Abstract
Africa accounts for the majority of global human immunodeficiency virus (HIV) infections, most of which affect women through heterosexual intercourse. Currently, there is no cure for HIV and the development of vaccines and microbicides remains the best solution to eradicate the pandemic. We and others have identified HIV highly-exposed seronegative (HESN) individuals among African female commercial sex workers (CSWs). Analyses of genital samples from HESNs have demonstrated potent innate and anti-inflammatory conditions, HIV-specific CD4+ and CD8+ T-cells as well as immunoglobulins (Igs), and increased regulatory cell populations, all of which support a delicate balance between strength and control against HIV intrusion. Moreover, we have recently shown that frequencies of innate marginal zone (MZ) B-cells are decreased in the blood of HESNs when compared to HIV-uninfected non-CSW women, suggesting their recruitment to peripheral sites. This coincides with the fact that levels of B lymphocyte stimulator (BLyS/BAFF), known to shape the MZ pool and whose overexpression leads to MZ deregulation in HIV-infected progressors, are significantly lower in the blood of HESNs when compared to both HIV-infected CSWs and HIV-uninfected non-CSW women. Interestingly, MZ B-cells can bind HIV gp120 and produce specific IgG and IgA, and have a propensity for B regulatory potential, which could help both the fight against HIV and maintenance of low inflammatory conditions in HESNs. HESN individuals provide an exceptional opportunity to identify important clues for the development of protective devices, and efforts should aim at soliciting immune responses observed in the context of their natural immunity to HIV.
1. Introduction
Worldwide, it is estimated that nearly 36.7 million people live with human immunodeficiency virus (HIV). In 2016, around 1.8 million became newly infected and 1 million died from AIDS. Africa accounts for 69% of global infections, most of which affect women through heterosexual intercourse [1]. Currently, there is no cure for HIV and the development of preventive strategies such as vaccines and microbicides remains the best solution to eradicate the pandemic. To date, the transmission mechanisms of the virus and immune responsiveness at the initial site of infection are not fully understood. Frequent mucosal exposure to HIV in the absence of infection was documented in different cohorts, including the Beninese commercial sex workers (CSWs) [2]. As such, individuals highly exposed to HIV and persistently seronegative (HESN) have been shown to possess low-inflammatory conditions and immune responsiveness towards the virus [2,3,4], which suggests that the capacity to maintain a low-key inflammatory profile along with anti-HIV responses is associated with protection against HIV infection. We believe that efforts to develop effective devices should aim at mimicking conditions and soliciting immune responses observed in the context of natural immunity to HIV.
2. Immunology of the Female Genital Tract
The female genital tract (FGT) is part of the major mucosal associated lymphoid tissue (MALT) [5]. The FGT constitutes a main portal of entry for many organisms and plays a role in protecting the host against pathogens while maintaining a tolerance to a commensal flora [5,6]. FGT immunity is tightly regulated by a hormonal/inflammatory process throughout the menstrual cycle, having to deal with the pressure of procreation and microbial control [7,8]. FGT is subdivided into 2 regions presenting distinct phenotypic profiles. The upper FGT consists of the sterile endometrium, fallopian tubes and the endocervix in which sterility may be temporally related to the menstrual cycle phase. In contrast, the lower FGT, which is composed of the non-sterile vagina and ectocervix is colonized by a commensal microflora [8]. FGT immunity involves genital epithelial cells (ECs) as well as dendritic cells (DCs), Langerhans cells (LC), macrophages, natural killer (NK) cells, neutrophils and lymphoid cells, which confer protection through the production of antimicrobial agents, antibodies, chemokines and cytokines [5]. Wira and colleagues have shown that the upper FGT contains unique lymphoid aggregates constituted of CD8+ T cells that surround a central B-cell core, which are encapsulated by macrophages [7]. Even if mechanisms of immune induction in the FGT remain poorly understood [2,6,7], the FGT is provided with an array of protective mechanisms from the innate and adaptive arms of the immune system to maintain a delicate balance between protection and tolerance [9].
Together with ECs, DCs are one of the earliest cell types to sense the virus through pattern recognition receptors (PRRs), such as toll-like receptors (TLRs), lectins and NOD-like receptors [2,10,11]. Cross-talks between ECs and sub-mucosal DCs involve immunomodulatory cytokines and lead to activation of effector and regulatory cells in the lamina propria [2,11]. It is well known that DCs are important for the generation of first-line innate as well as adaptive immune responses [11] during infections. Indeed, DCs are involved in the delivery of cognate and non-cognate molecular events as well as production of immunomodulatory molecules, such as cytokines and growth factors that can shape the overall outcome of T and B lymphocyte responses [11].
