Emerging Role of LY6E in Virus–Host Interactions
Abstract
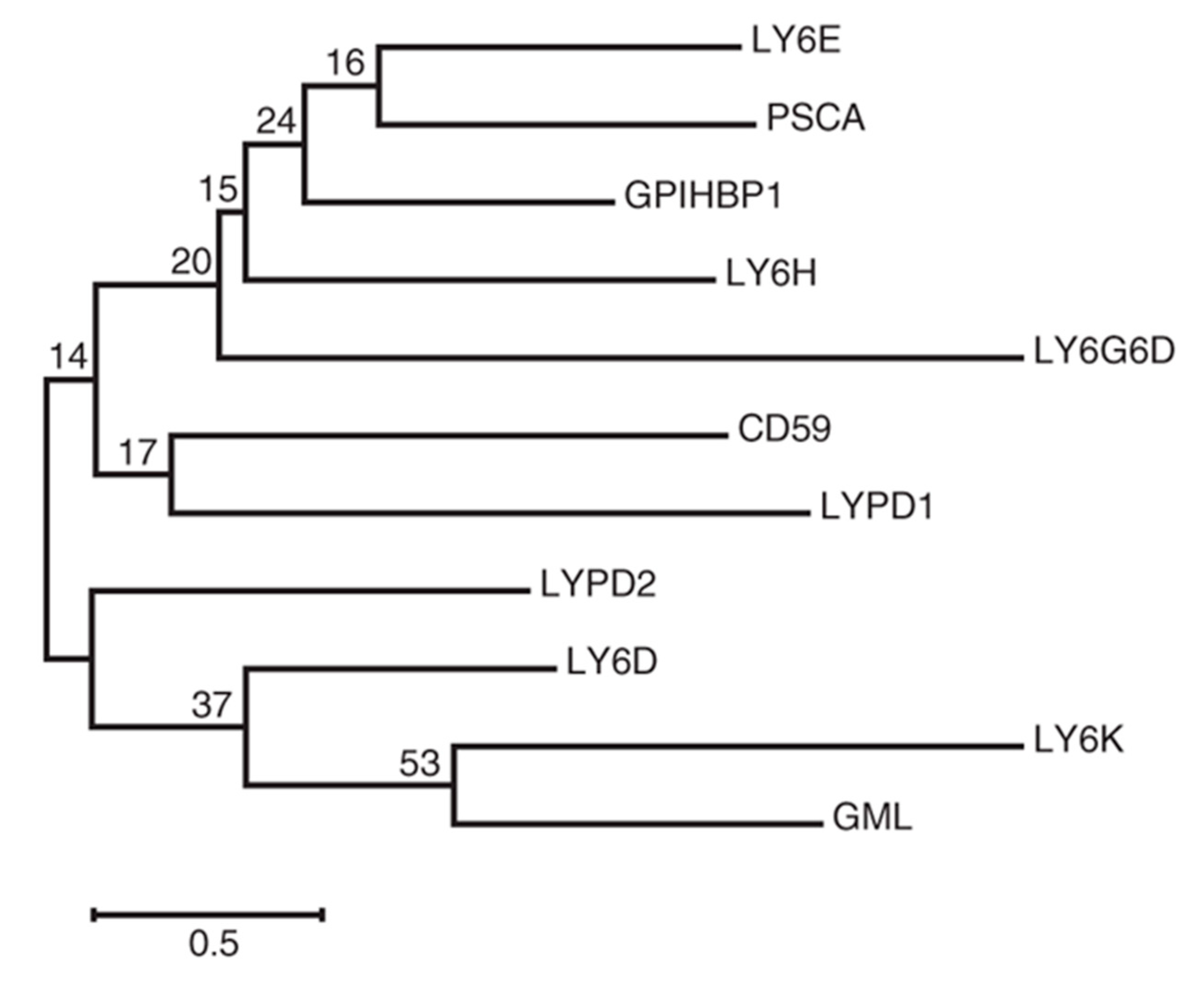
1. Introduction
2. Genetic Association of LY6E with MDV and MAV-1 Infection
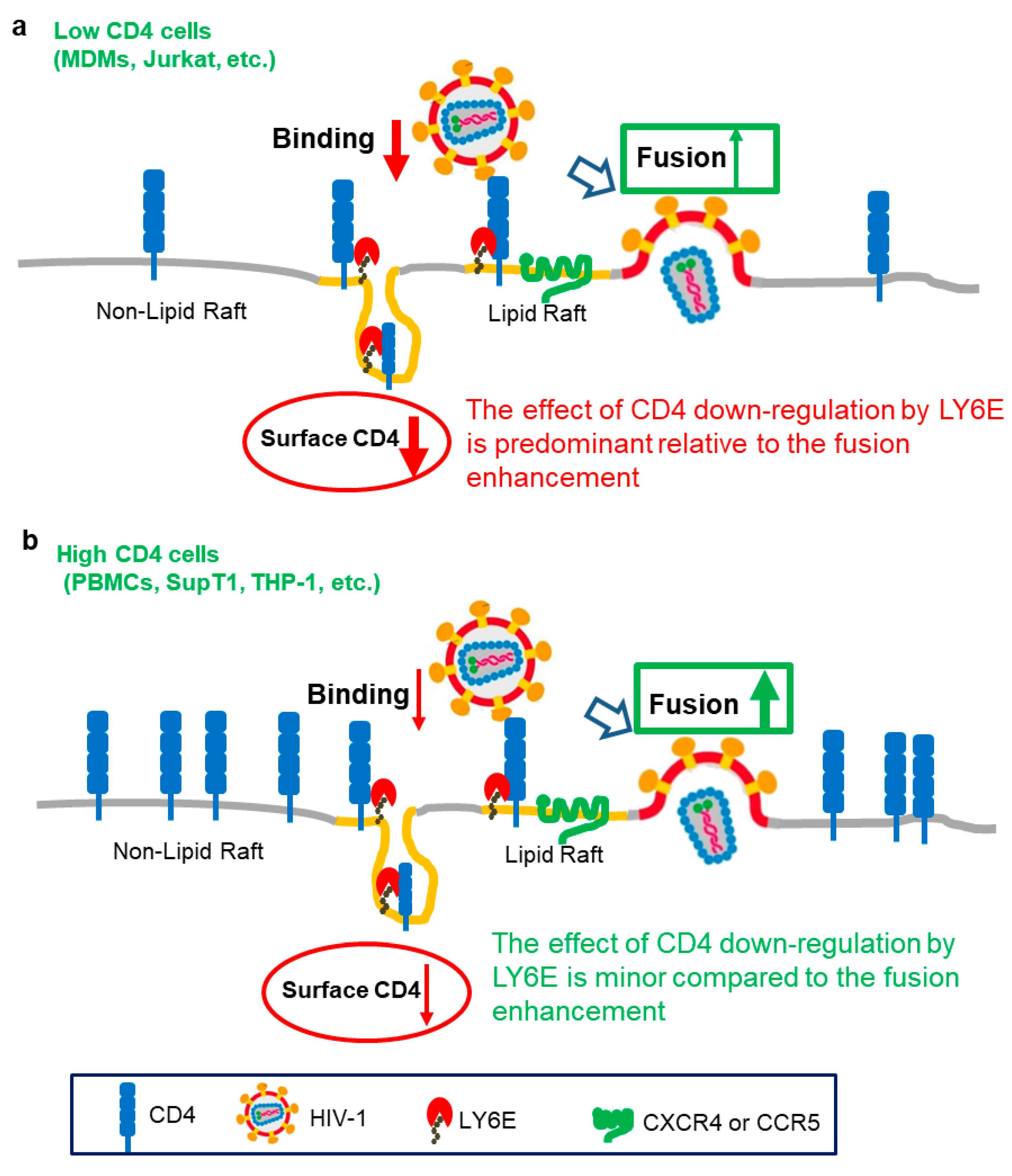
3. Opposing Roles of LY6E in HIV Entry
4. Modulation of Other Viral Infections by LY6E: Yellow Fever Virus (YFV), Dengue Virus (DENV), Influenza A Virus (IAV), and Vesicular Stomatitis Virus (VSV)
5. Mechanisms of Action by LY6E on Viral Infection: Direct vs. Indirect Effects
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Godfrey, D.I.; Masciantonio, M.; Tucek, C.L.; Malin, M.A.; Boyd, R.L.; Hugo, P. Thymic shared antigen-1. A novel thymocyte marker discriminating immature from mature thymocyte subsets. J. Immunol. 1992, 148, 2006–2011. [Google Scholar] [PubMed]
- MacNeil, I.; Kennedy, J.; Godfrey, D.I.; Jenkins, N.A.; Masciantonio, M.; Mineo, C.; Gilbert, D.J.; Copeland, N.G.; Boyd, R.L.; Zlotnik, A. Isolation of a cDNA encoding thymic shared antigen-1. A new member of the Ly6 family with a possible role in T cell development. J. Immunol. 1993, 151, 6913–6923. [Google Scholar] [PubMed]
- Classon, B.J.; Coverdale, L. Mouse stem cell antigen Sca-2 is a member of the Ly-6 family of cell surface proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 5296–5300. [Google Scholar] [CrossRef] [PubMed]
- Capone, M.C.; Gorman, D.M.; Ching, E.P.; Zlotnik, A. Identification through bioinformatics of cDNAs encoding human thymic shared Ag-1/stem cell Ag-2. A new member of the human Ly-6 family. J. Immunol. 1996, 157, 969–973. [Google Scholar] [PubMed]
- Mao, M.; Yu, M.; Tong, J.H.; Ye, J.; Zhu, J.; Huang, Q.H.; Fu, G.; Yu, L.; Zhao, S.Y.; Waxman, S.; et al. RIG-E, a human homolog of the murine Ly-6 family, is induced by retinoic acid during the differentiation of acute promyelocytic leukemia cell. Proc. Natl. Acad. Sci. USA 1996, 93, 5910–5914. [Google Scholar] [CrossRef]
- Noda, S.; Kosugi, A.; Saitoh, S.; Narumiya, S.; Hamaoka, T. Protection from anti-TCR/CD3-induced apoptosis in immature thymocytes by a signal through thymic shared antigen-1/stem cell antigen-2. J. Exp. Med. 1996, 183, 2355–2360. [Google Scholar] [CrossRef]
