Abstract
Although Hepatitis E is increasingly described as a major cause of liver disease in industrialized countries, the epidemiology is far from being fully elucidated. We provide here a comprehensive review of documented clusters of cases, and of serological studies conducted in populations with distinct types of exposure. Seroprevalence rates range from <5% to >50% depending on the countries and the groups of population. Such discrepancies can be attributed to the type of serological assay used, but this solves only a part of the problem. We performed a meta-analysis of studies performed with the broadly used Wantai HEV-IgG ELISA and found striking differences that remain difficult to understand with the current knowledge of transmission pathways.
1. Introduction
Hepatitis E virus (HEV) is the major cause of enterically transmitted hepatitis worldwide [1]. There are two contrasting situations around the world with regard to hepatitis E. On the one hand, in developing countries, transmission is mainly fecal-oral with poorly treated water playing a major role. In these countries, the incriminated genotypes are HEV-1 and HEV-2. On the other hand, in industrialized countries, although not all transmission pathways are well known [2], the main form of transmission is zoonotic with HEV-3 and HEV-4 genotypes [3,4]. A growing number of studies have been published concerning the seroprevalence of HEV antibodies in different human populations. Hyperendemic zones also appear in industrialized countries with seroprevalences of up to 70% in some regions. However, considerable heterogeneity exists depending upon either the type of method used or the population under study, or both. In high-income countries, where swine are the main reservoir, very high IgG anti-HEV seropositivity rates can be observed.
HEV is a small, single-stranded, positive-sense, RNA virus (~7.2 kb) [5]. HEV was initially known as a nonenveloped virus; however, another form has recently been identified. This form can be “masked” by the membrane of the host cells and is resistant to antibodies when it is in the blood [6,7]. When HEV particles are released by the cellular exosomal pathway, it is considered a “quasi-enveloped” virus. It contains genome coding for three open reading frames (ORF1, ORF2, and ORF3). In HEV genotype 1, a fourth ORF was discovered on ORF1, namely ORF4 [8]. HEV belongs to the Hepeviridae family [3]. Since 2015, this family has been divided into two genera, Orthohepevirus and Piscihepevirus [9,10]. Orthohepevirus contains four species named Orthohepevirus A to D. Currently, all strains isolated from humans belong to Orthohepevirus A in these five genotypes: HEV-1, HEV-2, HEV-3, HEV-4, and HEV-7. Genotypes 1 and 2 only affect humans but other genotypes can also infect other mammals. Orthohepevirus B, C, and D consist of avian, rat/carnivore, and bat viruses, respectively. The genus Piscihepevirus includes a single species (A) whose typical isolate, cutthroat trout virus, infects trout, although its pathogenicity and full host range are unknown [11].
The balance between symptomatic and asymptomatic forms is not yet well known. Certainly, with the very high seroprevalence observed in certain “hyperendemic” regions and the number of clinical cases recorded, infection by HEV is predominantly asymptomatic [12,13,14]. However, there are different forms of clinical manifestations that can range from acute hepatitis to fulminant hepatitis (1–2% of cases) [15]. Extrahepatic forms may also exist, including neurological disorders, thrombocytopenia, kidney injury, hemolytic anemia, and pancreatitis [16,17,18]. Chronic infections have been reported in most cases with genotype 3 infection in immunocompromised people in industrialized countries [19,20,21,22,23,24,25]. Recently, new chronic infections have been found to have been caused by genotype 4 [1,26,27,28,29,30,31].
Our understanding of the epidemiology of HEV in industrialized countries for human populations is complicated given the large number of studies, the heterogeneity of the laboratory tests used, and the different types of population included. The purpose of this review is to summarize the situation in industrialized countries with regard to humans. We review the recent detection of HEV markers and grouped cases among humans. Furthermore, a meta-analysis was performed to determine the overall seroprevalence of anti-HEV IgG in the general population in industrialized countries.
2. Seroprevalence of IgG anti-HEV
Anti-HEV IgG antibodies represent a marker of previous exposure to HEV. However, wide variations in sensitivity and specificity rates of various anti-HEV IgG assays make the interpretation of seroepidemiological studies of HEV infection difficult [32,33,34].
2.1. Seroprevalence of IgG anti-HEV with Wantai HEV-IgG ELISA Assay
Currently, the Wantai assay is the most commonly used assay with specificity and sensitivity for the HEV IgG of 97.96% and 99.6%, respectively [35,36]. In addition, other studies showed a better detection rate with the Wantai assay than other assays and this difference was probably not due to false positives [37,38]. Indeed, Wantai assay shows very low seroprevalences in children or some countries and increasing values with age [39,40]. Moreover, the main part of positives samples was confirmed by immunoblot [37].
As shown in Table 1, the non-Wantai assays underestimate HEV seroprevalence. A Danish study estimated seroprevalences of 10.7% and 19.8% for HEV antibodies depending on the assay used for NIH and Wantai, respectively [41]. The prevalence of IgG anti-HEV in Catalan blood donors was 19.96% (Wantai assay) and 10.72% (Mikrogen assay) [42]. We can also compare the use of different assays in different studies of the same population. Two studies in the Czech Republic, using different assays, found seroprevalences in BD of 5.7% and 6.7% [43,44]. Although the percentages here are similar, this is not always the case. The seroprevalence rate in France ranges from 3.2% to 22.4% depending on the type of detection assay (Genelabs Diagnostics vs. Wantai) [45,46].

Table 1.
HEV IgG prevalences observed when the same population is tested using different assays.
Of the 5 studies which have tested the same sera using at least 2 different methods, the Wantai IgG kit was used on 4 occasions: in all of these 4 cases, the prevalence of IgG observed with the Wantai IgG kit was higher than that obtained with other kits [41,42,43,47,48].
2.2. Meta-Analysis
A meta-analysis was carried out in accordance with the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guide [49].
2.2.1. Literature Search, Selection Criteria, and Study Quality
A literature search was made in PubMed using the keywords “hepatitis E seroprevalence” or “hepatitis E blood donors”.
Selection was based on title and abstract, and excluded studies older than 10 years and those where samples were collected before 2003, reviews, animal studies, and studies with a sample size smaller than 20 individuals or sera. The populations retained were the general population and the blood donors. No language restrictions were applied. Only studies regarding seroprevalence rates in industrialized countries with Wantai assay were included. Original abstracts were obtained and assessed in detail for inclusion. Following the abstract review, the full papers of the included studies were reviewed (Figure 1).
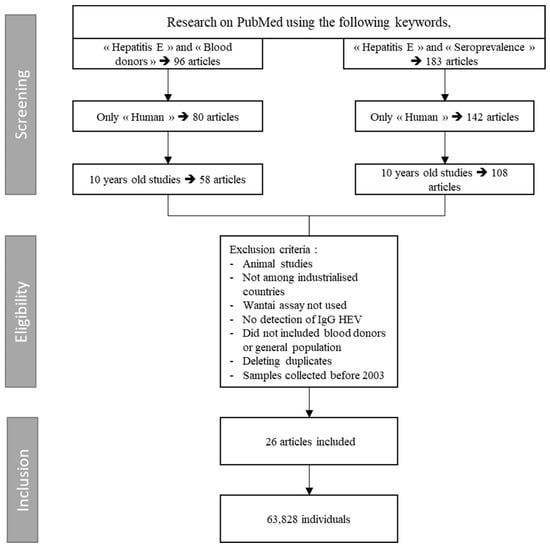
Figure 1.
