Tomato Yellow Leaf Curl Sardinia Virus, a Begomovirus Species Evolving by Mutation and Recombination: A Challenge for Virus Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Virus Sources
2.3. Cloning, Sequencing and Construction of an Infectious Clone of Isolate [ES-Mur-TY2-Tom-11]
2.4. Virus Inoculation, Detection and Symptoms Evaluation
2.5. Analysis of Sequence Data
3. Results
3.1. TYLCSV Population Is Composed of Isolates of Two Differentiated Strains
3.2. TYLCSV Is Evolving by Mutation and Recombination
3.3. A TYLCSV Recombinant Isolate Detected in Spain Putatively Resulted from a Genetic Exchange between Isolates of Strains ES and Sar of TYLCSV
3.4. An Infectious Clone of TYLCSV Recombinant Isolate [ES-Mur-TY2-Tom-11] Efficiently Infects Tomato and Solanum Nigrum Plants
3.5. Behaviour of Isolate TYLCSV[ES-Mur-TY2-Tom-11] in TYLCD-Resistant Tomatoes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rybicki, E.P.; Pietersen, G. Plant virus disease problems in the developing world. Adv. Virus Res. 1999, 53, 127–175. [Google Scholar] [PubMed]
- Chua, K.B.; Bellini, W.J.; Rota, P.A.; Harcourt, B.H.; Tamin, A.; Lam, S.K.; Ksiazek, T.G.; Rollin, P.E.; Zaki, S.R.; Shieh, W.-J.; et al. Nipah virus: A recently emergent deadly paramyxovirus. Science 2000, 288, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.H.; Shaw, G.M.; De Cock, K.M.; Sharp, P.M. AIDS as a zoonosis: Scientific and public health implications. Science 2000, 287, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Zaidi, S.S.; Shakir, S.; Farooq, M.; Amin, I.; Scheffler, J.A.; Scheffler, B.E.; Mansoor, S. Multiple begomoviruses found associated with cotton leaf curl disease in Pakistan in early 1990 are back in cultivated cotton. Sci. Rep. 2017, 7, 680. [Google Scholar] [CrossRef] [PubMed]
- Moriones, E.; Praveen, S.; Chakraborty, S. Tomato leaf curl New Delhi virus: An emerging virus complex threatening vegetable and fiber crops. Viruses 2017, 9, 264. [Google Scholar] [CrossRef]
- Mabvakure, B.; Martin, D.P.; Kraberger, S.; Cloete, L.; van Brunschot, S.; Geering, A.D.W.; Thomas, J.E.; Bananej, K.; Lett, J.M.; Lefeuvre, P.; et al. Ongoing geographical spread of Tomato yellow leaf curl virus. Virology 2016, 498, 257–264. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, A.; Harimalala, M.; Zinga, I.; Mabvakure, B.M.; Hoareau, M.; Ravigne, V.; Walters, M.; Reynaud, B.; Varsani, A.; Harkins, G.W.; et al. Divergent evolutionary and epidemiological dynamics of cassava mosaic geminiviruses in Madagascar. BMC Evol. Biol. 2016, 16, 1–21. [Google Scholar] [CrossRef]
- Legg, J.; Somado, E.A.; Barker, I.; Beach, L.; Ceballos, H.; Cuellar, W.; Elkhoury, W.; Gerling, D.; Helsen, J.; Hershey, C. A global alliance declaring war on cassava viruses in Africa. Food Secur. 2014, 6, 231–248. [Google Scholar] [CrossRef]
- Rocha, C.S.; Castillo-Urquiza, G.P.; Lima, A.T.M.; Silva, F.I.N.; Xavier, C.A.D.; Hora-Junior, B.T.; Beserra-Junior, J.E.A.; Malta, A.W.O.; Martin, D.P.; Varsani, A.; et al. Brazilian begomovirus ppopulations aare highly recombinant, rapidly evolving, and segregated based on geographical location. J. Virol. 2013, 87, 5784–5799. [Google Scholar] [CrossRef]
- Kenyon, L.; Tsai, W.S.; Shih, S.L.; Lee, L.M. Emergence and diversity of begomoviruses infecting solanaceous crops in East and Southeast Asia. Virus Res. 2014, 186, 104–113. [Google Scholar] [CrossRef] [PubMed]