- Saitoh, S.; Kosugi, A.; Noda, S.; Yamamoto, N.; Ogata, M.; Minami, Y.; Miyake, K.; Hamaoka, T. Modulation of TCR-mediated signaling pathway by thymic shared antigen-1 (TSA-1)/stem cell antigen-2 (Sca-2). J. Immunol. 1995, 155, 5574–5581. [Google Scholar]
- Kosugi, A.; Saitoh, S.; Narumiya, S.; Miyake, K.; Hamaoka, T. Activation-induced expression of thymic shared antigen-1 on T lymphocytes and its inhibitory role for TCR-mediated IL-2 production. Int. Immunol. 1994, 6, 1967–1976. [Google Scholar] [CrossRef]
- Hanke, T.; Mitnacht, R.; Boyd, R.; Hunig, T. Induction of interleukin 2 receptor beta chain expression by self-recognition in the thymus. J. Exp. Med. 1994, 180, 1629–1636. [Google Scholar] [CrossRef]
- Liu, H.C.; Cheng, H.H.; Tirunagaru, V.; Sofer, L.; Burnside, J. A strategy to identify positional candidate genes conferring Marek’s disease resistance by integrating DNA microarrays and genetic mapping. Anim. Genet. 2001, 32, 351–359. [Google Scholar] [CrossRef]
- Morgan, R.W.; Sofer, L.; Anderson, A.S.; Bernberg, E.L.; Cui, J.; Burnside, J. Induction of host gene expression following infection of chicken embryo fibroblasts with oncogenic Marek’s disease virus. J. Virol. 2001, 75, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Niikura, M.; Fulton, J.E.; Cheng, H.H. Identification of chicken lymphocyte antigen 6 complex, locus E (LY6E, alias SCA2) as a putative Marek’s disease resistance gene via a virus-host protein interaction screen. Cytogenet. Genome Res. 2003, 102, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Welton, A.R.; Chesler, E.J.; Sturkie, C.; Jackson, A.U.; Hirsch, G.N.; Spindler, K.R. Identification of quantitative trait loci for susceptibility to mouse adenovirus type 1. J. Virol. 2005, 79, 11517–11522. [Google Scholar] [CrossRef] [PubMed]
- Spindler, K.R.; Welton, A.R.; Lim, E.S.; Duvvuru, S.; Althaus, I.W.; Imperiale, J.E.; Daoud, A.I.; Chesler, E.J. The major locus for mouse adenovirus susceptibility maps to genes of the hematopoietic cell surface-expressed LY6 family. J. Immunol. 2010, 184, 3055–3062. [Google Scholar] [CrossRef] [PubMed]
- Stier, M.T.; Spindler, K.R. Polymorphisms in Ly6 genes in Msq1 encoding susceptibility to mouse adenovirus type 1. Mamm. Genome 2012, 23, 250–258. [Google Scholar] [CrossRef]