Search algorithm for meta-analysis: HEV IgG seroprevalence among blood donors.
2.2.2. Data Extraction
Information was extracted from each study included authors, journal, year of publication, country, diagnostic assay used, number of patients, positive events, seroprevalence, and kind of patient cohort. Data were stratified for three variables: assay employed, country of study, and nature of study cohort. We focused on different study cohorts: general population/blood donors, immunocompromised patients, and individuals with contact with swine/wild animals. The latter included veterinarians, farmers, hunters, forestry workers, and pig farm workers.
2.2.3. Statistical Analysis
The overall seroprevalence of anti-HEV IgG in industrialized countries was estimated by pooling the study data to run a meta-analysis. The analysis was conducted using the R statistical platform (version 3.1.2) and the metafor package (version 1.9-5) [50]. The I2 statistic was used to estimate the amount of heterogeneity accounted for by each model [51]. We used the double arcsine transformation method for variance stabilization [52] and a restricted maximum likelihood (REML) estimator for prevalence estimation.
2.2.4. Results of Meta-Analysis: Human Seroprevalence of IgG anti-HEV
A total of 26 studies from 15 countries were included in the meta-analysis of seroprevalence in industrialized countries using Wantai assays. The overall seroprevalence of IgG anti-HEV calculated was 19% [14–25%] and the I2 showed high heterogeneity (100%) (Figure 2). Given the heterogeneity between studies, the random effects model was chosen. Reported seroprevalences ranged from 4.2% to 52.5% [40,53]. The 26 studies comprised a total of 63,828 individuals. The list of references associated with the studies of the meta-analysis is present in the Table 2.
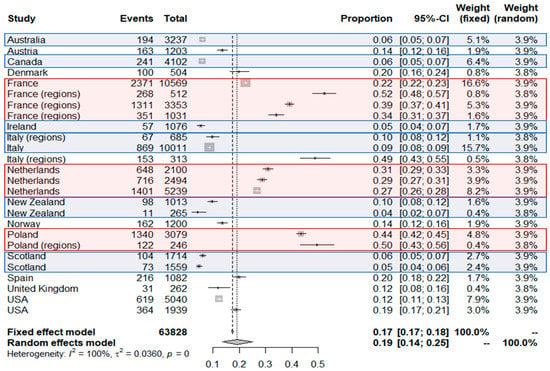
Figure 2.
Results of the meta-analysis: Seroprevalence among blood donors in industrialized countries using Wantai HEV-IgG ELISA assay. Red areas correspond to countries with seroprevalence higher than the overall seroprevalence and blue areas to those with lower value. Black crosses correspond to the seroprevalence value of each study. The size of the grey square around the cross depend on the number of samples of each study included (weight). Black bars around the point correspond to the confidential interval. The additional data for each study included in the meta-analysis are presented in Table 2.

Table 2.
Comparison of seroprevalence rates observed with the Wantai HEV-IgG ELISA assay in blood donors (BD) and the general population (GP) (data used for meta-analyses). “Country (regions)” means the seroprevalence is at regional level.
2.2.5. Interpretation of Meta-Analysis
France, Poland, and the Netherlands appear as countries with the highest seroprevalences. France has a fairly average national seroprevalence but regions of hyperendemias appear. For the Netherlands and Poland, the data of the different studies are homogeneous around 30% and 50% respectively. New Zealand, Scotland, Australia, Canada, and Ireland have seroprevalence under 10%. A special case appears with studies in Italy. In fact, for this country very distant seroprevalences have been observed, and hyperendemic zones could also exist in this country. Interestingly, in the 6 countries where at least 2 studies were done, the order of magnitude is well observed, specifically in the Netherlands (0.27; 0.29; 0.31), in Poland (0.44; 0.50), in Scotland (0.05; 0.06), in New Zealand (0.04; 0.1); in France, rates at 0.22 (national), 0.34 (Southwest France), 0.39 and 0.52 (Southern France) were observed; in Italy, the 3 studies provided very different rates at around 0.09 (national and Northern Italy) and 0.49 (Central Italy): These high differences in some regions may be explained by local eating habits or environmental HEV contamination.
2.3. High-Exposure Populations
People working in contact with animals (forest workers, hunters, pig and other farm workers) appeared as categories with higher seroprevalences (Table 3). It is impossible to compare studies that have not used the same assays. On the other hand, some studies have compared, under the same conditions, a GP with an “exposed population” (range 13.4–31.2%) [73,74]. While these studies were not conducted with the Wantai HEV-IgG ELISA assay, most of them were performed compared with a reference population. Accordingly, it can be deduced that selected populations are at higher risk of HEV infection such as pig farm workers or human with occupational exposure to pigs [73,75,76,77,78], forest workers [74,78,79], and farm workers [63]. Interestingly, veterinarians do not appear always as having a higher risk of infection [63,80,81]. However, the type of veterinarian activity (rural or urban) may greatly influence the risk of exposure to HEV as shown in Norway where seroprevalence in veterinarians working with swine was two times higher compared to those who did not work with swine [63].

Table 3.
HEV IgG rates observed in different populations.
2.4. Children
The few studies conducted in children consistently reported low seroprevalence rates: <5% in the USA, 4.2% in Turkey, 4.6% in Spain, 1.4% in Russia and 1.1% in Portugal [85,86,87,88,89] (Table 4).

Table 4.
HEV IgG prevalence observed in children.
2.5. Other Seroprevalences Observed in Industrialized Countries
Several studies have determined HEV seroprevalence through using assays other that the Wantai HEV-IgG ELISA. Seropositivity rates ranged between 1.9% in the Netherlands and 16.8% in Germany (Mikrogen) (Table 5) [90,91]. However, it is difficult to interpret these results and to compare results obtained by using different kits as previously indicated. These values may be useful whether the same kit is used in future studies or to compare exposure in different countries, in different populations or at different time points provided the same technique is used. These results should be considered as reference values for further studies performed using the same kit.

Table 5.
Prevalences of HEV IgG observed in industrialized countries with assays other that Wantai HEV-IgG ELISA.
2.6. Characteristics Associated with Higher Seroprevalence
The main characteristics associated with a high seropositivity rate in industrialized countries are increasing age, sex (male), contact with animals, consumption of raw or uncooked pork liver sausages (including ficatelli, a traditional Corsican pork liver sausage and traditional Dutch dry raw sausages), frequent consumption of bovine steak, frequent consumption of smoked beef offal, and consumption of oysters [45,59]. Conversely, bottled drinking water was associated with lower levels of anti-HEV IgG [45]. However, other factors could influence the exposure of the population to the virus.
