A Paradigmatic Interplay between Human Cytomegalovirus and Host Immune System: Possible Involvement of Viral Antigen-Driven CD8+ T Cell Responses in Systemic Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. HCMV Serology
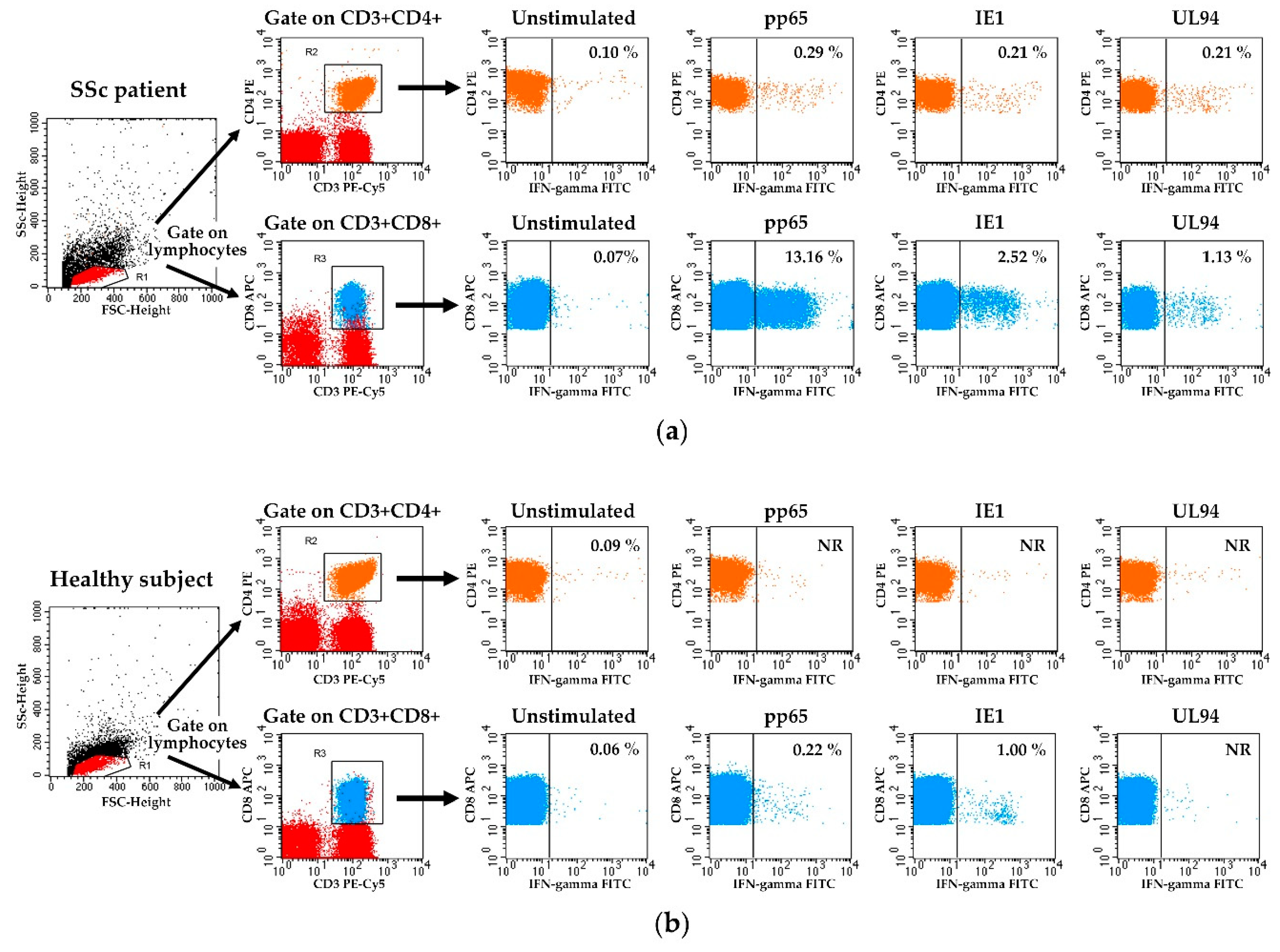
2.3. Detection of HCMV-Specific T Cell Responses
2.4. Statistical Analysis
3. Results
3.1. Study Population
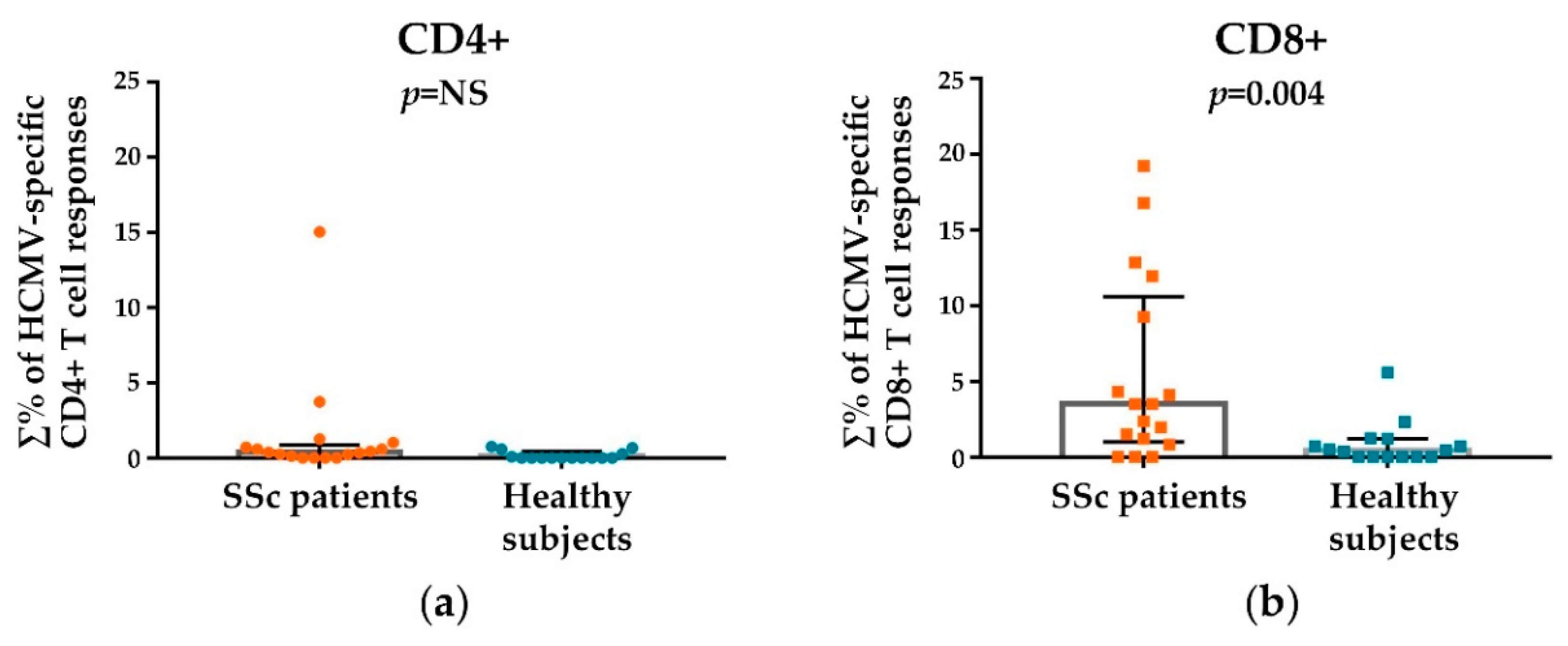
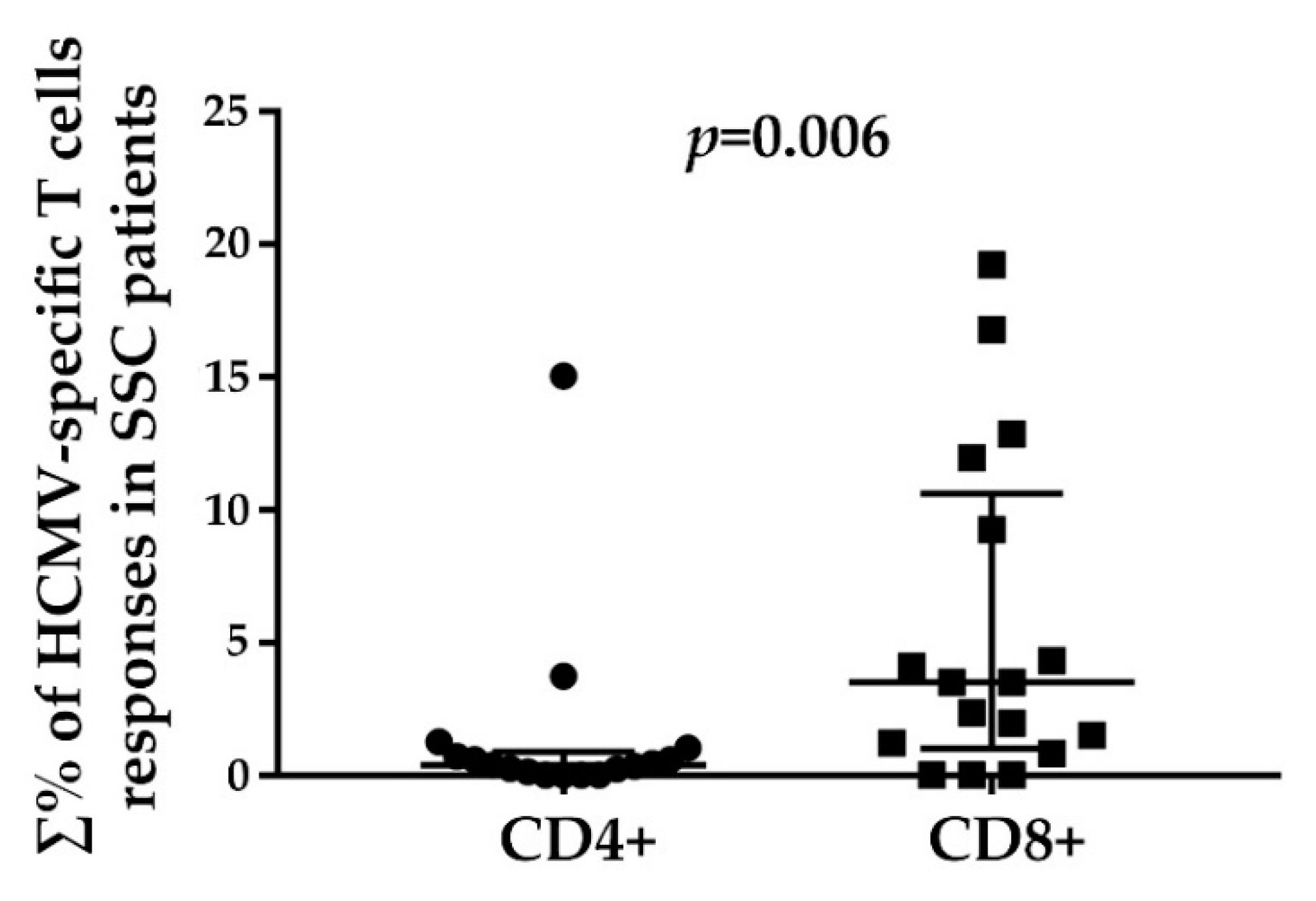
3.2. HCMV-Specifc CD4+ and CD8+ T Cell Responses
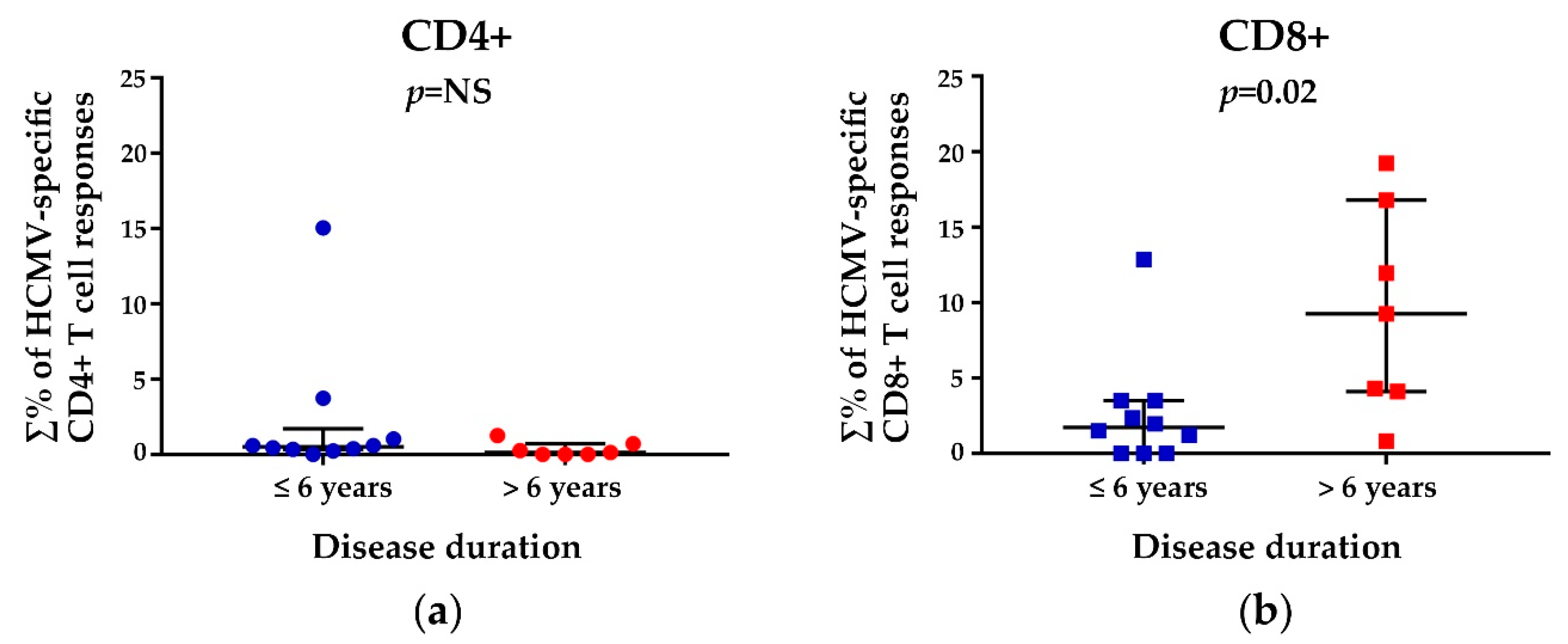
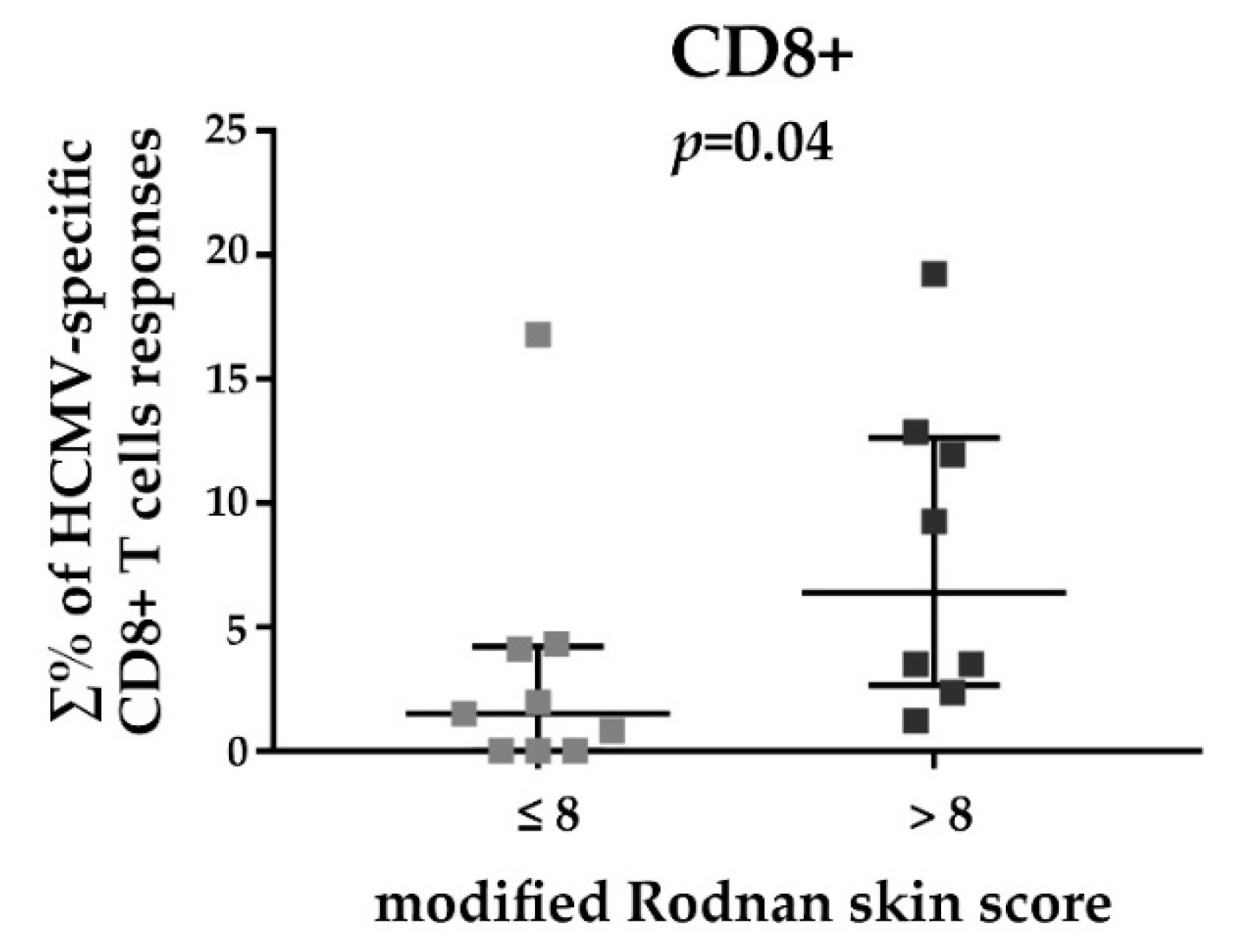
3.3. HCMV-Specific CD8+ T Cell Responses in Relation to the Disease Duration and Modified Rodnan Skin Score
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferri, C.; Valentini, G.; Cozzi, F.; Sebastiani, M.; Michelassi, C.; La Montagna, G.; Bullo, A.; Cazzato, M.; Tirri, E.; Storino, F.; et al. Systemic Sclerosis Study Group of the Italian Society of Rheumatology (SIR-GSSSc). Systemic sclerosis: Demographic, clinical, and serologic features and survival in 1012 Italian patients. Medicine 2002, 81, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Medsger, T. Natural history of systemic sclerosis and the assessment of disease activity, severity, functional status, and psychologic well-being. Rheum. Dis. Clin. N. Am. 2003, 29, 255–273. [Google Scholar] [CrossRef]
- Gabrielli, A.; Avvedimento, E.; Krieg, T. Scleroderma. N. Engl. J. Med. 2009, 360, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Ferri, C.; Sebastiani, M.; Lo Monaco, A.; Iudici, M.; Giuggioli, D.; Furini, F.; Manfredi, A.; Cuomo, G.; Spinella, A.; Colaci, M.; et al. Systemic sclerosis evolution of disease pathomorphosis and survival. Our experience on Italian patients’ population and review of the literature. Autoimmun. Rev. 2014, 13, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Barsotti, S.; Bruni, C.; Orlandi, M.; Della Rossa, A.; Marasco, E.; Codullo, V.; Guiducci, S. One year in review 2017: Systemic sclerosis. Clin. Exp. Rheumatol. 2017, 35, S3–S20. [Google Scholar]
- Giuggioli, D.; Colaci, M.; Cocchiara, E.; Spinella, A.; Lumetti, F.; Ferri, C. From Localized Scleroderma to Systemic Sclerosis: Coexistence or Possible Evolution. Dermatol. Res. Pract. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y. Systemic sclerosis. J. Dermatol. 2018, 45, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, L.I.; Platsoucas, C.D. Is systemic sclerosis an antigen-driven T cell disease? Arthritis Rheum. 2004, 50, 1721–1733. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, L. New developments in the pathogenesis of systemic sclerosis. Autoimmunity 2005, 38, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.; Krieg, T.; Distler, J.; Distler, O. Overview of pathogenesis of systemic sclerosis. Rheumatology 2006, 48, iii3–iii7. [Google Scholar] [CrossRef] [PubMed]