3. The Female Genital Tract in the Context of HIV
The study of the FGT in CSWs is often complicated by numerous difficulties associated with ethics and sampling, and therefore studies are often guided by observations from the gastro-intestinal lymphoid tissue (GALT) [12]. The FGT involves the mucous lining of a tight EC barrier, stratified at the vaginal and ectocervical levels [7]. Integrity of ECs can be influenced by pro-inflammatory factors such as tumor necrosis factor (TNF) [13] but also by sustained Th17 cell activity [14] in the context of early HIV infection. This could affect tight junctions of mucosal ECs leading to increase risks of microbial translocation and chronic infection [12], and also facilitate virus transcytosis across ECs [13,15,16], particularly via the galactosylceramide (GalCer) receptor [17]. In fact, although HIV does not productively infect ECs [2], GalCer, a glycosphingolipid highly enriched at the luminal pole of ECs, can bind both HIV glycoproteins gp120 and gp41 [18], and allows endocytosis by ECs, which subsequently transfer the virus to DCs and target cells [2,19,20]. HIV has also been shown to be internalized by FGT ECs via gp340, a scavenger receptor, subsequently promoting the production of pro-inflammatory thymic stromal lymphopoietin (TSLP) via TLR7 signaling, which then activates DCs and promotes HIV transmission to CD4+ T cells [21].
Although DCs can be infected by HIV, mostly at an immature stage, they express potent restriction factors such as SAMHD1 [22] and do not constitute the main target for the virus and are rather involved in its transmission to CD4+ T-cells [23]. Sub-mucosal DCs express high levels of C type lectins, such as DC-SIGN (CD209), which can bind gp120 [20,24]. This allows internalization of the virus and transfer to CD4+ CCR5+ effector target T-cells, either locally or following migration to draining lymphoid organs [25].
Using simian immunodeficiency virus (SIV)/macaque vaginal infection model [26,27], numerous infectious foci throughout the FTG were identified early after infection. It has been shown that CCR6+ Th17 cells are the preferential targets of SIV following vaginal transmission [26,27]. These Th17 targets express RORγt, a transcriptional regulator required for their generation and differentiation [28,29]. Th17 cells also express α4β7 another co-receptor for HIV [30]. Factors such as transforming growth factor (TGF)-β, interleukin (IL)-1, IL-6, IL-21 and IL-23 are required for Th17 differentiation [28]. CCR6 is a major ligand for the chemokine macrophage inflammatory protein-3alpha (MIP-3α/CCL20), which is secreted by mucosal ECs and is known to attract immature LCs and DCs [31,32]. It has been shown that strong doses of SIV in the vaginal mucosa caused an increase of the chemokine MIP-3α/CCL20 [19] and recruitment of plasmacytoid DCs, DCs and macrophages at the cervical epithelium [23]. Early blocking of MIP-3α and pro-inflammatory cytokines prevented cellular recruitment, establishment of an inflammatory milieu, and infection despite repeated intravaginal exposure to high doses of SIV [16,33]. CD4+ T cells can also be recruited via MIP-1α/CCL3 and MIP-1β/CCL4. Interferon (IFN)-α is an important promotor of CD4+T cells clonal expansion at the vaginal mucosa, and subsequently in the blood and secondary lymphoid organs [19]. Establishment of systematic infection eventually leads to a massive depletion of sub-mucosa CD4+ T cells, in particular Th17 cells (CCR4+CCR6+), that is associated with an increased regulatory T (Treg) cells, leading to an imbalance in the ratio of T effector vs Treg cell populations [2,14,16,23].