- De Barro, P.J. Bemisia tabaci, the Capacity to Invade. In The Whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) Interaction with Geminivirus-Infected Host Plants; Thompson, W.M.O., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 181–204. [Google Scholar]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, R.L.; Batuman, O.; Webster, C.G.; Adkins, S. Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the eemergence and global spread of plant viruses. Annu. Rev. Virol. 2015, 2, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Zerbini, F.; Briddon, R.W.; Idris, A.; Martin, D.P.; Moriones, E.; Navas-Castillo, J.; Rivera-Bustamante, R.; Roumagnac, P.; Varsani, A. ICTV Virus Taxonomy Profile: Geminiviridae. J. Gen. Virol. 2017, 98, 131–133. [Google Scholar] [CrossRef]
- Hanley-Bowdoin, L.; Settlage, S.B.; Orozco, B.M.; Nagar, S.; Robertson, D. Geminiviruses: Models for plant DNA replication, transcription, and cell cycle regulation. Critical Rev. Biochem. Mol. Biol. 1999, 35, 105–140. [Google Scholar] [CrossRef]
- Luna, A.P.; Morilla, G.; Voinnet, O.; Bejarano, E.R. Functional analysis of gene-silencing suppressors from tomato yellow leaf curl disease viruses. Mol. Plant-Microbe Interact. 2012, 25, 1294–1306. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Diaz, T.; Zhang, D.; Fan, P.; Wang, L.; Ding, X.; Jiang, Y.; Jimenez-Gongora, T.; Medina-Puche, L.; Zhao, X.; Feng, Z.; et al. A virus-targeted plant receptor-like kinase promotes cell-to-cell spread of RNAi. Proc. Natl. Acad. Sci. USA 2018, 115, 1388. [Google Scholar] [CrossRef]
- García-Arenal, F.; Fraile, A.; Malpica, J.M. Variability and genetic structure of plant virus populations. Annu. Rev. Phytopathol. 2001, 39, 157–186. [Google Scholar] [CrossRef]
- Botstein, D. A modular theory of virus evolution. In Animal Virus Genetics; Fields, B.N., Jaenisch, R., Eds.; Academic Press: Cambridge, MA, USA, 1980; pp. 11–20. [Google Scholar]
- Martin, D.P.; Van der Walt, E.; Posada, D.; Rybicki, E.P. The evolutionary value of recombination is constrained by genome modularity. PLoS Genet. 2005, 1, 475–479. [Google Scholar] [CrossRef]
- Lefeuvre, P.; Moriones, E. Recombination as a motor of host switches and virus emergence: Geminiviruses as case studies. Curr. Opin. Virol. 2015, 10, 14–19. [Google Scholar] [CrossRef]
- García-Andrés, S.; Tomás, D.M.; Sánchez-Campos, S.; Navas-Castillo, J.; Moriones, E. Frequent occurrence of recombinants in mixed infections of tomato yellow leaf curl disease-associated begomoviruses. Virology 2007, 365, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Monci, F.; Sánchez-Campos, S.; Navas-Castillo, J.; Moriones, E. A natural recombinant between the geminiviruses Tomato yellow leaf curl Sardinia virus and Tomato yellow leaf curl virus exhibits a novel pathogenic phenotype and is becoming prevalent in Spanish populations. Virology 2002, 303, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Davino, S.; Napoli, C.; Dellacroce, C.; Miozzi, L.; Noris, E.; Davino, M.; Accotto, G.P. Two new natural begomovirus recombinants associated with the tomato yellow leaf curl disease co-exist with parental viruses in tomato epidemics in Italy. Virus Res. 2009, 143, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Belabess, Z.; Peterschmitt, M.; Granier, M.; Tahiri, A.; Blenzar, A.; Urbino, C. The non-canonical tomato yellow leaf curl virus recombinant that displaced its parental viruses in Southern Morocco exhibits a high selective advantage in experimental conditions. J. Gen. Virol. 2016, 97, 3433–3445. [Google Scholar] [CrossRef] [PubMed]