3. Outbreaks in High-Income Countries
3.1. Confirmed and Grouped Cases
The European Centre for Disease Prevention and Control (ECDC) reported that the number of confirmed HEV cases had increased from 514 in 2005 to 5617 in 2015, with 21,018 total reported cases during the whole period [110]. Three countries (France, the UK, and Germany) accounted for 80% of all reported cases. The number of severe cases also increased between 2005 (n = 85) and 2015 (n = 1115). The sex ratio (male) ranged from 61% to 69%. The proportion of cases in people older than 50 increased from ~40% during the 2005–2008 period to >60% during the 2013–2015 period. During the 2005–2015 period, a total of 28 deaths attributable to HEV have been claimed, with a clear increase since 2009.
Although HEV epidemics are predominantly due to genotypes 1 and 2 in tropical countries, small outbreaks have been described in industrialized countries. The ECDC reported 37 outbreaks between 2005 and 2015 with an increasing trend after 2009 [111]. The size of the outbreaks reported is ranged from 2 to 47 cases per year. Data on HEV outbreaks are provided by 18 countries and among them, 11 did not report any outbreak.
3.2. Description of Outbreaks
Table 6 summarizes seven HEV outbreaks in industrialized countries that involved 4–17 cases. Two were caused by HEV genotype 3 with the common source of contamination being undercooked pig or wild boar.

Table 6.
List of HEV outbreaks for which peer-reviewed data are available.
In Spain, in October 2015, an HIV-infected patient presented at the Infectious Diseases Unit of the Hospital Universitario Reina Sofía de Córdoba with malaise, diarrhea, jaundice, vomiting, and fever. He tested positive for the presence of HEV RNA in her serum. The epidemiological investigation concluded that (i) all 8 family members had detectable HEV RNA in the serum, (ii) they were infected through the consumption of wild boar meat as demonstrated by HEV RNA detection in remaining frozen meat collected at home, (iii) genetic analysis showed that the 8 family members and the remaining frozen meat corresponded to the same viral strain (100% identity) belonging to genotype 3 [112]. This sequence was previously described in wild boars from south-central Spain and swine (GenBank accession number: EU723512).
In France, in 2013, a cluster of 17 cases was associated with consumption of a spit-roasted piglet during a wedding [113]. Twelve cases (70%) were non symptomatic, five were symptomatic, and among them two (11.7%) were hospitalized. All individuals recovered. The survey attributed this large HEV outbreak to the consumption of an undercooked pig liver-based stuffing.
In the UK, in 2008, four passengers on a cruise ship presented with HEV infection [14]. Because of the incubation period, the source of infection was not identified.
In Italy, in 2011, five persons living in the Lazio region (close to Roma) were infected by HEV [114]. Despite the source of infection not being identified, the causing strain belonged to genotype 4 and was genetically most closely related with strains from China; none of these persons had contact or consumption history of Chinese products. These results are in line with those provided by other studies indicating that genotype 4 is now established in Europe.
The Czech Republic was also affected by two outbreaks [115]. For the first 13-case outbreak, the source was not identified. In the second outbreak, a link between human cases and infection in farm pigs was revealed for the first time.
In Australia, 55 cases of HEV infection were confirmed, including 24 people who had not traveled during the incubation period [116]. Of these, 17 had eaten in the same restaurant. The HEV RNA detected was of genotype 3. The high attack rates and OR estimated in the study were for pork products.
In 2016, after the notification of hepatitis E cases to the Asahikawa City Center of Public Health (ACPH) in a nursing home for elderly in Hokkaido, Japan, 125 persons were tested for HEV infection markers, and 29 were found to be positive. Only four presented anorexia, fatigue, and abdominal pain. All cases were caused by strains belonging to genotype 3 [117] and amplified sequences presented 99.8 to 100% of homology.
4. Conclusions
The markers of HEV infection are found at an increasing frequency among humans in industrialized countries. In addition, the sporadic nature of human cases in these countries is no longer relevant; hyperendemic regions exist in France, Italy, and Poland, at least. The number of confirmed cases and of clusters of cases has also drastically increased during the last decade. Whether this is due to a true epidemiological trend or due to increased awareness to HEV is not clear. The meta-analysis of studies performed with the Wantai HEV-IgG ELISA on blood donors or healthy general populations allowed us to classify industrialized countries into one of the following 3 categories based on the 19% (CI95 14–26) cut-off prevalence rate: high risk (France, Netherlands, Poland), medium risk (Austria, Denmark, Norway, Spain, UK and USA), and low risk (Canada, Scotland, Ireland, Australia and New Zealand). In addition, there is clearly an occupational risk for forest workers, farm workers, and pig farm workers. Apart from the pork-eating route of infection, the high exposure of adult population in industrialized countries (lowest rate is 5%) demands future investigations to better understand how people acquire HEV infection. The great heterogeneity observed between the anti-HEV IgG detection assays makes the comparison of the different studies and the comprehension of HEV epidemiology difficult. The establishment of a reference method in seroprevalence studies could remedy this problem.
The markers of HEV infection are found at an increasing frequency among humans in industrialized countries. In addition, the sporadic nature of human cases in these countries is no longer relevant; hyperendemic regions exist in France, Italy, and Poland, at least. The number of confirmed cases and of clusters of cases has also drastically increased during the last decade. Whether this is due to a true epidemiological trend or due to increased awareness to HEV is not clear. The meta-analysis of studies performed with the Wantai HEV-IgG ELISA on blood donors or healthy general populations allowed us to classify industrialized countries into one of the following 3 categories based on the 19% (CI95 14–26) cut-off prevalence rate: high risk (France, Netherlands, Poland), medium risk (Austria, Denmark, Norway, Spain, UK and USA), and low risk (Canada, Scotland, Ireland, Australia and New Zealand). In addition, there is clearly an occupational risk for forest workers, farm workers, and pig farm workers. Apart from the pork-eating route of infection, the high exposure of adult population in industrialized countries (lowest rate is 5%) demands future investigations to better understand how people acquire HEV infection. The great heterogeneity observed between the anti-HEV IgG detection assays makes the comparison of the different studies and the comprehension of HEV epidemiology difficult. The establishment of a reference method in seroprevalence studies could remedy this problem.