4. Natural Immunity to HIV in the FGT of HESNs
Blood and genital mucosal factors that constitute a low-inflammatory “immune” profile have also been linked with protection in HESNs [2,34,35]. Indeed, it has been shown that high levels of anti-inflammatory and neutralizing proteins, such as anti-proteases are found in the genital mucosa of HESN CSWs [2,4,34]. HIV-Env reactive immunoglobulins (Igs) have also been documented in blood and FGT of HESNs, and will be discussed later in this review. Levels of pro-inflammatory cytokines such as IL-1α, IFN-γ and TNF-α, as well as monokine induced by IFN-γ (MIG) and IFN-γ induced protein (IP)-10 chemokines have been reported to be lower in cervicovaginal lavages (CVLs) of HESNs when compared to HIV-infected CSWs [36,37]. In fact, production of MIG and IP-10 is induced by expression of IFN-γ, and polymorphisms in the IRF-1 regulating IFN-γ were associated with protection to HIV [38,39]. In a Kenyan female CSW cohort, HIV-specific CD4+ and CD8+ T-cell responses have been found in both the blood and genital tract of HESN CSWs [40,41,42,43]. In these individuals a low activation T-cell profile corresponds with a greater ability to proliferate in response to HIV p24 peptides when compared to that observed in HIV-infected CSWs [44]. Moreover, these studies also identified poly-functional effector T-cells in HESNs.
Interestingly, we have shown that Beninese HESN CSWs presented higher levels of IFN-α in their CVLs when compared to those observed in HIV-infected CSWs and HIV-uninfected non-CSWs [45], which could be critical to sustain immune homeostasis, antiviral activity and restriction factors such as SAMHD1, APOBEC3, or tetherin in cells at the portal of entry for the virus. Indeed, type I interferons are indispensable to protect host against viruses [46]. Although in uncontrolled situations such as in the context of HIV-infection, type I IFNs promote inflammatory responses as well as recruitment of target cell, they can also induce a myriad of IFN-stimulated genes (ISGs), which have been shown to interfere with multiple viruses at various life cycle stages [47]. Type I IFNs can be triggered via TLRs 3, 7, 8 and 9 [48], and HIV ssRNA triggers TLR 7 and TLR 8 [15,46]. Interestingly, genital epithelial and myeloid cell populations of Beninese HESN CSWs expressed high levels of TLR 7 [45]. IL-10, which is known to promote immunoregulatory responses was elevated in the CVLs of Beninese HESNs, but lower than that observed in CVLs of HIV-infected CSWs [45]. IL-10 levels are often elevated in the context of HIV, unfortunately the overall outcome of excessive IL-10 may well be to sustain chronic activation and dysregulation associated with HIV disease progression, and may impede on viral eradication [49,50,51]. A more modest elevation of IL-10, such as observed for HESN CSWs, may be beneficial and promote an immunoregulatory microenvironment preventing HIV attempts to establish infection by lowering availability of targets [52]. Therefore, IFN-α and IL-10 levels measured in the CVLs of Beninese HESN CSWs likely promote a potent antiviral and yet at the same time immunoregulatory conditions.
5. Immunoregulatory Cell Populations in HESNs
Studies of the genital immune profile in HESN CSWs suggest that natural immunity in the context of HIV may be associated with the host’s capacity to orchestrate dynamics of cellular populations to maintain low inflammatory conditions at the initial site of exposition. In this view, we recently reported that endocervical myeloid HLA-DR+ cells from Beninese HESN CSWs expressed higher levels of IFN-α, TLR 7, IL-10 and HLA-G than those from both HIV-infected CSWs and HIV-uninfected non-CSWs. Further characterization of these cells in HESNs revealed a CD103+ CD14+ CD11c+ “DC-like” population expressing high levels of IFN-α and IL-10 [45], which is reminiscent of the recently described tolerogenic profile of “DC-10” [53]. Interestingly, the majority of the myeloid CD11c+CD14+IFN-α+IL-10+ cells also expressed HLA-G and ILT-4 [45], as do DC-10 [53,54]. Concomitantly, Beninese HESN CSWs had higher frequencies of endocervical regulatory CD4+ T-cells (Tregs) when compared to those from the two other groups of women [45], which is consistent with that reported in the blood of Kenyan CSWs [55]. Moreover, we found an increased frequency of endocervical Tregs expressing programmed cell death protein (PD)-1+, as well as higher intensity of PD-1, IL-10 and CTLA expression for both Tregs and type 1 regulatory cells (Tr1) (CD49b+ and LAG-3+) [56] in Beninese HESNs [45]. This could be reflective of their ongoing regulatory activity [57], which likely confers an advantage to these individuals.
Thus, to date, observations on HESNs suggest that natural immunity against HIV involves a capacity to induce/maintain strong innate and HIV-specific immune responses, and at the same time, regulatory populations such as “DC-10-like”, Treg and Tr1 to help control/maintain low inflammatory conditions at the initial site of exposure. Understanding how the potent antiviral but regulated inflammatory response observed in HESNs is achieved at the initial site of infection is crucial for the development of effective mucosal microbicides or vaccines.