- Moriones, E.; García-Andrés, S.; Navas-Castillo, J. Recombination in the TYLCV Complex: A Mechanism to Increase Genetic Diversity. Implications for Plant Resistance Development. In Tomato Yellow Leaf Curl Virus Disease Management, Molecular Biology, Breeding for Resistance; Czosnek, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 119–138. [Google Scholar]
- Moriones, E.; Navas-Castillo, J.; Díaz-Pendón, J.A. Emergence of Begomovirus Diseases; Caranta, C., Aranda, M.A., Tepfer, M., López-Moya, J.J., Eds.; Caister Acad: Norfolk, VA, USA, 2011; pp. 301–320. [Google Scholar]
- Duffy, S.; Holmes, E.C. Multiple Introductions of the Old World Begomovirus Tomato yellow leaf curl virus into the New World. Appl. Environ. Microbiol. 2007, 73, 7114–7117. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, B.; Luan, J.; Xie, Y.; Liu, S.; Zhou, X. Molecular variation of tomato yellow leaf curl virus in the insect vector Bemisia tabaci. Sci. Rep. 2017, 7, 16427. [Google Scholar] [CrossRef] [PubMed]
- Panno, S.; Caruso, A.G.; Davino, S. The nucleotide sequence of a recombinant tomato yellow leaf curl virus strain frequently detected in Sicily isolated from tomato plants carrying the Ty-1 resistance gene. Arch. Virol. 2018, 63, 795–797. [Google Scholar] [CrossRef]
- Kil, E.J.; Kim, S.; Lee, Y.J.; Byun, H.S.; Park, J.; Seo, H.; Kim, C.S.; Shim, J.K.; Lee, J.H.; Kim, J.K.; et al. Tomato yellow leaf curl virus (TYLCV-IL): A seed-transmissible geminivirus in tomatoes. Sci. Rep. 2016, 6, 19013. [Google Scholar] [CrossRef]
- Ohnishi, J.; Yamaguchi, H.; Saito, A. Analysis of the Mild strain of tomato yellow leaf curl virus, which overcomes Ty-2 gene-mediated resistance in tomato line H24. Arch. Virol. 2016, 161, 2207–2217. [Google Scholar] [CrossRef]
- Hariton Shalev, A.; Sobol, I.; Ghanim, M.; Liu, S.S.; Czosnek, H. The whitefly Bemisia tabaci Knottin-1 gene is implicated in regulating the quantity of Tomato yellow leaf curl virus ingested and transmitted by the insect. Viruses 2016, 8, 205. [Google Scholar] [CrossRef]
- Lefeuvre, P.; Martin, D.P.; Harkins, G.; Lemey, P.; Gray, A.J.A.; Meredith, S.; Lakay, F.; Monjane, A.; Lett, J.M.; Varsani, A.; et al. The spread of tomato yellow leaf curl virus from the Middle East to the world. PLoS Pathog. 2010, 6, e1001164. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Campos, S.; Díaz, J.A.; Monci, F.; Bejarano, E.R.; Reina, J.; Navas-Castillo, J.; Aranda, M.A.; Moriones, E. High genetic stability of the begomovirus Tomato yellow leaf curl Sardinia virus in southern Spain over an 8-year period. Phytopathology 2002, 92, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Mnari-Hattab, M.; Zammouri, S.; Pellegrin, F.; Gauthier, N. Natural ooccurrence of begomovirus recombinants associated with Tomato yellow leaf curl disease co-existing with parental viruses in tomato crops and weeds in Tunisia. J. Plant Pathol. 2014, 96, 195–200. [Google Scholar]
- Davino, S.; Miozzi, L.; Panno, S.; Rubio, L.; Davino, M.; Accotto, G.P. Recombination profiles between Tomato yellow leaf curl virus and Tomato yellow leaf curl Sardinia virus in laboratory and field conditions: Evolutionary and taxonomic implications. J. Gen. Virol. 2012, 93, 2712–2717. [Google Scholar] [CrossRef] [PubMed]
- Pellegrin, F.; Mnari-Hattab, M.; Tahiri, A.; Dalleau-Clouet, C.; Peterschmitt, M.; Bonato, O. First report of simultaneous presence of Tomato yellow leaf curl Sardinia virus and Tomato yellow leaf curl Israel virus infecting crops and weeds in Tunisia. J. Plant Pathol. 2008, 90, 145. [Google Scholar]