- Grossman, C.; Dovrish, Z.; Shoenfeld, Y.; Amital, H. Do infections facilitate the emergence of systemic sclerosis? Autoimmun. Rev. 2011, 10, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Fuschiotti, P. Current perspectives on the role of CD8+ T cells in systemic sclerosis. Immunol. Lett. 2018, 195, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Hasegawa, M.; Takehara, K.; Mukaida, N.; Sato, S. Abnormal expression of intracellular cytokines and chemokine receptors in peripheral blood T lymphocytes from patients with systemic sclerosis. Clin. Exp. Immunol. 2002, 130, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Radstake, T.; van Bon, L.; Broen, J.; Hussiani, A.; Hesselstrand, R.; Wuttge, D.; Deng, Y.; Simms, R.; Lubberts, E.; Lafyatis, R. The pronounced Th17 profile in systemic sclerosis (SSc) together with intracellular expression of TGFβ and IFNγ distinguishes SSc phenotypes. PLoS ONE 2009, 4, e5903. [Google Scholar] [CrossRef] [PubMed]
- Baraut, J.; Michel, L.; Verrecchia, F.; Farge, D. Relationship between cytokine profiles and clinical outcomes in patients with systemic sclerosis. Autoimmun. Rev. 2010, 10, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, D.; Battaglia, F.; Parodi, A.; Stringara, S.; Negrini, S.; Panico, N.; Rizzi, M.; Kalli, F.; Conteduca, G.; Ghio, M.; et al. Alteration of Th17 and Treg cell subpopulations co-exist in patients affected with systemic sclerosis. Clin. Immunol. 2011, 139, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Truchetet, M.; Brembilla, N.; Montanari, E.; Allanore, Y.; Chizzolini, C. Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis: Association with interstitial lung disease. Arthritis Res. Ther. 2011, 13, R166. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S.; Hugle, T.; van Laar, J. T cells in systemic sclerosis: A reappraisal. Rheumatology 2012, 51, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Brembilla, N.; Montanari, E.; Truchetet, M.; Raschi, E.; Meroni, P.; Chizzolini, C. Th17 cells favor inflammatory responses while inhibiting type I collagen deposition by dermal fibroblasts: Differential effects in healthy and systemic sclerosis fibroblasts. Arthritis Res. Ther. 2013, 15, R151. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, W.; Sun, X.; Yang, J.; Xu, J.; Fu, W.; Li, M. New insights into CD4+ T cell abnormalities in systemic sclerosis. Cytokine Growth Factor Rev. 2016, 28, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Krasimirova, E.; Velikova, T.; Ivanova-Todorova, E.; Tumangelova-Yuzeir, K.; Kalinova, D.; Boyadzhieva, V.; Stoilov, N.; Yoneva, T.; Rashkov, R.; Kyurkchiev, D. Treg/Th17 cell balance and phytohaemagglutinin activation of T lymphocytes in peripheral blood of systemic sclerosis patients. World J. Exp. Med. 2017, 7, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Larregina, A.; Domsic, R.; Stolz, D.; Medsger, T.; Lafyatis, R.; Fuschiotti, P. Skin-resident effector memory CD8+CD28– T cells exhibit a profibrotic phenotype in patients with systemic sclerosis. J. Investig. Dermatol. 2017, 137, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Fuschiotti, P.; Larregina, A.; Ho, J.; Feghali-Bostwick, C.; Medsger, T. Interleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum. 2013, 65, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Duffy, L.; O’Reilly, S. Toxic Memories in Systemic Sclerosis. J. Investig. Dermatol. 2017, 137, 1003–1004. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, R.; Tötterman, T.; Klareskog, L.; Hällgren, R. Increase in activated T cells and reduction in suppressor inducer T cells in systemic sclerosis. Ann. Rheum. Dis. 1990, 49, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Lympany, P.; Southcott, A.; Welsh, K.; Black, C.; Boylston, A.; du Bois, R. T-cell receptor gene usage in patients with fibrosing alveolitis and control subjects. Eur. J. Clin. Investig. 1999, 29, 173–181. [Google Scholar] [CrossRef]
- Tiev, K.; Abriol, J.; Burland, M.; Antonelli, D.; Klatzmann, D.; Cabane, J.; Boyer, O. T cell repertoire in patients with stable scleroderma. Clin. Exp. Immunol. 2005, 139, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Randone, S.; Guiducci, S.; Cerinic, M. Systemic sclerosis and infections. Autoimmun. Rev. 2008, 8, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Moroncini, G.; Mori, S.; Tonnini, C.; Gabrielli, A. Role of viral infections in the etiopathogenesis of systemic sclerosis. Clin. Exp. Rheumatol. 2013, 31, 3–7. [Google Scholar] [PubMed]
- Halenius, A.; Hengel, H. Human cytomegalovirus and autoimmune disease. Biomed. Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sinzger, C.; Grefte, A.; Plachter, B.; Gouw, A.; The, T.; Jahn, G. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J. Gen. Virol. 1995, 76, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Mostmans, Y.; Cutolo, M.; Giddelo, C.; Decuman, S.; Melsens, K.; Declercq, H.; Vandecasteele, E.; de Keyser, F.; Distler, O.; Gutermuth, J.; et al. The role of endothelial cells in the vasculopathy of systemic sclerosis: A systematic review. Autoimmun. Rev. 2017, 16, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, C.; Dolcino, M.; Peterlana, D.; Bason, C.; Navone, R.; Tamassia, N.; Beri, R.; Corrocher, R.; Puccetti, A. Antibodies against Human Cytomegalovirus in the Pathogenesis of Systemic Sclerosis: A Gene Array Approach. PLoS Med. 2006, 3, 94–1058. [Google Scholar] [CrossRef] [PubMed]