6. The Importance of HIV ENV Reactive Immunoglobulins: Lessons from Vaccination Trials
Although the induction of broadly neutralizing antibodies (bNAbs) is a main goal in vaccination [58], there is growing evidence suggesting that both neutralizing and non-neutralizing Abs can mediate some level of protection against HIV [59]. Analyses of correlates of protection from the RV144 vaccine trial suggested that increased blood derived IgG1 and IgG3-mediated Ab dependent cell cytotoxicity (ADCC) activity towards HIV ENV V1V2 region was linked with decreased HIV acquisition [60]. Genetic analyses demonstrated that RV144 vaccinees bearing HLA class II alleles such as DQB1*06 presented increased risk of HIV acquisition possibly associated with elevated ENV-specific IgA interfering with ADCC activity [59,60]. However, non-neutralizing ENV-reactive IgA derived from blood memory B-cells of RV144 vaccinees who did not bear predisposing HLA alleles, blocked in vitro HIV ENV binding to GalCer and mediated in vitro phagocytosis by monocytes expressing FcRα [59]. Raising the possibility that the RV144 regimen may have induced a certain level of non-neutralizing, protective IgA in some individuals [59]. Albeit, one of these ENV reactive IgA antibody did not bind to the RV144 immunogen, and was possibly derived from a pre-existing B-cell pool, expanded by vaccination and microbiota reactive [59], as it has been shown that some HIV ENV reactive Abs cross-react with microbiota [61].
Samples from mucosal ports of entry were not collected during the RV144 trial and the reactivity of mucosal Igs have not been assessed. The importance of mucosal Igs, in particular IgA, in fighting HIV has been highlighted by vaccination and passive immunization studies [62]. At mucosal sites, IgA is produced in the lamina propria by local plasma cells, mainly as dimeric (dIgA) containing a joining J chain. It can translocate across ECs to generate luminal secretory IgA (SIgA) via the polymeric Ig receptor (pIgR). Mucosal IgA can mediate protection by trapping, neutralizing and preventing transcytosis [62]. In 2011, the elegant study by the group of Morgan Bomsel had shown that vaccination of non-human primates with HIV gp41 virosomes induced mucosal IgA and IgG, which prevented systemic invasion following vaginal simian-HIV challenge, by blocking transcytosis and by mediating ADCC, respectively [63]. The fact that these animals lacked serum neutralizing antibody activity highlighted the importance of effector antibodies at the mucosal portal of entry [63]. More recently, passive immunization studies showed that rhesus macaques given anti-HIV-1 neutralizing monoclonal Ab (NmAb) HGN194 as mucosal dIgA2 together with systemic IgG1 with the same epitope specificity were completely protected against high-dose intra rectal SHIV-1157ipEL-p challenge [64,65]. Furthermore, the dIgA1 version of the same mAb was significantly more protective compared to the dIgA2 version, highlighting the importance of characterizing different isotypes of IgA, as they differ predominantly in the hinge region and may confer varying effector functions [62]. The fact that these NmAb were poorly efficient when used alone implies that inducing mucosal IgA as first-line defense in conjunction with immunity against HIV at the systemic level is required to prevent virus acquisition [64,65].
7. HIV ENV Reactive Immunoglobulins in HESNs
Given the 31% HIV protection conferred by the RV144 vaccine regimen, we were inclined to screen sera and genital samples from the Benin CSW cohort for the presence of anti-HIV trimeric ENV IgG as well as for their potential to neutralize HIV viral particles and/or mediate ADCC. Although anti-HIV ENV IgG, neutralizing or ADCC activities were detected in samples from HIV-infected CSWs, no such activities were observed in blood and CVLs from HESN CSWs [66]. Therefore, suggesting that natural immunity may not be associated with the production of HIV-specific IgG mediating neutralizing or ADCC activities. We and others have detected ENV reactive IgA in HESN individuals, which may help prevent HIV infection [37,62]. Indeed, it has been shown that HESNs present HIV cross-clade neutralizing-IgA in their blood and FGT, which are mostly directed towards ENV glycoproteins [67,68,69,70,71]. Furthermore, in a cohort of HESN women from Ivory Coast, mucosal IgA were shown to block HIV transcytosis through tight epithelial barriers [72,73]. Whether these IgA are generated and maintained in response to frequent HIV exposition and/or a contained local HIV reservoir and/or expanded from pre-existing microbiota reactive B-cell pools that cross-react with HIV-ENV remains to be established. The sol fact that sex-break will eventually lead to seroconversion in HESN CSWs who return to sex work, despite pre-existing HIV-specific responses [74], suggests frequent exposition to HIV is required for maintenance of immune populations and their protective responses in the mucosal niche. This could possibly operate via a mechanism analogous to that described in the GALT in the context of host-microbiota immune “tolerance” system [75].