- Loeuillet, C.; Deutsch, S.; Ciuffi, A.; Robyr, D.; Taffe, P.; Munoz, M.; Beckmann, J.S.; Antonarakis, S.E.; Telenti, A. In vitro whole-genome analysis identifies a susceptibility locus for HIV-1. PLoS Biol. 2008, 6, e32. [Google Scholar] [CrossRef]
- Xu, X.; Qiu, C.; Zhu, L.; Huang, J.; Li, L.; Fu, W.; Zhang, L.; Wei, J.; Wang, Y.; Geng, Y.; et al. IFN-stimulated gene LY6E in monocytes regulates the CD14/TLR4 pathway but inadequately restrains the hyperactivation of monocytes during chronic HIV-1 infection. J. Immunol. 2014, 193, 4125–4136. [Google Scholar] [CrossRef]
- Yu, J.; Liang, C.; Liu, S.L. Interferon-inducible LY6E Protein Promotes HIV-1 Infection. J. Biol. Chem. 2017, 292, 4674–4685. [Google Scholar] [CrossRef]
- Del Real, G.; Jimenez-Baranda, S.; Lacalle, R.A.; Mira, E.; Lucas, P.; Gomez-Mouton, C.; Carrera, A.C.; Martinez, A.C.; Manes, S. Blocking of HIV-1 infection by targeting CD4 to nonraft membrane domains. J. Exp. Med. 2002, 196, 293–301. [Google Scholar] [CrossRef]
- Popik, W.; Alce, T.M.; Au, W.C. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4(+) T cells. J. Virol. 2002, 76, 4709–4722. [Google Scholar] [CrossRef]
- Van Wilgenburg, B.; Moore, M.D.; James, W.S.; Cowley, S.A. The productive entry pathway of HIV-1 in macrophages is dependent on endocytosis through lipid rafts containing CD4. PLoS ONE 2014, 9, e86071. [Google Scholar] [CrossRef] [PubMed]
- Bacquin, A.; Bireau, C.; Tanguy, M.; Romanet, C.; Vernochet, C.; Dupressoir, A.; Heidmann, T. A Cell Fusion-Based Screening Method Identifies Glycosylphosphatidylinositol-Anchored Protein Ly6e as the Receptor for Mouse Endogenous Retroviral Envelope Syncytin-A. J. Virol. 2017, 91, e00832-17. [Google Scholar] [CrossRef] [PubMed]
- Langford, M.B.; Outhwaite, J.E.; Hughes, M.; Natale, D.R.C.; Simmons, D.G. Deletion of the Syncytin A receptor Ly6e impairs syncytiotrophoblast fusion and placental morphogenesis causing embryonic lethality in mice. Sci. Rep. 2018, 8, 3961. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liang, C.; Liu, S.L. CD4-dependent Modulation of HIV-1 Entry by LY6E. J. Virol. 2019, 93, e01866-18. [Google Scholar] [CrossRef]