- Arnson, Y.; Amital, H.; Guiducci, S.; Matucci-Cerinic, M.; Valentini, G.; Barzilai, O.; Maya, R.; Shoenfeld, Y. The role of infections in the immunopathogensis of systemic sclerosis-evidence from serological studies. Ann. N. Y. Acad. Sci. 2009, 1173, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Marou, E.; Liaskos, C.; Simopoulou, T.; Efthymiou, G.; Dardiotis, E.; Katsiari, C.; Scheper, T.; Meyer, W.; Hadjigeorgiou, G.; Bogdanos, D.; et al. Human cytomegalovirus (HCMV) UL44 and UL57 specific antibody responses in anti-HCMV-positive patients with systemic sclerosis. Clin. Rheumatol. 2017, 36, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Vescovini, R.; Biasini, C.; Fagnoni, F.; Telera, A.; Zanlari, L.; Pedrazzoni, M.; Bucci, L.; Monti, D.; Medici, M.; Chezzi, C.; et al. Massive load of functional effector CD4+ and CD8+ T cells against cytomegalovirus in very old subjects. J. Immunol. 2007, 179, 4283–4291. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C. Buffered memory: A hypothesis for the maintenance of functional, virus-specific CD8(+) T cells during cytomegalovirus infection. Immunol. Res. 2011, 51, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Seckert, C.; Grießl, M.; Büttner, J.; Scheller, S.; Simon, C.; Kropp, K.; Renzaho, A.; Kühnapfel, B.; Grzimek, N.; Reddehase, M. Viral latency drives “memory inflation”: A unifying hypothesis linking two hallmarks of cytomegalovirus infection. Med. Microbiol. Immunol. 2012, 201, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Vescovini, R.; Fagnoni, F.F.; Telera, A.R.; Bucci, L.; Pedrazzoni, M.; Magalini, F.; Stella, A.; Pasin, F.; Medici, M.C.; Calderaro, A.; et al. Naïve and memory CD8 T cell pool homeostasis in advanced aging: Impact of age and of antigen-specific responses to cytomegalovirus. Age 2014, 36, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Sansoni, P.; Vescovini, R.; Fagnoni, F.F.; Akabar, A.; Arens, R.; Chiu, Y.L. New advances in CMV and immunosenescence. Exp. Gerontol. 2014, 55, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Klenerman, P.; Oxenius, A. T cell responses to cytomegalovirus. Nat. Rev. Immunol. 2016, 16, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.; Medsger, T.; Carreira, P.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Clements, P.; Lachenbruch, P.; Siebold, J.; White, B.; Weiner, S.; Martin, R.; Weinstein, A.; Weisman, M.; Mayes, M.; Collier, D. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J. Rheumatol. 1995, 22, 1281–1285. [Google Scholar] [PubMed]