8. Innate B-Cells and BLyS/BAFF in Natural Immunity to HIV
Until now, few studies have assessed the nature of B-cells involved in production of Abs in the context of natural immunity against HIV. The detailed characterization of the Ig repertoire of cervical and systemic B-cells from a Kenyan HESN individual revealed that site-specific responses occur with unique regulation of tolerance and recruitment into local memory or blast B-cell compartments, and the infusion of systemic post-germinal center (GC) B-cells to the cervix seems to be a common event [76]. Further understanding the nature and how B-cell populations are solicited to fight against HIV appears important to the design of preventive approaches.
Growing importance is given to innate marginal zone (MZ) type B-cells in health and disease [77]. Indeed, given their location in lymphoid organs and mucosal-associated structures, human MZ B-cells constitute early first-line defense against invading pathogens. Although the Abs generated from innate B-cells may be important in some circumstances, such as with MZ B-cells in the context of encapsulated bacteria [77], in others such as with viral infections more refined adaptive Ab responses are also required to eradicate the infection. In viral infections, innate populations such as MZ B-cells likely provide a quick efficient first-line response and participate in the development of the adaptive Ab responses. Indeed, MZ B-cells can traffic Ag to follicular B-cell areas of lymphoid structures and promote GC reactions, where T-cell dependent B-cell class switch recombination (CSR) and affinity maturation lead to the production of highly specific Abs with potent neutralizing and ADCC effector functions [78]. MZ B-cells are capable of CSR, mostly towards IgG and IgA in humans [77]. MZ B-cells express a polyreactive BCR repertoire, which comprises usage of the IGHV1-2 gene [79], whose product has been shown to take part to HIV-ENV reactive broadly neutralizing Abs (bNAbs) such as VRC01 [80]. Moreover, human MZ B-cells have been shown to naturally bind to fully glycosylated gp120 via surface lectins, and in presence of BLyS/BAFF subsequently produced polyreactive IgG and IgA, of which a fraction recognized gp120 [81].
BLyS/BAFF is highly recognized for its role in B-cell ontogenesis, as well as cell fate decision towards the innate MZ B-cell pool [77,82]. Interestingly, repeated treatment of mice with BLyS/BAFF increased their MZ B-cell compartment and generated an increased response to ENV immunization and bNAbs [83]. Although the capacity of MZ B-cells to bind gp120 suggest they could possibly transfer HIV, it is unlikely that they get infected by the virus. Indeed, despite the fact that HIV has been shown to replicate in CD40 stimulated B-cells in vitro [84], the virus has not yet been convincingly shown to infect or replicate in B-cells in vivo [85]. Moreover, HiSeq gene expression analysis of MZ B-cells reveals relatively high levels of viral restriction factors such as SAMHD1 (Poudrier J and Roger M, preliminary data 2016).
Previous reports by us and others demonstrated that BLyS/BAFF expression is increased in the context of HIV disease, and not fully restored following therapy [86,87]. This is likely due to direct and indirect factors associated with HIV infection. Indeed, soluble HIV-Nef can directly modulate BLyS/BAFF membrane expression and soluble release by monocyte derived DCs [88], and HIV-ENV has been shown to upregulate BLyS/BAFF expression by macrophages [81]. Furthermore, BLyS/BAFF has been shown to be directly induced by type I IFNs [89,90]. Elements of microbial translocation, such as LPS are also known to up-regulate BLyS/BAFF expression and release [88,91]. We have previously shown that BLyS/BAFF overexpression in blood of HIV-infected progressors coincides with hyperglobulinemia and increased frequencies of IL-10 expressing precursor-like MZ B-cells [50,86], which HiSeq gene expression analyses reveal several dysregulated genes (Poudrier J and Roger, preliminary data 2016). We also found overexpression of BLyS/BAFF and increased frequencies of precursor-like MZ B-cells in the blood of HIV-infected CSWs from Benin [92]. Elevated BLyS/BAFF levels likely favor expansion, activation and dysregulation of innate B-cell populations such as MZ, contributing to the over-representation of polyreactive and auto-reactive Abs [93] at the expense of eradicative anti-HIV Ab responses. Interestingly, cumulating evidence point to the fact that increased BLyS/BAFF and dysregulated B-cells sharing similar features with the precursor-like MZ B-cells we identified in the blood of HIV-infected individuals are a common trait of chronic inflammatory diseases [94].