- Schoggins, J.W.; Wilson, S.J.; Panis, M.; Murphy, M.Y.; Jones, C.T.; Bieniasz, P.; Rice, C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 2011, 472, 481–485. [Google Scholar] [CrossRef]
- Schoggins, J.W.; Dorner, M.; Feulner, M.; Imanaka, N.; Murphy, M.Y.; Ploss, A.; Rice, C.M. Dengue reporter viruses reveal viral dynamics in interferon receptor-deficient mice and sensitivity to interferon effectors in vitro. Proc. Natl. Acad. Sci. USA 2012, 109, 14610–14615. [Google Scholar] [CrossRef]
- Mar, K.B.; Rinkenberger, N.R.; Boys, I.N.; Eitson, J.L.; McDougal, M.B.; Richardson, R.B.; Schoggins, J.W. LY6E mediates an evolutionarily conserved enhancement of virus infection by targeting a late entry step. Nat. Commun. 2018, 9, 3603. [Google Scholar] [CrossRef]
- Hackett, B.A.; Cherry, S. Flavivirus internalization is regulated by a size-dependent endocytic pathway. Proc. Natl. Acad. Sci. USA 2018, 115, 4246–4251. [Google Scholar] [CrossRef]
- Sharonov, G.V.; Balatskaya, M.N.; Tkachuk, V.A. Glycosylphosphatidylinositol-Anchored Proteins as Regulators of Cortical Cytoskeleton. Biochem. Biokhimiia 2016, 81, 636–650. [Google Scholar] [CrossRef]
- Liu, S.Y.; Sanchez, D.J.; Aliyari, R.; Lu, S.; Cheng, G. Systematic identification of type I and type II interferon-induced antiviral factors. Proc. Natl. Acad. Sci. USA 2012, 109, 4239–4244. [Google Scholar] [CrossRef]
- Ferguson, M.A.J.; Williams, A.F. Cell-Surface Anchoring of Proteins via Glycosyl-Phosphatidylinositol Structures. Annu. Rev. Biochem. 1988, 57, 285–320. [Google Scholar] [CrossRef] [PubMed]
- Head, B.P.; Patel, H.H.; Insel, P.A. Interaction of membrane/lipid rafts with the cytoskeleton: Impact on signaling and function: Membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim. Biophys. Acta 2014, 1838, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, I.; Horejsi, V.; Ansotegui, I.; Knapp, W.; Stockinger, H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science 1991, 254, 1016–1019. [Google Scholar] [CrossRef]
- Wei, Y.; Lukashev, M.; Simon, D.I.; Bodary, S.C.; Rosenberg, S.; Doyle, M.V.; Chapman, H.A. Regulation of integrin function by the urokinase receptor. Science 1996, 273, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Zurzolo, C.; Simons, K. Glycosylphosphatidylinositol-anchored proteins: Membrane organization and transport. Biochim. Biophys. Acta 2016, 1858, 632–639. [Google Scholar] [CrossRef]