Author Contributions
L.C.: data mining and drafting of the manuscript; A.F. and R.C.: supervision manuscript. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Debing, Y.; Moradpour, D.; Neyts, J.; Gouttenoire, J. Update on hepatitis e virology: Implications for clinical practice. J. Hepatol. 2016, 65, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Purcell, R.H.; Emerson, S.U. Hepatitis e: An emerging awareness of an old disease. J. Hepatol. 2008, 48, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Bendall, R.; Legrand-Abravanel, F.; Xia, N.S.; Ijaz, S.; Izopet, J.; Dalton, H.R. Hepatitis e. Lancet 2012, 379, 2477–2488. [Google Scholar] [CrossRef]
- Dalton, H.R.; Bendall, R.; Ijaz, S.; Banks, M. Hepatitis e: An emerging infection in developed countries. Lancet Infect Dis. 2008, 8, 698–709. [Google Scholar] [CrossRef]
- Tam, A.W.; Smith, M.M.; Guerra, M.E.; Huang, C.C.; Bradley, D.W.; Fry, K.E.; Reyes, G.R. Hepatitis e virus (hev): Molecular cloning and sequencing of the full-length viral genome. Virology 1991, 185, 120–131. [Google Scholar] [CrossRef]
- Takahashi, M.; Tanaka, T.; Takahashi, H.; Hoshino, Y.; Nagashima, S.; Jirintai; Mizuo, H.; Yazaki, Y.; Takagi, T.; Azuma, M.; et al. Hepatitis e virus (hev) strains in serum samples can replicate efficiently in cultured cells despite the coexistence of hev antibodies: Characterization of hev virions in blood circulation. J. Clin. Microbiol. 2010, 48, 1112–1125. [Google Scholar] [CrossRef] [PubMed]
- Chapuy-Regaud, S.; Dubois, M.; Plisson-Chastang, C.; Bonnefois, T.; Lhomme, S.; Bertrand-Michel, J.; You, B.; Simoneau, S.; Gleizes, P.E.; Flan, B.; et al. Characterization of the lipid envelope of exosome encapsulated hev particles protected from the immune response. Biochimie 2017, 141, 70–79. [Google Scholar] [CrossRef]
- Nair, V.P.; Anang, S.; Subramani, C.; Madhvi, A.; Bakshi, K.; Srivastava, A.; Shalimar; Nayak, B.; Ranjith Kumar, C.T.; Surjit, M. Endoplasmic reticulum stress induced synthesis of a novel viral factor mediates efficient replication of genotype-1 hepatitis e virus. PLoS Pathog. 2016, 12, e1005521. [Google Scholar] [CrossRef]
- Smith, D.B. International committee on taxonomy of viruses hepeviridae study g, jameel s, emerson su, harrison tj, meng xj, okamoto h, van der poel wh, purdy ma. Consensus proposals for classification of the family hepeviridae. J. Gen. Virol. 2014, 95, 2223–2232. [Google Scholar] [CrossRef]
- Smith, D.B.; Simmonds, P.; Izopet, J.; Oliveira-Filho, E.F.; Ulrich, R.G.; Johne, R.; Koenig, M.; Jameel, S.; Harrison, T.J.; Meng, X.-J.; et al. Proposed reference sequences for hepatitis e virus subtypes. J. Gen. Virol. 2016, 97, 537–542. [Google Scholar] [CrossRef]
- Purdy, M.A.; Harrison, T.J.; Jameel, S.; Meng, X.J.; Okamoto, H.; Van der Poel, W.H.M.; Smith, D.B.; Ictv Report, C. Ictv virus taxonomy profile: Hepeviridae. J. Gen. Virol. 2017, 98, 2645–2646. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R. Clinical presentation of hepatitis e. Virus Res. 2011, 161, 15–22. [Google Scholar] [CrossRef]
- Echevarria, J.M. Light and darkness: Prevalence of hepatitis e virus infection among the general population. Scientifica (Cairo) 2014, 2014, 481016. [Google Scholar] [CrossRef]
- Said, B.; Ijaz, S.; Kafatos, G.; Booth, L.; Thomas, H.L.; Walsh, A.; Ramsay, M.; Morgan, D.; Hepatitis, E.I.I.T. Hepatitis e outbreak on cruise ship. Emerg. Infect. Dis. 2009, 15, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Hazeldine, S.; Banks, M.; Ijaz, S.; Bendall, R. Locally acquired hepatitis e in chronic liver disease. Lancet 2007, 369, 1260. [Google Scholar] [CrossRef]
- Colson, P.; Dhiver, C.; Gerolami, R. Hepatitis e virus as a newly identified cause of acute viral hepatitis during human immunodeficiency virus infection. Clin. Microbiol. Infect. 2008, 14, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Abid, S.; Khan, A.H. Severe hemolysis and renal failure in glucose-6-phosphate dehydrogenase deficient patients with hepatitis e. Am. J. Gastroenterol. 2002, 97, 1544–1547. [Google Scholar] [CrossRef]
- Kamar, N.; Marion, O.; Abravanel, F.; Izopet, J.; Dalton, H.R. Extrahepatic manifestations of hepatitis e virus. Liver Int. 2016, 36, 467–472. [Google Scholar] [CrossRef]
- Hoofnagle, J.H.; Nelson, K.E.; Purcell, R.H. Hepatitis e. N. Engl. J. Med. 2012, 367, 1237–1244. [Google Scholar] [CrossRef]
- de Niet, A.; Zaaijer, H.L.; ten Berge, I.; Weegink, C.J.; Reesink, H.W.; Beuers, U. Chronic hepatitis e after solid organ transplantation. Neth. J. Med. 2012, 70, 261–266. [Google Scholar]
- Gerolami, R.; Moal, V.; Colson, P. Chronic hepatitis e with cirrhosis in a kidney-transplant recipient. N. Engl. J. Med. 2008, 358, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Te, H.S.; Drobeniuc, J.; Kamili, S.; Dong, C.; Hart, J.; Sharapov, U.M. Hepatitis e virus infection in a liver transplant recipient in the United States: A case report. Transplant Proc. 2013, 45, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Renou, C.; Lafeuillade, A.; Cadranel, J.F.; Pavio, N.; Pariente, A.; Allegre, T.; Poggi, C.; Penaranda, G.; Cordier, F.; Nicand, E. Hepatitis e virus in hiv-infected patients. AIDS 2010, 24, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Tavitian, S.; Peron, J.M.; Huynh, A.; Mansuy, J.M.; Ysebaert, L.; Huguet, F.; Vinel, J.P.; Attal, M.; Izopet, J.; Recher, C. Hepatitis e virus excretion can be prolonged in patients with hematological malignancies. J. Clin. Virol. 2010, 49, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Gauss, A.; Wenzel, J.J.; Flechtenmacher, C.; Navid, M.H.; Eisenbach, C.; Jilg, W.; Stremmel, W.; Schnitzler, P. Chronic hepatitis e virus infection in a patient with leukemia and elevated transaminases: A case report. J. Med. Case Rep. 2012, 6, 334. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Zhang, H.; Huang, W.; T, J.H.; Geng, K.; Li, Z.; Wang, Y. Persistent hepatitis e virus genotype 4 infection in a child with acute lymphoblastic leukemia. Hepat. Mon. 2014, 14, e15618. [Google Scholar] [CrossRef]