- Khanna, D.; Furst, D.; Clements, P.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Maccari, C.; University-Hospital of Parma, Department of Medicine and Surgery, University of Parma, Parma, Italy; Vescovini, R.; Department of Medicine and Surgery, University of Parma, Parma, Italy; Arcangeletti, M.-C.; University-Hospital of Parma, Department of Medicine and Surgery, University of Parma, Parma, Italy. Unpublished work. 2018.
- Ferri, C.; Cazzato, M.; Giuggioli, D.; Sebastiani, M.; Magro, C. Systemic sclerosis following human cytomegalovirus infection. Ann. Rheum. Dis. 2002, 61, 937–938. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, C.; Bason, C.; Navone, R.; Millo, E.; Damonte, G.; Corrocher, R.; Puccetti, A. Systemic sclerosis immunoglobulin G autoantibodies bind the human cytomegalovirus late protein UL94 and induce apoptosis in human endothelial cells. Nat. Med. 2000, 6, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.; Redeker, A.; Arens, R.; van Baarle, D.; van den Berg, S.; Benedict, C.; Čičin-Šain, L.; Hill, A.; Wills, M. CMV immune evasion and manipulation of the immune system with aging. Geroscience 2017, 39, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Souquette, A.; Frere, J.; Smithey, M.; Sauce, D.; Thomas, P. A constant companion: Immune recognition and response to cytomegalovirus with aging and implications for immune fitness. Geroscience 2017, 39, 293–303. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, C.; Diamond, D. The immune response to human CMV. Future Virol. 2012, 7, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Torti, N.; Oxenius, A. T cell memory in the context of persistent herpes viral infections. Viruses 2012, 4, 1116–1143. [Google Scholar] [CrossRef] [PubMed]
- Vescovini, R.; Telera, A.; Fagnoni, F.; Biasini, C.; Medici, Mc.; Valcavi, P.; di Pede, P.; Lucchini, G.; Zanlari, L.; Passeri, G.; et al. Different contribution of EBV and CMV infections in very long-term carriers to age-related alterations of CD8+ T cells. Exp. Gerontol. 2004, 39, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Hayakawa, H.; Sato, A.; Chida, K.; Nakamura, H.; Miura, K. Involvement of Epstein-Barr virus latent membrane protein 1 in disease progression in patients with idiopathic pulmonary fibrosis. Thorax 2000, 55, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.; Peruzzi, G.; Lacconi, V.; Lenna, S.; Quarta, S.; Rosato, E.; Vestri, A.; York, M.; Dreyfus, D.; Faggioni, A.; et al. Epstein-Barr virus lytic infection promotes activation of Toll-like receptor 8 innate immune response in systemic sclerosis monocytes. Arthritis Res. Ther. 2017, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Cicin-Sain, L.; Brien, J.; Uhrlaub, J.; Drabig, A.; Marandu, T.; Nikolich-Zugich, J. Cytomegalovirus Infection Impairs Immune Responses and Accentuates T-cell Pool Changes Observed in Mice with Aging. PLoS Pathog. 2012, 8, e1002849. [Google Scholar] [CrossRef] [PubMed]