- Belabess, Z.; Dallot, S.; El-Montaser, S.; Granier, M.; Majde, M.; Tahiri, A.; Blenzar, A.; Urbino, C.; Peterschmitt, M. Monitoring the dynamics of emergence of a non-canonical recombinant of Tomato yellow leaf curl virus and displacement of its parental viruses in tomato. Virology 2015, 486, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, M.; Legg, J.P.; Wintermantel, W.M.; Polston, J.E. Management of whitefly-transmitted viruses in open-field production systems. Adv. Virus Res. 2014, 90, 147–206. [Google Scholar] [PubMed]
- Rojas, M.R.; Macedo, M.A.; Maliano, M.R.; Soto-Aguilar, M.; Souza, J.O.; Briddon, R.W.; Kenyon, L.; Bustamante, R.F.R.; Zerbini, F.M.; Adkins, S.; et al. World management of geminiviruses. Annu. Rev. Phytopathol. 2018, 56, 637–677. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, M.G.; Hutton, S.F.; Ibrahem, R.M.; Kormelink, R.; Visser, R.G.; Scott, J.W.; Edwards, J.D.; Bai, Y. The Tomato yellow leaf curl virus resistance genes Ty-1 and Ty-3 are allelic and code for DFDGD-class RNA-dependent RNA polymerases. PLoS Genet. 2013, 9, e1003399. [Google Scholar] [CrossRef] [PubMed]
- Tomás, D.M.; Cañizares, C.; Abad, J.; Fernández-Muñoz, R.; Moriones, E. Resistance to tomato yellow leaf curl virus accumulation in the tomato wild relative Solanum habrochaites associated with the C4 viral protein. Mol. Plant-Microbe Interact. 2011, 24, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.M.; Bernacchi, D.; Green, S.; Tanksley, S.D.; Muniyappa, V.; Padmaja, S.; Chen, H.M.; Kuo, G.; Fang, D.; Chen, J.T. Mapping a wild tomato introgression associated with tomato yellow leaf curl virus resistance in a cultivated tomato line. J. Am. Soc. Hort. Sci. 2000, 125, 15–20. [Google Scholar]
- García-Cano, E.; Resende, R.O.; Boiteux, L.S.; Giordano, L.B.; Fernandez-Muñoz, R.; Moriones, E. Phenotypic expression, stability, and inheritance of a recessive resistance to monopartite begomoviruses associated with Tomato yellow leaf curl disease in tomato. Phytopathology 2008, 98, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Hutton, S.F.; Scott, J.W.; Schuster, D.J. Recessive resistance to Tomato yellow leaf curl virus from the tomato cultivar Tyking is located in the same region as Ty-5 on chromosome 4. Hortscience 2012, 47, 324–327. [Google Scholar]
- Morilla, G.; Janssen, D.; García-Andrés, S.; Moriones, E.; Cuadrado, I.M.; Bejarano, E.R. Pepper (Capsicum annuum) is a dead-end host for tomato yellow leaf curl virus. Phytopathology 2005, 95, 1089–1097. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Sánchez-Campos, S.; Díaz, J.A.; Saez-Alonso, E.; Moriones, E. Tomato yellow leaf curl virus-Is causes a novel disease of common bean and severe epidemics in tomato in Spain. Plant Dis. 1999, 83, 29–32. [Google Scholar] [CrossRef]
- Noris, E.; Hidalgo, E.; Accotto, G.P.; Moriones, E. High similarity among the tomato yellow leaf curl virus isolates from the West Mediterranean Basin - The nucleotide sequence of an infectious clone from Spain. Arch. Virol. 1994, 135, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Campos, S.; Navas-Castillo, J.; Camero, R.; Soria, C.; Díaz, J.A.; Moriones, E. Displacement of tomato yellow leaf curl virus (TYLCV)-Sr by TYLCV-Is in tomato epidemics in spain. Phytopathology 1999, 89, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- García-Andrés, S.; Accotto, G.P.; Navas-Castillo, J.; Moriones, E. Founder effect, plant host, and recombination shape the emergent population of begomoviruses that cause the tomato yellow leaf curl disease in the Mediterranean basin. Virology 2007, 359, 302–312. [Google Scholar] [CrossRef]
- Permingeat, H.R.; Romagnoli, M.V.; Sesma, J.I.; Vallejos, R.H. A simple method for Isolating DNA of high yield and quality from cotton (shape Gossypium hirsutum L.) leaves. Plant Mol. Biol. Rep. 1998, 16, 89. [Google Scholar] [CrossRef]