In contrast to that observed in HIV-infected progressors, both blood BLyS/BAFF levels and frequencies of precursor-like MZ B-cells remained unaltered in HIV-infected Elite-Controllers [50,86]. Rather, frequencies of more mature MZ B-cells were found to be decreased in blood, implying that their recruitment to periphery, as suggested by their elevated migratory potential [95], may be involved in control of HIV disease progression. Although the mechanisms conferring natural immunity against HIV remain to be elucidated, we hypothesize they may share some similarities with that we observed in HIV-infected Elite-Controllers. Accordingly, in the Benin cohort, plasma and cellular BLyS/BAFF levels were significantly lower in the blood of HESN CSWs when compared to those measured in HIV-infected CSWs and HIV-uninfected non-CSW women [92]. These low BLyS/BAFF levels are consistent with the low-inflammatory response we have previously described in these individuals [37], and may be linked to the modulation of the intracellular machinery leading to BLyS/BAFF expression and/or release. Frequencies of precursor-like MZ B-cells remained unaltered in HESNs when compared to HIV-infected CSWs [92]. However, as for HIV-infected Elite-Controllers, frequencies of more mature MZ B-cells were decreased in their blood when compared to HIV-uninfected non-CSWs [92]. It is conceivable that these cells are recruited to the periphery, where they generate first-line responses against HIV. Moreover, given their propensity for B regulatory potential [50] they could also contribute to the maintenance of low inflammatory conditions observed in HESNs. Furthermore, since MZ B cells promote GC reactions, it is likely that they also participate in the development of adaptive B-cell responses against HIV. Therefore, immunomodulation of BLyS/BAFF levels and innate MZ-like B-cell populations may benefit to mucosal preventive devices viewed to produce a rapid and potent first line antiviral immune response at the initial site of exposure, and which could be combined with refined adaptive immunity. Understanding the dynamics of BLyS/BAFF and its role in homeostasis of immune responsiveness appears pivotal to the design of vaccine strategies soliciting first-line B-cell responses. Based on our observations, the capacity to contain BLyS/BAFF expression levels seems concomitant with natural immunity against HIV, whereas excessive BLyS/BAFF may promote immune dysregulation and disease progression.
9. Concluding Remarks
As depicted in Figure 1, natural genital mucosal immunity to HIV in HESN CSWs likely implies a strong capacity to generate efficient anti-viral responses and at the same time to prevent excessive inflammation. It likely involves orchestration of first-line innate immune responses in conjunction with matured high-affinity adaptive responses, which is expected to operate at cervicovaginal mucosal sites, which are ports of entry and replication for the virus. Promotion of regulatory DC-10-like, Treg, Tr1 and possibly Breg cells locally may contribute to the maintenance of a low-inflammatory genital milieu. This allows balanced responses from effector populations. The fact that BLyS/BAFF levels are contained helps to maintain the integrity of innate, possibly MZ-like, B-cell responses, the latter of which likely produce IgG and/or IgA capable of binding to HIV Env.
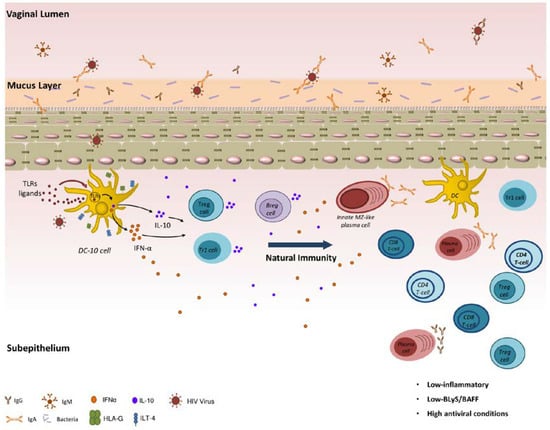
Figure 1.
Natural genital mucosal immunity to HIV.
Understanding the nature and how immune populations are recruited and maintained within mucosal niche to fight HIV is important to the design of effective preventive/therapeutic approaches. This is critical, especially for innate first-line B-cell populations, such as MZ, which do not generate memory, and possibly require a frequent degree of Ag exposure to be maintained in the mucosal niche. This suggests that any protective device soliciting first-line responses will likely require frequent boosting to the vaccine regimen.