- Janahi, E.; Das, S.; Bhattacharya, S.; Haque, S.; Akhter, N.; Jawed, A.; Wahid, M.; Mandal, R.; Lohani, M.; Areeshi, M.; et al. Cytomegalovirus aggravates the autoimmune phenomenon in systemic autoimmune diseases. Microb. Pathog. 2018, 120, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Maecker, H.; Dunn, H.; Suni, M.; Khatamzas, E.; Pitcher, C.; Bunde, T.; Persaud, N.; Trigona, W.; Fu, T.; Sinclair, E.; et al. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods 2001, 255, 27–40. [Google Scholar] [CrossRef]
- Komatsu, H.; Sierro, S.; V Cuero, A.; Klenerman, P. Population analysis of antiviral T cell responses using MHC class I-peptide tetramers. Clin. Exp. Immunol. 2003, 134, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Slezak, S.; Bettinotti, M.; Selleri, S.; Adams, S.; Marincola, F.; Stroncek, D. CMV pp65 and IE-1 T cell epitopes recognized by healthy subjects. J. Transl. Med. 2007, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Bentz, G.; Jarquin-Pardo, M.; Chan, G.; Smith, M.; Sinzger, C.; Yurochko, A. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J. Virol. 2006, 80, 11539–11555. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.; Sissons, P. Latent and persistent infections of monocytes and macrophages. Intervirology 1996, 39, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Arcangeletti, M.; Vasile Simone, R.; Rodighiero, I.; De Conto, F.; Medici, M.; Maccari, C.; Chezzi, C.; Calderaro, A. Human cytomegalovirus reactivation from latency: Validation of a “switch” model in vitro. Virol. J. 2016, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Bentz, G.; Alexander, J.; Yurochko, A. Human cytomegalovirus induces monocyte differentiation and migration as a strategy for dissemination and persistence. J. Virol. 2004, 78, 4444–4453. [Google Scholar] [CrossRef] [PubMed]
- Waldman, W.; Knight, D.; Adams, P. Cytolytic activity against allogeneic human endothelia: Resistance of cytomegalovirus-infected cells and virally activated lysis of uninfected cells. Transplantation 1998, 66, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Steen, V.D.; Medsger, T.; Rodnan, G. D-Penicillamine therapy in progressive systemic sclerosis (scleroderma): A retrospective analysis. Ann. Intern. Med. 1982, 97, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Clements, P.; Hurwitz, E.; Wong, W.; Seibold, J.; Mayes, M.; White, B.; Wigley, F.; Weisman, M.; Barr, W.; Moreland, L.; et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: High-dose versus low-dose penicillamine trial. Arthritis Rheum. 2000, 43, 2445–2454. [Google Scholar] [CrossRef]