- Monci, F.; García-Andrés, S.; Maldonado, J.A.; Moriones, E. Resistance to monopartite begomoviruses associated with the bean leaf crumple disease in Phaseolus vulgaris controlled by a single dominant gene. Phytopathology 2005, 95, 819–826. [Google Scholar] [CrossRef]
- Díaz-Pendón, J.A.; Fernández-Muñoz, R.; Gómez-Guillamón, M.L.; Moriones, E. Inheritance of resistance to Watermelon mosaic virus in Cucumis melo that impairs virus accumulation, symptom expression, and aphid transmission. Phytopathology 2005, 95, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stercher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Lole, K.S.; Bollinger, R.C.; Paranjape, R.S.; Gadkari, D.; Kulkarni, S.S.; Novak, N.G.; Ingersoll, R.; Sheppard, H.W.; Ray, S.C. Full-length human immunodeficiency virus Type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999, 73, 152–160. [Google Scholar] [PubMed]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Reina, J.; Jiménez, J.J.; Bejarano, E.R.; Guerra, J.M.; Cuadrado, I.M.; García, C. El virus del rizado amarillo del tomate (TYLCV). Hortofruticultura 1994, 5, 36–40. [Google Scholar]
- Kheyr-Pour, A.; Bendahmane, M.; Matzeit, V.; Accotto, G.P.; Crespi, S.; Gronenborn, B. Tomato yellow leaf curl virus from Sardinia is a whitefly-transmitted monoparatite geminivirus. Nucleic Acids Res. 1991, 19, 6763. [Google Scholar] [CrossRef]
- Crespi, S.; Noris, E.; Vaira, A.M.; Accotto, G.P. Molecular characterization of cloned DNA from a tomato yellow leaf curl virus isolate from Sicily. Phytopathol. Med. 1995, 34, 93–99. [Google Scholar]
- Davino, S.; Miozzi, L.; Accotto, G.P. The complete nucleotide sequence of an isolate of Tomato yellow leaf curl Sardinia virus found in Sicily. Arch. Virol. 2010, 155, 1539–1542. [Google Scholar] [CrossRef]
- El Mehrachi, K.; Sedegui, M.; Hatimi, A.; Tahrouch, S.; Arifi, A.; Czosnek, H.; Nakhla, M.K.; Maxwell, D.P. Molecular characterization of a Moroccan isolate of Tomato yellow leaf curl Sardinia virus and differentiation of the Tomato yellow leaf curl virus complex by the polymerase chain reaction. Phytopathol. Med. 2007, 46, 185–194. [Google Scholar]
- Chouchane, S.G.; Gorsane, F.; Nakhla, M.K.; Maxwell, D.P.; Marrakchi, M.; Fakhfakh, H. Complete nucleotide sequence and construction of an infectious clone of a Tunisian isolate of Tomato yellow leaf curl Sardinia virus. J. Phytopathol. 2006, 154, 626–631. [Google Scholar] [CrossRef]
- Anfoka, G.; Altaleb, M.; Haj Ahmad, F.; Abu Obaida, M. Charlock mustard (Sinapis arvensis): A weed reservoir for begomoviruses and associated betasatellite in Jordan. Can. J. Plant Pathol. 2017, 39, 325–333. [Google Scholar] [CrossRef]
- Brown, J.; Zerbini, F.M.; Navas-Castillo, J.; Moriones, E.; Ramos-Sobrinho, R.; Silva, J.; Fiallo-Olivé, E.; Briddon, R.; Hernandez-Zepeda, C.; Idris, A.; et al. Revision of Begomovirus taxonomy based on pairwise sequence comparisons. Arch. Virol. 2015, 160, 1593–1619. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.; Fauquet, C.M.; Briddon, R.W.; Zerbini, F.M.; Moriones, E.; Navas-Castillo, J. Family Geminiviridae. In Virus Taxonomy—Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier Inc.: San Diego, CA, USA, 2012; pp. 351–373. [Google Scholar]