Acknowledgments
This work was supported by Grants from the Canadian Institutes of Health Research (MOP-119406, PJT-148529) and the Réseau SIDA et Maladies Infectieuses, Fonds de Recherche du Québec en Santé (FRQS).
Conflicts of Interest
The authors declare no conflict of interest.
References
- UNAIDS Unaids Data 2017. Available online: http://www.unaids.org/en/resources/documents/2017/2017_data_book (accessed on 20 March 2018).
- Poudrier, J.; Thibodeau, V.; Roger, M. Natural immunity to HIV: A delicate balance between strength and control. Clin. Dev. Immunol. 2012, 2012, 875821. [Google Scholar] [CrossRef] [PubMed]
- Fowke, K.R.; Nagelkerke, N.J.; Kimani, J.; Simonsen, J.N.; Anzala, A.O.; Bwayo, J.J.; MacDonald, K.S.; Ngugi, E.N.; Plummer, F.A. Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet 1996, 348, 1347–1351. [Google Scholar] [CrossRef]
- Yao, X.D.; Omange, R.W.; Henrick, B.M.; Lester, R.T.; Kimani, J.; Ball, T.B.; Plummer, F.A.; Rosenthal, K.L. Acting locally: Innate mucosal immunity in resistance to HIV-1 infection in Kenyan commercial sex workers. Mucosal Immunol. 2014, 7, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Wira, C.R.; Fahey, J.V.; Sentman, C.L.; Pioli, P.A.; Shen, L. Innate and adaptive immunity in female genital tract: Cellular responses and interactions. Immunol. Rev. 2005, 206, 306–335. [Google Scholar] [CrossRef] [PubMed]
- Shacklett, B.L. Cell-mediated immunity to hiv in the female reproductive tract. J. Reprod. Immunol. 2009, 83, 190–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wira, C.R.; Fahey, J.V. A new strategy to understand how HIV infects women: Identification of a window of vulnerability during the menstrual cycle. Aids 2008, 22, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Wira, C.R.; Rodriguez-Garcia, M.; Patel, M.V. The role of sex hormones in immune protection of the female reproductive tract. Nat. Rev. Immunol. 2015, 15, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Hickey, D.K.; Patel, M.V.; Fahey, J.V.; Wira, C.R. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: Stratification and integration of immune protection against the transmission of sexually transmitted infections. J. Reprod. Immunol. 2011, 88, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Poudrier, J.; Chagnon-Choquet, J.; Roger, M. Influence of dendritic cells on b-cell responses during hiv infection. Clin. Dev. Immunol. 2012, 2012, 592187. [Google Scholar] [CrossRef] [PubMed]
- Nazli, A.; Chan, O.; Dobson-Belaire, W.N.; Ouellet, M.; Tremblay, M.J.; Gray-Owen, S.D.; Arsenault, A.L.; Kaushic, C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010, 6, e1000852. [Google Scholar] [CrossRef] [PubMed]
- Grant-Tschudy, K.S.; Wira, C.R. Paracrine mediators of mouse uterine epithelial cell transepithelial resistance in culture. J. Reprod. Immunol. 2005, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cleret-Buhot, A.; Zhang, Y.; Planas, D.; Goulet, J.P.; Monteiro, P.; Gosselin, A.; Wacleche, V.S.; Tremblay, C.L.; Jenabian, M.A.; Routy, J.P.; et al. Identification of novel HIV-1 dependency factors in primary CCR4+CCR6+Th17 cells via a genome-wide transcriptional approach. Retrovirology 2015, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Gringhuis, S.I.; van der Vlist, M.; van den Berg, L.M.; den Dunnen, J.; Litjens, M.; Geijtenbeek, T.B. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat. Immunol. 2010, 11, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Haase, A.T. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 2010, 464, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Magerus-Chatinet, A.; Yu, H.; Garcia, S.; Ducloux, E.; Terris, B.; Bomsel, M. Galactosyl ceramide expressed on dendritic cells can mediate HIV-1 transfer from monocyte derived dendritic cells to autologous T cells. Virology 2007, 362, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bomsel, M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 1997, 3, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Duan, L.; Perkey, K.E.; Wietgrefe, S.; Zupancic, M.; Smith, A.J.; Southern, P.J.; Johnson, R.P.; Haase, A.T. Epithelium-innate immune cell axis in mucosal responses to siv. Mucosal Immunol. 2017, 10, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.; Kwon, D.S.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.; Middel, J.; Cornelissen, I.L.; Nottet, H.S.; KewalRamani, V.N.; Littman, D.R.; et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 2000, 100, 587–597. [Google Scholar] [CrossRef]