- Samuel, C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001, 14, 778–809. [Google Scholar] [CrossRef]
- Su, B.; Waneck, G.L.; Flavell, R.A.; Bothwell, A.L. The glycosyl phosphatidylinositol anchor is critical for Ly-6A/E-mediated T cell activation. J. Cell Biol. 1991, 112, 377–384. [Google Scholar] [CrossRef]
- Sussman, J.J.; Saito, T.; Shevach, E.M.; Germain, R.N.; Ashwell, J.D. Thy-1-and Ly-6-mediated lymphokine production and growth inhibition of a T cell hybridoma require co-expression of the T cell antigen receptor complex. J. Immunol. 1988, 140, 2520–2526. [Google Scholar]
- Kosugi, A.; Saitoh, S.; Noda, S.; Miyake, K.; Yamashita, Y.; Kimoto, M.; Ogata, M.; Hamaoka, T. Physical and functional association between thymic shared antigen-1/stem cell antigen-2 and the T cell receptor complex. J. Biol. Chem. 1998, 273, 12301–12306. [Google Scholar] [CrossRef]
- Moran, M.; Miceli, M.C. Engagement of GPI-linked CD48 contributes to TCR signals and cytoskeletal reorganization: A role for lipid rafts in T cell activation. Immunity 1998, 9, 787–796. [Google Scholar] [CrossRef]
- Stevenson, M.; Stanwick, T.L.; Dempsey, M.P.; Lamonica, C.A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990, 9, 1551–1560. [Google Scholar] [CrossRef]
- Yeom, C.J.; Zeng, L.; Goto, Y.; Morinibu, A.; Zhu, Y.; Shinomiya, K.; Kobayashi, M.; Itasaka, S.; Yoshimura, M.; Hur, C.G.; et al. LY6E: A conductor of malignant tumor growth through modulation of the PTEN/PI3K/Akt/HIF-1 axis. Oncotarget 2016, 7, 65837–65848. [Google Scholar] [CrossRef] [PubMed]
- AlHossiny, M.; Luo, L.; Frazier, W.R.; Steiner, N.; Gusev, Y.; Kallakury, B.; Glasgow, E.; Creswell, K.; Madhavan, S.; Kumar, R.; et al. Ly6E/K Signaling to TGFbeta Promotes Breast Cancer Progression, Immune Escape, and Drug Resistance. Cancer Res. 2016, 76, 3376–3386. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zhang, L.; Yang, G.; Zhao, L.; Peng, F.; Tian, Y.; Xiao, X.; Chung, R.T.; Gong, G. Hepatitis C virus NS5A drives a PTEN-PI3K/Akt feedback loop to support cell survival. Liver Int. 2015, 35, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Yang, G.D.; Huang, T.J.; Li, R.; Chu, Q.Q.; Xu, L.; Wang, M.S.; Cai, M.D.; Zhong, L.; Wei, H.J.; et al. EB-virus latent membrane protein 1 potentiates the stemness of nasopharyngeal carcinoma via preferential activation of PI3K/AKT pathway by a positive feedback loop. Oncogene 2015, 35, 3419. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Feng, N.; Fan, W.; Ma, X.; Yan, Q.; Lv, Z.; Zeng, Y.; Zhu, J.; Lu, C. Activation of PI3K/AKT and ERK MAPK signal pathways is required for the induction of lytic cycle replication of Kaposi’s Sarcoma-associated herpesvirus by herpes simplex virus type 1. BMC Microbiol. 2011, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G. TGF-β in infections and infectious diseases. Microbes Infect. 1999, 1, 1313–1325. [Google Scholar] [CrossRef]
- Griffin, G.E.; Leung, K.; Folks, T.M.; Kunkel, S.; Nabel, G.J. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature 1989, 339, 70–73. [Google Scholar] [CrossRef]
- Nabel, G.; Baltimore, D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 1987, 326, 711–713. [Google Scholar] [CrossRef]
- Lv, Y.; Song, Y.; Ni, C.; Wang, S.; Chen, Z.; Shi, X.; Jiang, Q.; Cao, C.; Zuo, Y. Overexpression of Lymphocyte Antigen 6 Complex, Locus E in Gastric Cancer Promotes Cancer Cell Growth and Metastasis. Cell. Physiol. Biochem. 2018, 45, 1219–1229. [Google Scholar] [CrossRef]
| Virus | Family | Mode of Action | Mechanism of Action | Tissue/Cell Type Tested | Reference |
|---|---|---|---|---|---|
| Mouse Adenovirus Type 1 (MAV-1) | Adenovirus | Enhanced | Enhance mouse susceptibility by genetic mapping | BALB/cJ Mice | [14,15] |
| Marek’s Disease Virus (MDV) | Herpesvirus | Enhanced | Enhance chicken susceptibility by genetic mapping | Chicken | [12] |
| Vesicular Stomatitis Virus (VSV) | Rhabdovirus | Restricted | Unknown | HEK293 | [30] |
| Enhanced | Unknown | STAT1-/- fibroblasts, THP-1, U2OS | [27] | ||
| Zika Virus (ZIKV) | Flavivirus | Enhanced | LY6E tubularization facilitates the uptake of large clathrin-dependent endocytosed cargoes | U2OS, STAT1-/- fibroblasts | [27,28] |