- García-Andrés, S.; Monci, F.; Navas-Castillo, J.; Moriones, E. Begomovirus genetic diversity in the native plant reservoir Solanum nigrum: Evidence for the presence of a new virus species of recombinant nature. Virology 2006, 350, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Acciarri, N.; Sabatini, E.; Sardo, L.; Accotto, G.P.; Pecchioni, N. Introgression of resistance to two Mediterranean virus species causing tomato yellow leaf curl into a valuable traditional tomato variety. J. Plant Pathol. 2010, 92, 485–493. [Google Scholar]
- Fortes, M.I.; Sánchez-Campos, S.; Fiallo-Olivé, E.; Díaz-Pendón, J.A.; Navas-Castillo, J.; Moriones, E. A novel strain of tomato leaf curl New Delhi virus has spread to the Mediterranean basin. Viruses 2016, 8, 307. [Google Scholar] [CrossRef]
- Melgarejo, T.A.; Kon, T.; Gilbertson, R.L. Molecular and biological characterization of distinct strains of Jatropha mosaic virus from the Dominican Republic reveal a potential to infect crop plants. Phytopathology 2014, 105, 141–153. [Google Scholar] [CrossRef]
- Lefeuvre, P.; Lett, J.M.; Varsani, A.; Martin, D.P. Widely conserved recombination patterns among single-stranded DNA viruses. J. Virol. 2009, 83, 2697–2707. [Google Scholar] [CrossRef]
- Martin, D.P.; Lefeuvre, P.; Varsani, A.; Hoareau, M.; Semegni, J.Y.; Dijoux, B.; Vincent, C.; Reynaud, B.; Lett, J.M. Complex recombination patterns arising during geminivirus coinfections preserve and demarcate biologically important intra-genome interaction networks. PLoS Pathog. 2011, 7, e1002203. [Google Scholar] [CrossRef]
- Leke, W.N.; Mignouna, D.B.; Brown, J.K.; Kvarnheden, A. Begomovirus disease complex: Emerging threat to vegetable production systems of West and Central Africa. Agric. Food Secur. 2015, 4, 1–14. [Google Scholar] [CrossRef]
- Silva, F.; Lima, A.; Rocha, C.; Castillo-Urquiza, G.; Alves-Junior, M.; Zerbini, F. Recombination and pseudorecombination driving the evolution of the begomoviruses Tomato severe rugose virus (ToSRV) and Tomato rugose mosaic virus (ToRMV): Two recombinant DNA-A components sharing the same DNA-B. Virol. J. 2014, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Stam, R.; McDonald, B.A. When resistance gene pyramids are not durable—The role of pathogen diversity. Mol. Plant Pathol. 2018, 19, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Navas-Castillo, J.; Sánchez-Campos, S.; Noris, E.; Louro, D.; Accotto, G.P.; Moriones, E. Natural recombination between Tomato yellow leaf curl virus-Is and Tomato leaf curl virus. J. Gen. Virol. 2000, 81, 2797–2801. [Google Scholar] [CrossRef] [PubMed]
- Pagán, I. The diversity, evolution and epidemiology of plant viruses: A phylogenetic view. Infect. Gen. Evol. 2018, 65, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Fargette, D.; Konate, G.; Fauquet, C.; Muller, E.; Peterschmitt, M.; Thresh, J.M. Molecular ecology and emergence of tropical plant viruses. Annu. Rev. Phytopathol. 2006, 44, 235–260. [Google Scholar] [CrossRef]
- van der Walt, E.; Rybicki, E.P.; Varsani, A.; Polston, J.E.; Billharz, R.; Donaldson, L.; Monjane, A.L.; Martin, D.P. Rapid host adaptation by extensive recombination. J. Gen. Virol. 2009, 90, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Noris, E.; Lucioli, A.; Tavazza, R.; Caciagli, P.; Accotto, G.P.; Tavazza, M. Tomato yellow leaf curl Sardinia virus can overcome transgene-mediated RNA silencing of two essential viral genes. J. Gen. Virol. 2004, 85, 1745–1749. [Google Scholar] [CrossRef]
- Lucioli, A.; Noris, E.; Brunetti, A.; Tavazza, R.; Ruzza, V.; Castillo, A.G.; Bejarano, E.R.; Accotto, G.P.; Tavazza, M. Tomato yellow leaf curl Sardinia virus Rep-derived resistance to homologous and heterologous geminiviruses occurs by different mechanisms and is overcome if virus-mediated transgene silencing is activated. J. Virol. 2003, 77, 6785–6798. [Google Scholar] [CrossRef]