- Steen, V.; Medsger, T. Improvement in skin thickening in systemic sclerosis associated with improved survival. Arthritis Rheum. 2001, 44, 2828–2835. [Google Scholar] [CrossRef]
- Shand, L.; Lunt, M.; Nihtyanova, S.; Hoseini, M.; Silman, A.; Black, C.; Denton, C. Relationship between change in skin score and disease outcome in diffuse cutaneous systemic sclerosis: Application of a latent linear trajectory model. Arthritis Rheum. 2007, 56, 2422–2431. [Google Scholar] [CrossRef] [PubMed]
| SSc Patients | Healthy Subjects | |
|---|---|---|
| Number (n) | 20 | 18 |
| Age, years | ||
| Median (range) | 54 (37–73) | 55 (39–62) |
| Gender, n (%) | ||
| Male | 4 (20) | 2 (11) |
| Female | 16 (80) | 16 (89) |
| HCMV serology, n (%) | ||
| IgG+ IgM- | 16 (80) | 15 (83) |
| IgG- IgM- | 3 (15) | 3 (17) |
| IgG- IgM+ | 1 (5) | |
| Disease duration, years | ||
| Median (range) | 4 (1–19) | |
| Clinical subgroups, n (%) | ||
| Diffuse cutaneous | 4 (20) | |
| Limited cutaneous | 16 (80) | |
| Clinical manifestations, n (%) | ||
| Raynaud’s phenomenon | 20 (100) | |
| Digital ulcer | 7 (35) | |
| Puffy fingers | 10 (50) | |
| Pitting | 7 (35) | |
| Telangiectasia | 12 (60) | |
| Arthralgia | 11 (55) | |
| Interstitial lung disease | 11 (55) | |
| Heart involvement | 8 (40) | |
| Esophageal dysfunction | 14 (70) | |
| FVC% 1, median (range) | 101 (86–130) | |
| FEV1% 2, median (range) | 103 (84–126) | |
| DLCO Sb% 3, median (range) | 65 (31–94) | |
| Modified Rodnan skin score | ||
| Median (range) | 8 (0–22) | |
| Autoantibodies, n (%) | ||
| Positive ANA 4 | 19 (95) | |
| Positive ACA 5 | 8 (40) | |
| Positive anti-SCl70 | 5 (25) | |
| Treatment, n (%) | ||
| Steroids | 2 (10) | |
| Prostanoids | 16 (80) | |
| Bosentan | 5 (25) | |
| Calcium antagonist | 12 (60) |
| Blood Samples | CD4+ T Cell Responses (%) | CD8+ T Cell Responses (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| ∑% 1 | pp65 | IE1 | UL94 | ∑% | pp65 | IE1 | UL94 | |
| SSc pz 2 1 | 15.06 | 3.11 | 5.28 | 6.67 | 12.86 | 2.65 | 5.20 | 5.01 |
| SSc pz 2 | 0.25 | 0.25 | NR 3 | NR | 19.23 | 19.23 | NR | NR |
| SSc pz 3 | 0.59 | NR | NR | 0.59 | 1.21 | NR | 1.21 | NR |
| SSc pz 4 | 3.74 | 0.52 | NR | 3.22 | 3.52 | 0.74 | NR | 2.78 |
| SSc pz 5 | 1.04 | 0.43 | 0.45 | 0.6 | NR | NR | NR | NR |
| SSc pz 6 | 0.71 | 0.29 | 0.21 | 0.21 | 16.81 | 13.16 | 2.52 | 1.13 |
| SSc pz 7 | 1.26 | 0.4 | 0.28 | 0.58 | 9.27 | 4.08 | 4.64 | 0.55 |
| SSc pz 8 | NR | NR | NR | NR | 4.33 | 0.98 | 3.35 | NR |
| SSc pz 9 | 0.38 | NR | 0.1 | 0.28 | 1.96 | 1.1 | 0.86 | NR |
| SSc pz 10 | NR | NR | NR | NR | 11.95 | 1.05 | 10.9 | NR |
| SSc pz 11 | 0.13 | 0.13 | NR | NR | 4.12 | 3.13 | 0.77 | NR |
| SSc pz 12 | NR | NR | NR | NR | NR | NR | NR | NR |