- Kamar, N.; Selves, J.; Mansuy, J.-M.; Ouezzani, L.; Péron, J.-M.; Guitard, J.; Cointault, O.; Esposito, L.; Abravanel, F.; Danjoux, M.; et al. Hepatitis e virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 2008, 358, 811–817. [Google Scholar] [CrossRef]
- Péron, J.-M.; Mansuy, J.-M.; Récher, C.; Bureau, C.; Poirson, H.; Alric, L.; Izopet, J.; Vinel, J.-P. Prolonged hepatitis e in an immunocompromised patient. J. Gastroenterol. Hepatol. 2006, 21, 1223–1224. [Google Scholar] [CrossRef]
- Dalton, H.R.; Bendall, R.P.; Keane, F.E.; Tedder, R.S.; Ijaz, S. Persistent carriage of hepatitis e virus in patients with hiv infection. N. Engl. J. Med. 2009, 361, 1025–1027. [Google Scholar] [CrossRef]
- Behrendt, P.; Steinmann, E.; Manns, M.P.; Wedemeyer, H. The impact of hepatitis e in the liver transplant setting. J. Hepatol. 2014, 61, 1418–1429. [Google Scholar] [CrossRef]
- Tamura, A.; Shimizu, Y.K.; Tanaka, T.; Kuroda, K.; Arakawa, Y.; Takahashi, K.; Mishiro, S.; Shimizu, K.; Moriyama, M. Persistent infection of hepatitis e virus transmitted by blood transfusion in a patient with t-cell lymphoma. Hepatol. Res. 2007, 37, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.C.; Flower, R.L.; Seed, C.R.; Stramer, S.L.; Faddy, H.M. A comparative study of assay performance of commercial hepatitis e virus enzyme-linked immunosorbent assay kits in Australian blood donor samples. J. Blood Transfus. 2016, 2016, 9647675. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Jeong, S.H.; Kim, J.W.; Woo, B.H.; Lee, D.H.; Kim, H.Y.; Ahn, S. Seroprevalence of anti-hepatitis e virus (hev) in a korean population: Comparison of two commercial anti-hev assays. BMC Infect. Dis. 2012, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.J.; Preiss, J.; Schemmerer, M.; Huber, B.; Jilg, W. Test performance characteristics of anti-hev igg assays strongly influence hepatitis e seroprevalence estimates. J. Infect. Dis. 2013, 207, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Du, H.; Wang, Y. Comparison of two diagnostic reagents to detect anti-hepatitis e virus igg antibodies. Chin. J. Zoon. 2008, 24, 1087–1089. [Google Scholar]
- Beijing Wantai Biological Pharmacy Enterprise Co., Ltd. Wantai HEV-IgM ELISA. Available online: http://wantaibio.company.weiku.com/item/HEV-IgM-Elisa-kit-18043455.html (accessed on 3 December 2018).
- Rossi-Tamisier, M.; Moal, V.; Gerolami, R.; Colson, P. Discrepancy between anti-hepatitis e virus immunoglobulin g prevalence assessed by two assays in kidney and liver transplant recipients. J. Clin. Virol. 2013, 56, 62–64. [Google Scholar] [CrossRef]
- Hartl, J.; Otto, B.; Madden, R.G.; Webb, G.; Woolson, K.L.; Kriston, L.; Vettorazzi, E.; Lohse, A.W.; Dalton, H.R.; Pischke, S. Hepatitis e seroprevalence in europe: A meta-analysis. Viruses 2016, 8, 211. [Google Scholar] [CrossRef]
- Christensen, P.B.; Engle, R.E.; Hjort, C.; Homburg, K.M.; Vach, W.; Georgsen, J.; Purcell, R.H. Time trend of the prevalence of hepatitis e antibodies among farmers and blood donors: A potential zoonosis in Denmark. Clin. Infect Dis. 2008, 47, 1026–1031. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Bendall, R.; Legrand-Abravanel, F.; Saune, K.; Miedouge, M.; Ellis, V.; Rech, H.; Destruel, F.; Kamar, N.; Dalton, H.R.; et al. Hepatitis e virus antibodies in blood donors, France. Emerg. Infect. Dis. 2011, 17, 2309–2312. [Google Scholar] [CrossRef]
- Holm, D.K.; Moessner, B.K.; Engle, R.E.; Zaaijer, H.L.; Georgsen, J.; Purcell, R.H.; Christensen, P.B. Declining prevalence of hepatitis e antibodies among danish blood donors. Transfusion 2015, 55, 1662–1667. [Google Scholar] [CrossRef]
- Sauleda, S.; Ong, E.; Bes, M.; Janssen, A.; Cory, R.; Babizki, M.; Shin, T.; Lindquist, A.; Hoang, A.; Vang, L.; et al. Seroprevalence of hepatitis e virus (hev) and detection of hev rna with a transcription-mediated amplification assay in blood donors from Catalonia (Spain). Transfusion 2015, 55, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Nemecek, V.; Butovicova, P.; Maly, M.; Dite, P.; Vertatova, M.; Vodickova, I.; Kriz, B. The prevalence of antibodies against hepatitis e virus in the Czech Republic: Serological survey. Epidemiol. Mikrobiol. Imunol. 2017, 66, 3–7. [Google Scholar] [PubMed]
- Strakova, P.; Kriz, B.; Rudolf, I.; Hubalek, Z. Seroprevalence study of hepatitis e virus infection in two districts of the Czech Republic. Epidemiol. Mikrobiol. Imunol. 2014, 63, 92–94. [Google Scholar] [PubMed]
- Mansuy, J.M.; Gallian, P.; Dimeglio, C.; Saune, K.; Arnaud, C.; Pelletier, B.; Morel, P.; Legrand, D.; Tiberghien, P.; Izopet, J. A nationwide survey of hepatitis e viral infection in French blood donors. Hepatology 2016, 63, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Boutrouille, A.; Bakkali-Kassimi, L.; Cruciere, C.; Pavio, N. Prevalence of anti-hepatitis e virus antibodies in French blood donors. J. Clin. Microbiol. 2007, 45, 2009–2010. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, J.; Harte, D.; Sutherland, M.; Croucher, D.; Fouche, L.; Flanagan, P.; Williamson, D. Prevalence of hepatitis e virus antibodies and infection in New Zealand blood donors. N. Z. Med. J. 2018, 131, 38–43. [Google Scholar] [PubMed]