- Urbino, C.; Gutiérrez, S.; Antolik, A.; Bouazza, N.; Doumayrou, J.; Granier, M.; Martin, D.P.; Peterschmitt, M. Within-host dynamics of the emergence of tomato yellow leaf curl Virus recombinants. PLoS ONE 2013, 8, e58375. [Google Scholar] [CrossRef]
- García-Andrés, S.; Tomás, D.M.; Navas-Castillo, J.; Moriones, E. Resistance-driven selection of begomoviruses associated with the tomato yellow leaf curl disease. Virus Res. 2009, 146, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Butterbach, P.; Verlaan, M.G.; Dullemans, A.M.; Lohuis, D.; Visser, R.G.F.; Bai, Y.; Kormelink, R. Tomato yellow leaf curl virus resistance by Ty-1 involves increased cytosine methylation of viral genomes and is compromised by cucumber mosaic virus infection. Proc. Natl. Acad. Sci. USA 2014, 111, 12942–12947. [Google Scholar] [CrossRef] [PubMed]
- Gallois, J.L.; Moury, B.; German-Retana, S. Role of the genetic background in resistance to plant viruses. Int. J. Mol. Sci. 2018, 19, 2856. [Google Scholar] [CrossRef] [PubMed]
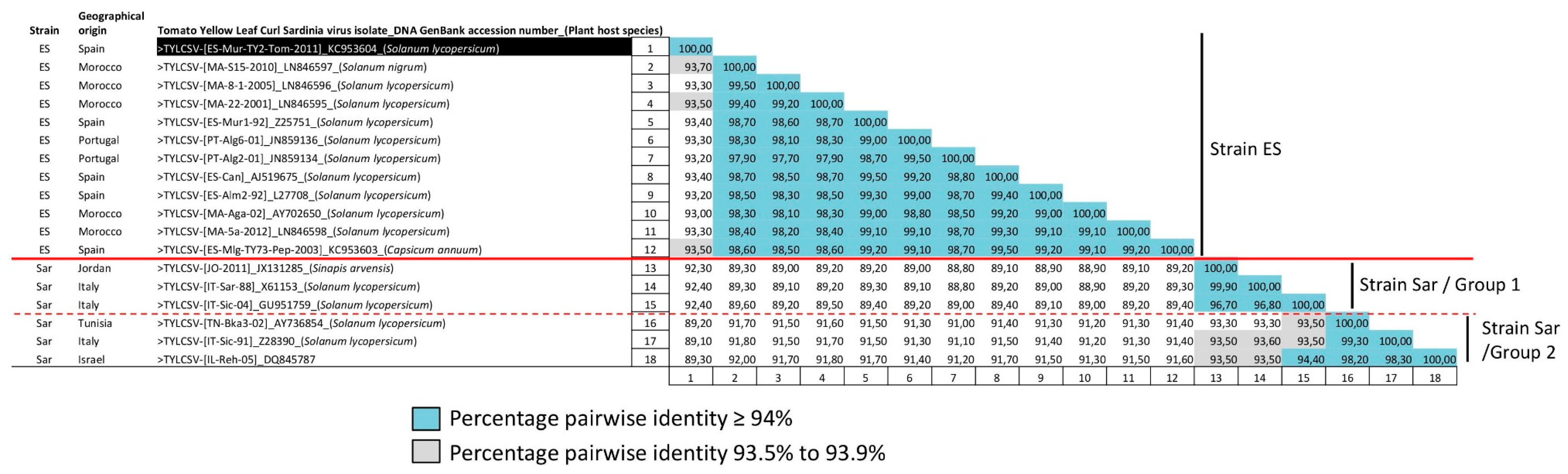
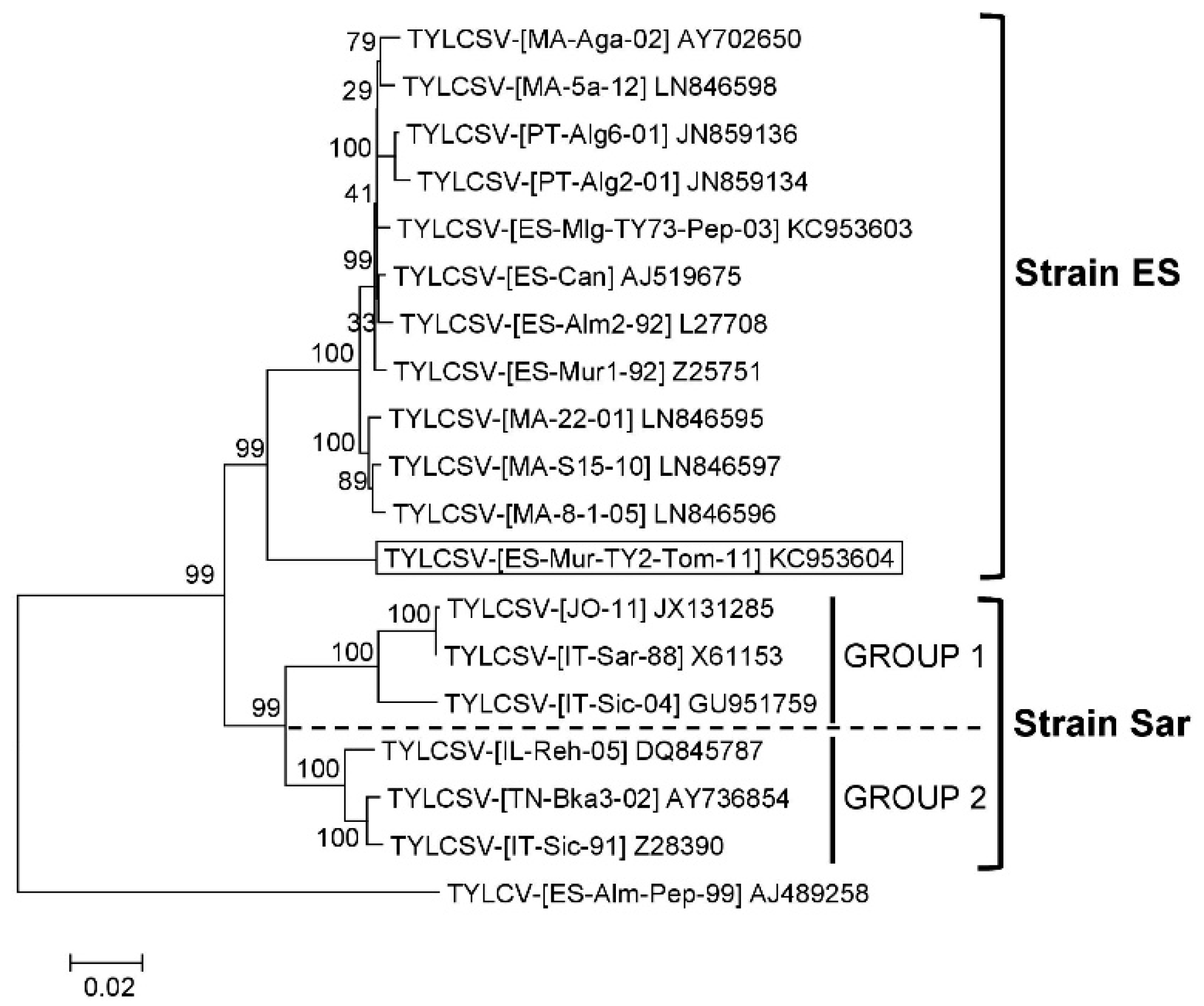
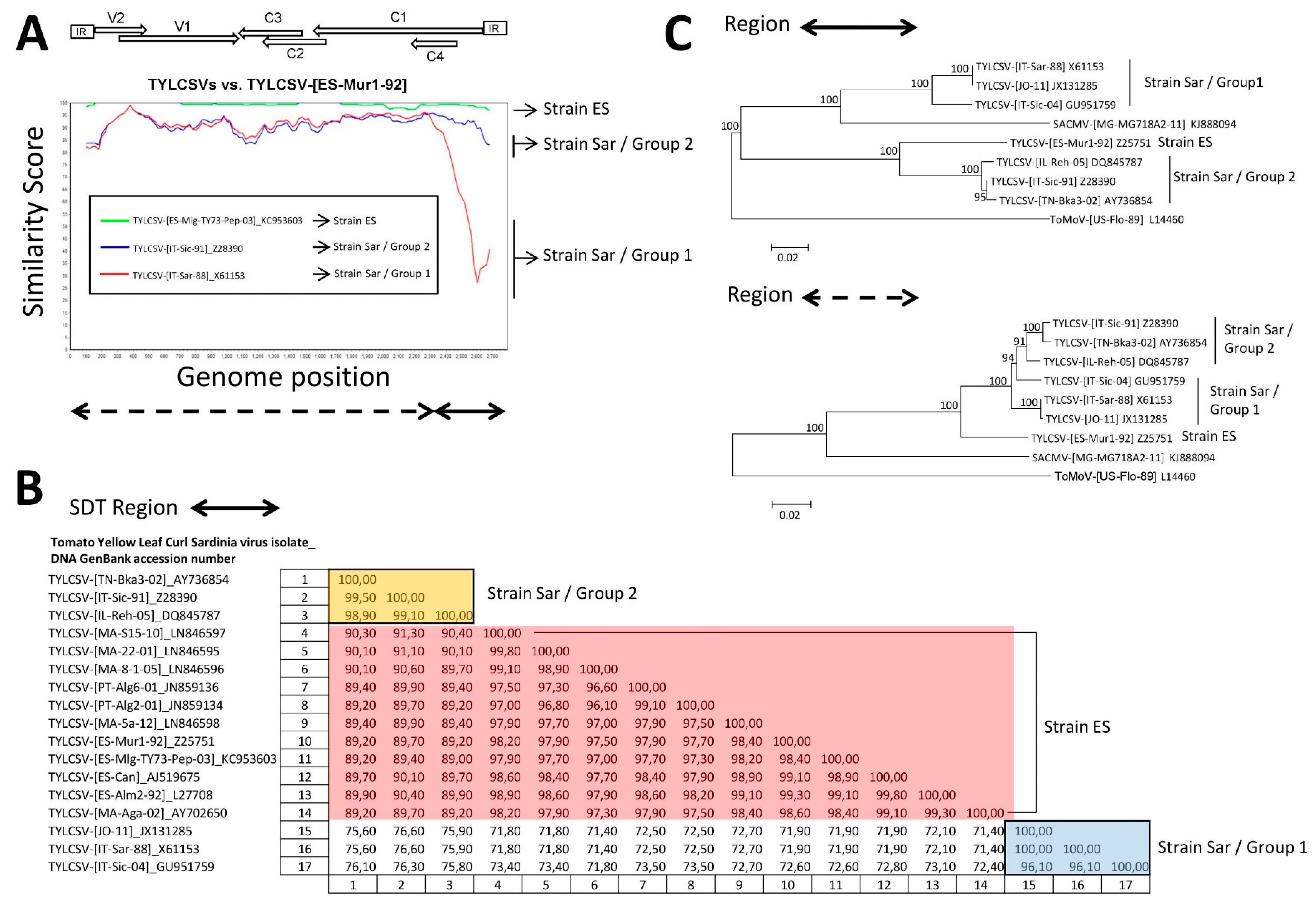

| Tomato Yellow Leaf Curl Sardinia Virus Isolate | Acronym | GenBank Accession Number | Host Species | Geographical Origin | Collection Date | Reference |
|---|---|---|---|---|---|---|
| Tomato yellow leaf curl Sardinia virus-[Spain-Murcia 1-1992] | TYLCSV-[ES-Mur1-92] | Z25751 | Solanum lycopersicum | Spain | 1992 | [50] |
| Tomato yellow leaf curl Sardinia virus-[Spain-Almeria 2-1992] | TYLCSV-[ES-Alm2-92] | L27708 | Solanum lycopersicum | Spain | 1992 | [61] |
| Tomato yellow leaf curl Sardinia virus-[Spain-Canary] | TYLCSV-[ES-Can] | AJ519675 | Solanum lycopersicum | Spain | NA | NA a |
| Tomato yellow leaf curl Sardinia virus-[Spain-Malaga-TY73-Pepper-2003] | TYLCSV-[ES-Mlg-TY73-Pep-03] | KC953603 | Capsicum annuum | Spain | 2003 | NA |
| Tomato yellow leaf curl Sardinia virus-[Spain-Murcia-TY2-Tomato-2011] | TYLCSV-[ES-Mur-TY2-Tom-11] | KC953604 | Solanum lycopersicum | Spain | 2011 | Present study |
| Tomato yellow leaf curl Sardinia virus-[Italy-Sardinia-1988] | TYLCSV-[IT-Sar-88] | X61153 | Solanum lycopersicum | Italy | 1988 | [62] |
| Tomato yellow leaf curl Sardinia virus-[Italy-Sicily-1991] | TYLCSV-[IT-Sic-91] | Z28390 | Solanum lycopersicum | Italy | 1991 | [63] |
| Tomato yellow leaf curl Sardinia virus-[Italy-Sicily-2004] | TYLCSV-[IT-Sic-04] | GU951759 | Solanum lycopersicum | Italy | 2004 | [64] |
| Tomato yellow leaf curl Sardinia virus-[Portugal-Algarve 6-2001] | TYLCSV-[PT-Alg6-01] | JN859136 | Solanum lycopersicum | Portugal | 2001 | NA |
| Tomato yellow leaf curl Sardinia virus-[Portugal-Algarve 2-2001] | TYLCSV-[PT-Alg2-01] | JN859134 | Solanum lycopersicum | Portugal | 2001 | NA |
| Tomato yellow leaf curl Sardinia virus-[Morocco-22-2001] | TYLCSV-[MA-22-01] | LN846595 | Solanum lycopersicum | Morocco | 2001 | [40] |
| Tomato yellow leaf curl Sardinia virus-[Morocco-Agadir-2002] | TYLCSV-[MA-Aga-02] | AY702650 | Solanum lycopersicum | Morocco | 2002 | [65] |
| Tomato yellow leaf curl Sardinia virus-[Morocco-8-1-2005] | TYLCSV-[MA-8-1-05] | LN846596 | Solanum lycopersicum | Morocco | 2005 | [40] |
| Tomato yellow leaf curl Sardinia virus-[Morocco-S15-2010] | TYLCSV-[MA-S15-10] | LN846597 | Solanum nigrum | Morocco | 2010 | [40] |
| Tomato yellow leaf curl Sardinia virus-[Morocco-5a-2012] | TYLCSV-[MA-5a-12] | LN846598 | Solanum lycopersicum | Morocco | 2012 | [40] |
