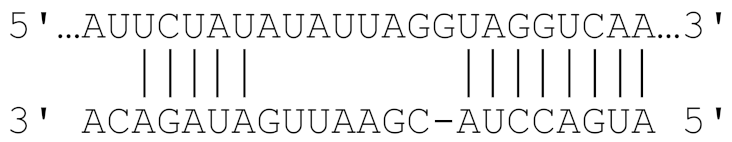 | −22.7 |
| 2784–2790 | 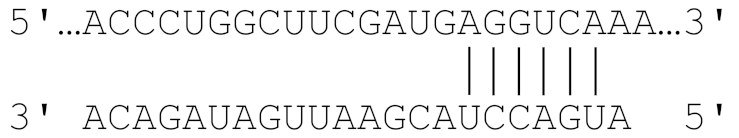 | −20.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saengchoowong, S.; Khongnomnan, K.; Poomipak, W.; Praianantathavorn, K.; Poovorawan, Y.; Zhang, Q.; Payungporn, S. High-Throughput MicroRNA Profiles of Permissive Madin-Darby Canine Kidney Cell Line Infected with Influenza B Viruses. Viruses 2019, 11, 986. https://doi.org/10.3390/v11110986
Saengchoowong S, Khongnomnan K, Poomipak W, Praianantathavorn K, Poovorawan Y, Zhang Q, Payungporn S. High-Throughput MicroRNA Profiles of Permissive Madin-Darby Canine Kidney Cell Line Infected with Influenza B Viruses. Viruses. 2019; 11(11):986. https://doi.org/10.3390/v11110986
Chicago/Turabian StyleSaengchoowong, Suthat, Kritsada Khongnomnan, Witthaya Poomipak, Kesmanee Praianantathavorn, Yong Poovorawan, Qibo Zhang, and Sunchai Payungporn. 2019. "High-Throughput MicroRNA Profiles of Permissive Madin-Darby Canine Kidney Cell Line Infected with Influenza B Viruses" Viruses 11, no. 11: 986. https://doi.org/10.3390/v11110986
APA StyleSaengchoowong, S., Khongnomnan, K., Poomipak, W., Praianantathavorn, K., Poovorawan, Y., Zhang, Q., & Payungporn, S. (2019). High-Throughput MicroRNA Profiles of Permissive Madin-Darby Canine Kidney Cell Line Infected with Influenza B Viruses. Viruses, 11(11), 986. https://doi.org/10.3390/v11110986