| Dengue Virus (DENV) | Flavivirus | Enhanced | LY6E tubularization facilitates the uptake of large clathrin-dependent endocytosed cargoes | U2OS, STAT1-/- fibroblasts | [27,28] |
| Yellow Fever Virus (YFV) | Flavivirus | Enhanced | Enhancing an early stage of life cycle that is after attachment but before viral translation | STAT1-/- fibroblasts, THP-1, U2OS | [27] |
| West Nile Virus (WNV) | Flavivirus | Enhanced | LY6E tubularization facilitates the uptake of large clathrin-dependent endocytosed cargoes | U2OS | [28] |
| Human Immunodeficiency Virus (HIV-1) | Lentivirus | Enhanced | Enhance viral entry, possibly acting on virus–cell membrane fusion | CD4 high T cells and PBMCs | [18] |
| Restricted | Restricting HIV-1 infection by lowing the cell surface CD4 | CD4 low Macrophages | [24] | ||
| Endogenous Retroviral Envelope, Syncytin-A | Retrovirus | Enhanced | Facilitating cell–cell fusion by serving as the syncytin-A receptor | Murine syncytiotrophoblast | [22] |
| Influenza A Virus (IAV) | Orthomyxovirus | Enhanced | Enhancing uncoating | U2OS | [27] |
| O'nyong'nyong Virus (ONNV) | Alphavirus | Resistant | Unknown | STAT1-/- fibroblasts | [27] |
| Sindbis Virus (SINV) | Alphavirus | Resistant | Unknown | STAT1-/- fibroblasts | [27] |
| Equine Arteritis Virus (EAV) | Alphaarterivirus | Resistant | Unknown | STAT1-/- fibroblasts | [27] |
| Measles Virus (MV) | Paramyxovirus | Resistant | Unknown | STAT1-/- fibroblasts | [27] |
| Parainfluenza Virus-5 (PIV5) | Paramyxovirus | Resistant | Unknown | U2OS | [28] |
| Replication-Defective Adenovirus Serotype 5 Vector (AdV5) | Adenovirus | Resistant | Unknown | STAT1-/- fibroblasts | [27] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Liu, S.-L. Emerging Role of LY6E in Virus–Host Interactions. Viruses 2019, 11, 1020. https://doi.org/10.3390/v11111020
Yu J, Liu S-L. Emerging Role of LY6E in Virus–Host Interactions. Viruses. 2019; 11(11):1020. https://doi.org/10.3390/v11111020
Chicago/Turabian StyleYu, Jingyou, and Shan-Lu Liu. 2019. "Emerging Role of LY6E in Virus–Host Interactions" Viruses 11, no. 11: 1020. https://doi.org/10.3390/v11111020
APA StyleYu, J., & Liu, S.-L. (2019). Emerging Role of LY6E in Virus–Host Interactions. Viruses, 11(11), 1020. https://doi.org/10.3390/v11111020