| Tomato yellow leaf curl Sardinia virus-[Tunisia-Bkalta 3-2002] | TYLCSV-[TN-Bka3-02] | AY736854 | Solanum lycopersicum | Tunisia | 2002 | [66] |
| Tomato yellow leaf curl Sardinia virus-[Jordan-2011] | TYLCSV-[JO-11] | JX131285 | Sinapis arvensis | Jordan | 2011 | [67] |
| Tomato yellow leaf curl Sardinia virus-[Israel-Rehovot-2005] | TYLCSV-[IL-Reh-05] | DQ845787 | NA | Israel | 2005 | NA |
| Recombinant Sequence | Major Parent | Minor Parent | Breakpoints a | Methods b | p-Value c | |
|---|---|---|---|---|---|---|
| Begin | End | |||||
| TYLCSV-[ES-Mur-TY2-Tom-11] (KC953604) | TYLCSV-[ES-Mur1-92] (Z25751) | TYLCSV-[IT-Sar-88] (X61153) | 1951 | 56 | RGBMCT | 1221 × 10−2 |
| Plant Species | TYLCSV-[ES-Mur-TY2-Tom-11] | TYLCSV-[ES-Mur1-92] | TYLCV-Mld[ES-72-97] | |||
|---|---|---|---|---|---|---|
| Assay 1 | Assay 2 | Assay 1 | Assay 2 | Assay 1 | Assay 2 | |
| Solanum nigrum | 10/10 b | 8/9 | 10/10 | 9/9 | 0/8 | 0/9 |
| Common bean | 0/10 | 1/9 | 0/10 | 0/9 | 7/8 | 9/9 |
| Tomato (Moneymaker) | 10/10 | 10/10 | 10/10 | 9/10 | 9/10 | 10/10 |
| TYLCSV-[ES-Mur-TY2-Tom-11] | TYLCSV-[ES-Mur1-92] | TYLCV-Mld[ES-72-97] | TYLCV-[ES-Alm-Pep-99] | |||||
|---|---|---|---|---|---|---|---|---|
| No of Plants Infected/No Inoculated | AUSPC (DSI) | No of Plants Infected/No Inoculated | AUSPC (DSI) | No of Plants Infected/No Inoculated | AUSPC (DSI) | No of Plants Infected/No Inoculated | AUSPC (DSI) | |
| ty-SkS | 10/10 | 70.4 ± 4.1 (98%) | 10/10 | 59.2 ± 0.8 (90%) | 10/10 | 100.1 ± 0.7 (100%) | 8/10 | 100.6 ± 0.9 (100%) |
| TY-Sk1 (Ty-1/ty-1) | 0/9 | 0 (0%) | 0/10 | 0 (0%) | 3/10 * | 0 (0%) | 5/10 * | 0 (0%) |
| TY-Sk3 (Ty-3/ty-3) | 7/10 | 0 (0%) | 9/10 | 0 (0%) | 10/10 | 40.7 ± 4.7 (46%) | 6/10 | 43.4 ± 8.7 (32%) |
| TY-Sk13 (Ty-1/Ty-3) | 8/10 | 0 (0%) | 8/10 | 0 (0%) | 9/10 | 0 (0%) | 7/10 | 11.8 ± 0.6 (5.9%) |
| ty-1S (ty-1/ty-1) | 10/10 | 64.4 ± 2.6 (100%) | 10/10 | 43.1 ± 5.1 (78%) | 10/10 | 84.0 ± 0.9 (100%) | 6/10 | 72.9 ± 0.8 (90%) |
| Ty-1F1 (Ty-1/ty-1) | 9/10 | 0 (0%) | 7/10 | 0 (0%) | 9/10 | 0 (0%) | 8/10 | 19.3 ± 8.3 (15%) |
| Ty-1R (Ty-1/Ty-1) | 2/10 * | 0 (0%) | 2/10 * | 0 (0%) | 10/10 | 0 (0%) | 4/10 * | 0 (0%) |
| S. habrochaites EELM-889 | 4/10 | 17.9 ± 3.1 (53%) | 5/10 | 38.2 ± 14.3 (46%) | 9/10 | 64.0 ± 0.8 (70%) | 0/10 | 0 (0%) |
| H24 (Ty-2/Ty-2) | 10/10 | 85.4 ± 2.3 (100%) | 10/10 | 82.6 ± 0.6 (100%) | 10/10 | 69.0 ± 1.2 (80%) | 0/10 | 0 (0%) |
| TX 468-RG | 9/10 * | 9.5 ± 3.2 (12%) | 10/10 * | 19.9 ± 3.9 (20%) | 8/10 * | 15.7 ± 5.1 (15%) | 5/10 * | 10.2 ± 3.4 (14%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Pendón, J.A.; Sánchez-Campos, S.; Fortes, I.M.; Moriones, E. Tomato Yellow Leaf Curl Sardinia Virus, a Begomovirus Species Evolving by Mutation and Recombination: A Challenge for Virus Control. Viruses 2019, 11, 45. https://doi.org/10.3390/v11010045
Díaz-Pendón JA, Sánchez-Campos S, Fortes IM, Moriones E. Tomato Yellow Leaf Curl Sardinia Virus, a Begomovirus Species Evolving by Mutation and Recombination: A Challenge for Virus Control. Viruses. 2019; 11(1):45. https://doi.org/10.3390/v11010045
Chicago/Turabian StyleDíaz-Pendón, Juan A., Sonia Sánchez-Campos, Isabel María Fortes, and Enrique Moriones. 2019. "Tomato Yellow Leaf Curl Sardinia Virus, a Begomovirus Species Evolving by Mutation and Recombination: A Challenge for Virus Control" Viruses 11, no. 1: 45. https://doi.org/10.3390/v11010045
APA StyleDíaz-Pendón, J. A., Sánchez-Campos, S., Fortes, I. M., & Moriones, E. (2019). Tomato Yellow Leaf Curl Sardinia Virus, a Begomovirus Species Evolving by Mutation and Recombination: A Challenge for Virus Control. Viruses, 11(1), 45. https://doi.org/10.3390/v11010045