- Zafrullah, M.; Zhang, X.; Tran, C.; Nguyen, M.; Kamili, S.; Purdy, M.A.; Stramer, S.L. Disparities in detection of antibodies against hepatitis e virus in US blood donor samples using commercial assays. Transfusion 2018, 58, 1254–1263. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting meta-analyses in r with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Fellows, H.J.; Gane, E.J.; Wong, P.; Gerred, S.; Schroeder, B.; Croxson, M.C.; Garkavenko, O. Hepatitis e in New Zealand. J. Gastroenterol. Hepatol. 2007, 22, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Bura, M.; Lagiedo, M.; Michalak, M.; Sikora, J.; Mozer-Lisewska, I. Hepatitis e virus igg seroprevalence in hiv patients and blood donors, west-central Poland. Int. J. Infect. Dis. 2017, 61, 20–22. [Google Scholar] [CrossRef]
- Lucarelli, C.; Spada, E.; Taliani, G.; Chionne, P.; Madonna, E.; Marcantonio, C.; Pezzotti, P.; Bruni, R.; La Rosa, G.; Pisani, G.; et al. High prevalence of anti-hepatitis e virus antibodies among blood donors in central Italy, February to March 2014. Euro Surveill. 2016, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Grabarczyk, P.; Sulkowska, E.; Gdowska, J.; Kopacz, A.; Liszewski, G.; Kubicka-Russel, D.; Baylis, S.A.; Corman, V.M.; Nocen, E.; Piotrowski, D.; et al. Molecular and serological infection marker screening in blood donors indicates high endemicity of hepatitis e virus in Poland. Transfusion 2018, 58, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, J.M.; Saune, K.; Rech, H.; Abravanel, F.; Mengelle, C.; S, L.H.; Destruel, F.; Kamar, N.; Izopet, J. Seroprevalence in blood donors reveals widespread, multi-source exposure to hepatitis e virus, southern France, October 2011. Euro Surveill. 2015, 20, 27–34. [Google Scholar] [CrossRef]
- Izopet, J.; Labrique, A.B.; Basnyat, B.; Dalton, H.R.; Kmush, B.; Heaney, C.D.; Nelson, K.E.; Ahmed, Z.B.; Zaman, K.; Mansuy, J.M.; et al. Hepatitis e virus seroprevalence in three hyperendemic areas: Nepal, Bangladesh and southwest France. J. Clin. Virol. 2015, 70, 39–42. [Google Scholar] [CrossRef]
- Mooij, S.H.; Hogema, B.M.; Tulen, A.D.; van Pelt, W.; Franz, E.; Zaaijer, H.L.; Molier, M.; Hofhuis, A. Risk factors for hepatitis e virus seropositivity in Dutch blood donors. BMC Infect. Dis. 2018, 18, 173. [Google Scholar] [CrossRef]
- Van Gageldonk-Lafeber, A.B.; van der Hoek, W.; Borlee, F.; Heederik, D.J.; Mooi, S.H.; Maassen, C.B.; Yzermans, C.J.; Rockx, B.; Smit, L.A.; Reimerink, J.H. Hepatitis e virus seroprevalence among the general population in a livestock-dense area in The Netherlands: A cross-sectional population-based serological survey. BMC Infect. Dis. 2017, 17, 21. [Google Scholar] [CrossRef]
- Slot, E.; Hogema, B.M.; Riezebos-Brilman, A.; Kok, T.M.; Molier, M.; Zaaijer, H.L. Silent hepatitis e virus infection in Dutch blood donors, 2011 to 2012. Euro Surveill. 2013, 18, 20550. [Google Scholar] [CrossRef]
- Xu, C.; Wang, R.Y.; Schechterly, C.A.; Ge, S.; Shih, J.W.; Xia, N.S.; Luban, N.L.; Alter, H.J. An assessment of hepatitis e virus (hev) in US blood donors and recipients: No detectable hev rna in 1939 donors tested and no evidence for hev transmission to 362 prospectively followed recipients. Transfusion 2013, 53, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Lange, H.; Overbo, J.; Borgen, K.; Dudman, S.; Hoddevik, G.; Urdahl, A.M.; Vold, L.; Sjurseth, S.K. Hepatitis e in norway: Seroprevalence in humans and swine. Epidemiol. Infect. 2017, 145, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Hofmann, M.; Danzer, M.; Hofer, K.; Kaar, J.; Gabriel, C. Seroprevalence and incidence of hepatitis e in blood donors in upper Austria. PLoS ONE 2015, 10, e0119576. [Google Scholar] [CrossRef] [PubMed]
- Beale, M.A.; Tettmar, K.; Szypulska, R.; Tedder, R.S.; Ijaz, S. Is there evidence of recent hepatitis e virus infection in english and north welsh blood donors? Vox Sang. 2011, 100, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Fomiatti, L.; Tagliacarne, C.; Velati, C.; Zanetti, A.R.; Castaldi, S.; Romano, L. Seroprevalence of hepatitis e virus among blood donors in northern Italy (Sondrio, Lombardy) determined by three different assays. Blood Transfus 2017, 15, 502–505. [Google Scholar] [PubMed]
- Spada, E.; Pupella, S.; Pisani, G.; Bruni, R.; Chionne, P.; Madonna, E.; Villano, U.; Simeoni, M.; Fabi, S.; Marano, G.; et al. A nationwide retrospective study on prevalence of hepatitis e virus infection in Italian blood donors. Blood Transfus 2018, 1–9. [Google Scholar] [CrossRef]
- Thom, K.; Gilhooly, P.; McGowan, K.; Malloy, K.; Jarvis, L.M.; Crossan, C.; Scobie, L.; Blatchford, O.; Smith-Palmer, A.; Donnelly, M.C.; et al. Hepatitis e virus (hev) in scotland: Evidence of recent increase in viral circulation in humans. Euro Surveill. 2018, 23, 12. [Google Scholar] [CrossRef]
- Shrestha, A.C.; Seed, C.R.; Flower, R.L.; Rooks, K.M.; Keller, A.J.; Harley, R.J.; Chan, H.T.; Holmberg, J.A.; Faddy, H.M. Hepatitis e virus and implications for blood supply safety, Australia. Emerg. Infect. Dis. 2014, 20, 1940–1942. [Google Scholar] [CrossRef]
- Fearon, M.A.; O’Brien, S.F.; Delage, G.; Scalia, V.; Bernier, F.; Bigham, M.; Weger, S.; Prabhu, S.; Andonov, A. Hepatitis e in Canadian blood donors. Transfusion 2017, 57, 1420–1425. [Google Scholar] [CrossRef]
- O’Riordan, J.; Boland, F.; Williams, P.; Donnellan, J.; Hogema, B.M.; Ijaz, S.; Murphy, W.G. Hepatitis e virus infection in the Irish blood donor population. Transfusion 2016, 56, 2868–2876. [Google Scholar] [CrossRef]
- Cleland, A.; Smith, L.; Crossan, C.; Blatchford, O.; Dalton, H.R.; Scobie, L.; Petrik, J. Hepatitis e virus in Scottish blood donors. Vox Sang. 2013, 105, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Tefanova, V.; Reshetnjak, I.; Kuznetsova, T.; Geller, J.; Lundkvist, A.; Janson, M.; Neare, K.; Velstrom, K.; Jokelainen, P.; et al. Hepatitis e virus in domestic pigs, wild boars, pig farm workers, and hunters in Estonia. Food Environ. Virol. 2015, 7, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Chaussade, H.; Rigaud, E.; Rodriguez, J.; Berthault, C.; Boue, F.; Tognon, M.; Touze, A.; Garcia-Bonnet, N.; Choutet, P.; et al. High hepatitis e virus seroprevalence in forestry workers and in wild boars in France. J. Clin. Microbiol. 2012, 50, 2888–2893. [Google Scholar] [CrossRef] [PubMed]