| SSc pz 13 | 0.44 | 0.44 | NR | NR | 2.37 | 0.42 | 1.95 | NR |
| SSc pz 14 | 0.23 | 0.23 | NR | NR | 3.51 | 3.36 | 0.15 | NR |
| SSc pz 15 | 0.35 | NR | 0.13 | 0.22 | 1.51 | 0.61 | 0.90 | NR |
| SSc pz 16 | 0.6 | 0.28 | 0.32 | NR | NR | NR | NR | NR |
| SSc pz 17 | NR | NR | NR | NR | 0.83 | NR | 0.19 | 0.64 |
| Hs 4 1 | NR | NR | NR | NR | NR | NR | NR | NR |
| Hs 2 | NR | NR | NR | NR | NR | NR | NR | NR |
| Hs 3 | 0.67 | 0.33 | 0.23 | 0.11 | 1.23 | 0.5 | 0.45 | 0.28 |
| Hs 4 | NR | NR | NR | NR | NR | NR | NR | NR |
| Hs 5 | 0.60 | NR | NR | 0.60 | 0.71 | 0.71 | NR | NR |
| Hs 6 | NR | NR | NR | NR | 5.60 | 5.60 | NR | NR |
| Hs 7 | 0.77 | 0.56 | 0.08 | 0.13 | 0.72 | 0.72 | NR | NR |
| Hs 8 | NR | NR | NR | NR | NR | NR | NR | NR |
| Hs 9 | NR | NR | NR | NR | NR | NR | NR | NR |
| Hs 10 | NR | NR | NR | NR | 1.22 | 0.22 | 1.00 | NR |
| Hs 11 | 0.28 | 0.28 | NR | NR | 0.52 | 0.52 | NR | NR |
| Hs 12 | NR | NR | NR | NR | NR | NR | NR | NR |
| Hs 13 | 0.09 | 0.09 | NR | NR | 2.32 | NR | 2.32 | NR |
| Hs 14 | NR | NR | NR | NR | 0.45 | 0.45 | NR | NR |
| Hs 15 | NR | NR | NR | NR | 0.37 | 0.11 | 0.26 | NR |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcangeletti, M.-C.; Maccari, C.; Vescovini, R.; Volpi, R.; Giuggioli, D.; Sighinolfi, G.; De Conto, F.; Chezzi, C.; Calderaro, A.; Ferri, C. A Paradigmatic Interplay between Human Cytomegalovirus and Host Immune System: Possible Involvement of Viral Antigen-Driven CD8+ T Cell Responses in Systemic Sclerosis. Viruses 2018, 10, 508. https://doi.org/10.3390/v10090508
Arcangeletti M-C, Maccari C, Vescovini R, Volpi R, Giuggioli D, Sighinolfi G, De Conto F, Chezzi C, Calderaro A, Ferri C. A Paradigmatic Interplay between Human Cytomegalovirus and Host Immune System: Possible Involvement of Viral Antigen-Driven CD8+ T Cell Responses in Systemic Sclerosis. Viruses. 2018; 10(9):508. https://doi.org/10.3390/v10090508
Chicago/Turabian StyleArcangeletti, Maria-Cristina, Clara Maccari, Rosanna Vescovini, Riccardo Volpi, Dilia Giuggioli, Gianluca Sighinolfi, Flora De Conto, Carlo Chezzi, Adriana Calderaro, and Clodoveo Ferri. 2018. "A Paradigmatic Interplay between Human Cytomegalovirus and Host Immune System: Possible Involvement of Viral Antigen-Driven CD8+ T Cell Responses in Systemic Sclerosis" Viruses 10, no. 9: 508. https://doi.org/10.3390/v10090508
APA StyleArcangeletti, M.-C., Maccari, C., Vescovini, R., Volpi, R., Giuggioli, D., Sighinolfi, G., De Conto, F., Chezzi, C., Calderaro, A., & Ferri, C. (2018). A Paradigmatic Interplay between Human Cytomegalovirus and Host Immune System: Possible Involvement of Viral Antigen-Driven CD8+ T Cell Responses in Systemic Sclerosis. Viruses, 10(9), 508. https://doi.org/10.3390/v10090508