- Krumbholz, A.; Joel, S.; Dremsek, P.; Neubert, A.; Johne, R.; Durrwald, R.; Walther, M.; Muller, T.H.; Kuhnel, D.; Lange, J.; et al. Seroprevalence of hepatitis e virus (hev) in humans living in high pig density areas of Germany. Med. Microbiol. Immunol. 2014, 203, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Mesquita, J.R.; Pereira, S.S.; Oliveira, R.M.; Abreu-Silva, J.; Rodrigues, A.; Myrmel, M.; Stene-Johansen, K.; Overbo, J.; Goncalves, G.; et al. Prevalence of hepatitis e virus antibodies in workers occupationally exposed to swine in Portugal. Med. Microbiol. Immunol. 2017, 206, 77–81. [Google Scholar] [CrossRef]
- Meng, X.J.; Wiseman, B.; Elvinger, F.; Guenette, D.K.; Toth, T.E.; Engle, R.E.; Emerson, S.U.; Purcell, R.H. Prevalence of antibodies to hepatitis e virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J. Clin. Microbiol. 2002, 40, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Chaussade, H.; Rigaud, E.; Allix, A.; Carpentier, A.; Touze, A.; Delzescaux, D.; Choutet, P.; Garcia-Bonnet, N.; Coursaget, P. Hepatitis e virus seroprevalence and risk factors for individuals in working contact with animals. J. Clin. Virol. 2013, 58, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Dremsek, P.; Wenzel, J.J.; Johne, R.; Ziller, M.; Hofmann, J.; Groschup, M.H.; Werdermann, S.; Mohn, U.; Dorn, S.; Motz, M.; et al. Seroprevalence study in forestry workers from eastern Germany using novel genotype 3- and rat hepatitis e virus-specific immunoglobulin g elisas. Med. Microbiol. Immunol. 2012, 201, 189–200. [Google Scholar] [CrossRef]
- Kantala, T.; Kinnunen, P.M.; Oristo, S.; Jokelainen, P.; Vapalahti, O.; Maunula, L. Hepatitis e virus antibodies in Finnish veterinarians. Zoon. Public Health 2017, 64, 232–238. [Google Scholar] [CrossRef]
- De Sabato, L.; Di Bartolo, I.; Montomoli, E.; Trombetta, C.; Ruggeri, F.M.; Ostanello, F. Retrospective study evaluating seroprevalence of hepatitis e virus in blood donors and in swine veterinarians in Italy (2004). Zoon. Public Health 2017, 64, 308–312. [Google Scholar] [CrossRef]
- Krumbholz, A.; Mohn, U.; Lange, J.; Motz, M.; Wenzel, J.J.; Jilg, W.; Walther, M.; Straube, E.; Wutzler, P.; Zell, R. Prevalence of hepatitis e virus-specific antibodies in humans with occupational exposure to pigs. Med. Microbiol. Immunol. 2012, 201, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Riveiro-Barciela, M.; Rodriguez-Frias, F.; Buti, M. Hepatitis e: Scale of the problem in Spain. Gastroenterol. Hepatol. 2012, 35, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.; Tokarska-Rodak, M.; Plewik, D.; Pańczuk, A.; Szepeluk, A.; Krajewska, M. The serological surveillance of hepatitis e virus among hunters and foresters in eastern Poland. Pol. J. Microbiol. 2017, 66, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Kuniholm, M.H.; Purcell, R.H.; McQuillan, G.M.; Engle, R.E.; Wasley, A.; Nelson, K.E. Epidemiology of hepatitis e virus in the United States: Results from the third national health and nutrition examination survey, 1988–1994. J. Infect. Dis. 2009, 200, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Buti, M.; Plans, P.; Dominguez, A.; Jardi, R.; Rodriguez Frias, F.; Esteban, R.; Salleras, L.; Plasencia, A. Prevalence of hepatitis e virus infection in children in the northeast of Spain. Clin. Vaccine Immunol. 2008, 15, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Bayhan, G.I.; Demioren, K.; Guducuoglu, H. Epidemiology of hepatitis e virus in children in the province of van, Turkey. Turk. Pediatr. Ars. 2016, 51, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Potemkin, I.A.; Lopatukhina, M.A.; Gadzhieva, O.A.; Prokhorova, E.L.; Diyarrassuba, A.; Isaeva, O.A.; Kozhanova, T.V.; Ivanova, O.E.; Silenova, O.V.; Setdikova, N.; et al. Prevalence of hepatitis e markers in children. Zh. Mikrobiol. Epidemiol. Immunobiol. 2015, 2, 38–46. [Google Scholar]
- Oliveira, R.; Mesquita, J.R.; Pereira, S.; Abreu-Silva, J.; Teixeira, J.; Nascimento, M.S.J. Seroprevalence of hepatitis e virus antibodies in Portuguese children. Pediatr. Infect. Dis. J. 2017, 36, 623–626. [Google Scholar] [CrossRef]
- Faber, M.S.; Wenzel, J.J.; Jilg, W.; Thamm, M.; Hohle, M.; Stark, K. Hepatitis e virus seroprevalence among adults, Germany. Emerg. Infect. Dis. 2012, 18, 1654–1657. [Google Scholar] [CrossRef]
- Verhoef, L.; Koopmans, M.; Duizer, E.; Bakker, J.; Reimerink, J.; Van Pelt, W. Seroprevalence of hepatitis e antibodies and risk profile of hev seropositivity in The Netherlands, 2006–2007. Epidemiol. Infect. 2012, 140, 1838–1847. [Google Scholar] [CrossRef]
- Van Hoecke, F.; Van Maerken, T.; De Boulle, M.; Geerts, A.; Vlierberghe, V.; Colle, I.; Padalko, H.E. Hepatitis e seroprevalence in east and west Flanders, Belgium. Acta Gastroenterol. Belg. 2012, 75, 322–324. [Google Scholar]
- Faber, M.; Willrich, N.; Schemmerer, M.; Rauh, C.; Kuhnert, R.; Stark, K.; Wenzel, J.J. Hepatitis e virus seroprevalence, seroincidence and seroreversion in the German adult population. J. Viral. Hepat. 2018, 25, 752–758. [Google Scholar] [CrossRef]
- Pittaras, T.; Valsami, S.; Mavrouli, M.; Kapsimali, V.; Tsakris, A.; Politou, M. Seroprevalence of hepatitis e virus in blood donors in Greece. Vox Sang. 2014, 106, 387. [Google Scholar] [CrossRef]
- Haagsman, A.; Reuter, G.; Duizer, E.; Nagy, G.; Herremans, T.; Koopmans, M.; Szucs, G. Seroepidemiology of hepatitis e virus in patients with non-a, non-b, non-c hepatitis in Hungary. J. Med. Virol. 2007, 79, 927–930. [Google Scholar] [CrossRef]
- Hickey, C.; Spillane, D.; Benson, J.; Levis, J.; Fanning, L.J.; Cryan, B.; Prentice, M.B. Hepatitis e virus (hev) infection in Ireland. Ir. Med. J. 2016, 109, 451. [Google Scholar] [PubMed]
- Mor, O.; Bassal, R.; Michaeli, M.; Wax, M.; Ram, D.; Cohen-Ezra, O.; Cohen, D.; Mendelson, E.; Ben-Ari, Z.; Shohat, T. Prevalence of hepatitis e virus antibodies, Israel, 2009–2010. Emerg. Infect. Dis. 2015, 21, 692–694. [Google Scholar] [CrossRef]
- Ricco, G.; Bonino, F.; Lanza, M.; Scatena, F.; Alfieri, C.M.; Messa, P.; Marchisio, E.; Mascolo, G.; Romano, L.; Galli, C.; et al. New immunoassays for total, iga and igm antibodies against hepatitis e virus: Prevalence in Italian blood donors and patients with chronic liver or kidney diseases. Dig. Liver Dis. 2016, 48, 536–541. [Google Scholar] [CrossRef]
- Takeda, H.; Matsubayashi, K.; Sakata, H.; Sato, S.; Kato, T.; Hino, S.; Tadokoro, K.; Ikeda, H. A nationwide survey for prevalence of hepatitis e virus antibody in qualified blood donors in Japan. Vox Sang. 2010, 99, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Tamura, K.; Hoshino, Y.; Nagashima, S.; Yazaki, Y.; Mizuo, H.; Iwamoto, S.; Okayama, M.; Nakamura, Y.; Kajii, E.; et al. A nationwide survey of hepatitis e virus infection in the general population of Japan. J. Med. Virol. 2010, 82, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bura, M.; Bukowska, A.; Bura, A.; Michalak, M.; Mozer-Lisewska, I. Hepatitis e virus antibodies in hiv-infected patients and blood donors from western Poland—A preliminary report. Adv. Clin. Exp. Med. 2017, 26, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Queiros, L.; Condeco, J.; Tender, A.; Mateus, M.; Teixeira, A.; Pascoal, H. The seroprevalence for hepatitis e viral antibodies in the northern region of Portugal (among the donor population). Acta Med. Port. 1997, 10, 447–453. [Google Scholar] [PubMed]
- Mateos, M.L.; Camarero, C.; Lasa, E.; Teruel, J.L.; Mir, N.; Baquero, F. Hepatitis e virus: Relevance in blood donors and risk groups. Vox Sang. 1999, 76, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Echevarria, J.M.; Fogeda, M.; Avellon, A. Epidemiology of hepatitis e virus infection in Spain. Enferm. Infecc. Microbiol. Clin. 2015, 33, 281–286. [Google Scholar] [PubMed]
- Fogeda, M.; Avellon, A.; Echevarria, J.M. Prevalence of specific antibody to hepatitis e virus in the general population of the community of Madrid, Spain. J. Med. Virol. 2012, 84, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A.; Kenfak-Foguena, A.; Andre, C.; Canellini, G.; Burgisser, P.; Moradpour, D.; Darling, K.E.; Cavassini, M. Hepatitis e virus seroprevalence among blood donors in southwest Switzerland. PLoS ONE 2011, 6, e21150. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Stableforth, W.; Thurairajah, P.; Hazeldine, S.; Remnarace, R.; Usama, W.; Farrington, L.; Hamad, N.; Sieberhagen, C.; Ellis, V.; et al. Autochthonous hepatitis e in southwest england: Natural history, complications and seasonal variation, and hepatitis e virus igg seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur. J. Gastroenterol. Hepatol. 2008, 20, 784–790. [Google Scholar] [CrossRef]
- Teshale, E.H.; Denniston, M.M.; Drobeniuc, J.; Kamili, S.; Teo, C.G.; Holmberg, S.D. Decline in hepatitis e virus antibody prevalence in the United States from 1988–1994 to 2009–2010. J. Infect. Dis. 2015, 211, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Stramer, S.L.; Moritz, E.D.; Foster, G.A.; Ong, E.; Linnen, J.M.; Hogema, B.M.; Mak, M.; Chia, C.P.; Dodd, R.Y. Hepatitis e virus: Seroprevalence and frequency of viral rna detection among US blood donors. Transfusion 2016, 56, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, E.J.; Couturier, E.; Faber, M.; Said, B.; Ijaz, S.; Tavoschi, L.; Takkinen, J.; Adlhoch, C. Hepatitis e virus infection in europe: Surveillance and descriptive epidemiology of confirmed cases, 2005 to 2015. Euro Surveill. 2016, 22. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Hepatitis E in the EU/EEA, 2005–2015 Baseline Assessment of Testing, Diagnosis, Surveillance and Epidemiology; European Center for Disease Prevention and Control: Stockholm, Sweden, 2017.
- Rivero-Juarez, A.; Frias, M.; Martinez-Peinado, A.; Risalde, M.A.; Rodriguez-Cano, D.; Camacho, A.; Garcia-Bocanegra, I.; Cuenca-Lopez, F.; Gomez-Villamandos, J.C.; Rivero, A. Familial hepatitis e outbreak linked to wild boar meat consumption. Zoon. Public Health 2017, 64, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Guillois, Y.; Abravanel, F.; Miura, T.; Pavio, N.; Vaillant, V.; Lhomme, S.; Le Guyader, F.S.; Rose, N.; Le Saux, J.C.; King, L.A.; et al. High proportion of asymptomatic infections in an outbreak of hepatitis e associated with a spit-roasted piglet, France, 2013. Clin. Infect. Dis. 2016, 62, 351–357. [Google Scholar] [CrossRef]
- Garbuglia, A.R.; Scognamiglio, P.; Petrosillo, N.; Mastroianni, C.M.; Sordillo, P.; Gentile, D.; La Scala, P.; Girardi, E.; Capobianchi, M.R. Hepatitis e virus genotype 4 outbreak, Italy, 2011. Emerg. Infect. Dis. 2013, 19, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Trmal, J.; Pavlik, I.; Vasickova, P.; Matejickova, L.; Simunkova, L.; Luks, S.; Pazderkova, J. Outbreaks of viral hepatitis e in the Czech Republic? Epidemiol. Mikrobiol. Imunol. 2012, 61, 15–20. [Google Scholar] [PubMed]
- Yapa, C.M.; Furlong, C.; Rosewell, A.; Ward, K.A.; Adamson, S.; Shadbolt, C.; Kok, J.; Tracy, S.L.; Bowden, S.; Smedley, E.J.; et al. First reported outbreak of locally acquired hepatitis e virus infection in Australia. Med. J. Aust. 2016, 204, 274. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Matsuura, K.; Yoshizumi, S.; Miyoshi, M.; Sugisawa, T.; Tanida, M.; Okano, M. Hepatitis e outbreak at a nursing home for aged people in Hokkaido, Japan, between February and March 2016. J. Clin. Virol. 2018, 101, 23–28. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).