- Fontenot, D.; He, H.; Hanabuchi, S.; Nehete, P.N.; Zhang, M.; Chang, M.; Nehete, B.; Wang, Y.H.; Wang, Y.H.; Ma, Z.M.; et al. TSLP production by epithelial cells exposed to immunodeficiency virus triggers DC-mediated mucosal infection of CD4+ T cells. Proc. Natl. Acad. Sci. USA 2009, 106, 16776–16781. [Google Scholar] [CrossRef] [PubMed]
- Malim, M.H.; Bieniasz, P.D. HIV restriction factors and mechanisms of evasion. Cold Spring Harb. Perspect. Med. 2012, 2, a006940. [Google Scholar] [CrossRef] [PubMed]
- Haase, A.T. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu. Rev. Med. 2011, 62, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Gringhuis, S.I.; den Dunnen, J.; Litjens, M.; van der Vlist, M.; Geijtenbeek, T.B. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat. Immunol. 2009, 10, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Teijeira, A.; Russo, E.; Halin, C. Taking the lymphatic route: Dendritic cell migration to draining lymph nodes. Semin. Immunopathol. 2014, 36, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.J.; Li, Q.; Abel, K.; Kim, E.Y.; Ma, Z.M.; Wietgrefe, S.; La Franco-Scheuch, L.; Compton, L.; Duan, L.; Shore, M.D.; et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 2005, 79, 9217–9227. [Google Scholar] [CrossRef] [PubMed]
- Stieh, D.J.; Matias, E.; Xu, H.; Fought, A.J.; Blanchard, J.L.; Marx, P.A.; Veazey, R.S.; Hope, T.J. Th17 cells are preferentially infected very early after vaginal transmission of SIV in macaques. Cell Host Microbe 2016, 19, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor rorgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Manel, N.; Unutmaz, D.; Littman, D.R. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008, 9, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.; Wang, X.; Piatak, M.; Lifson, J.; Roederer, M.; Veazey, R.; Mattapallil, J.J. α4+β7hiCD4+ memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009, 2, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Cremel, M.; Berlier, W.; Hamzeh, H.; Cognasse, F.; Lawrence, P.; Genin, C.; Bernengo, J.C.; Lambert, C.; Dieu-Nosjean, M.C.; Delezay, O. Characterization of CCL20 secretion by human epithelial vaginal cells: Involvement in langerhans cell precursor attraction. J. Leukoc. Biol. 2005, 78, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, E.; Struyf, S.; Van Damme, J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003, 14, 409–426. [Google Scholar] [CrossRef]
- Li, Q.; Estes, J.D.; Schlievert, P.M.; Duan, L.; Brosnahan, A.J.; Southern, P.J.; Reilly, C.S.; Peterson, M.L.; Schultz-Darken, N.; Brunner, K.G.; et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009, 458, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, J.J.; Plummer, F.A. The microbiological context of HIV resistance: Vaginal microbiota and mucosal inflammation at the viral point of entry. Int. J. Inflam. 2012, 2012, 131243. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.; Juno, J.; Burgener, A.; Rahman, S.; Mogk, K.; Wachihi, C.; Mwanjewe, J.; Plummer, F.A.; Kimani, J.; Ball, T.B.; et al. A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers. Mucosal Immunol. 2012, 5, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.; Poudrier, J.; Massinga Loembe, M.; Guedou, F.; Leblond, F.; Labbe, A.C.; Alary, M.; Roger, M. Chemokine expression patterns in the systemic and genital tract compartments are associated with HIV-1 infection in women from Benin. J. Clin. Immunol. 2010, 30, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.; Poudrier, J.; Massinga-Loembe, M.; Guedou, F.; Agossa-Gbenafa, C.; Labbe, A.C.; Alary, M.; Roger, M. Differences in immunoregulatory cytokine expression patterns in the systemic and genital tract compartments of HIV-1-infected commercial sex workers in Benin. Mucosal Immunol. 2008, 1, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Ball, T.B.; Ji, H.; Kimani, J.; McLaren, P.; Marlin, C.; Hill, A.V.; Plummer, F.A. Polymorphisms in IRF-1 associated with resistance to HIV-1 infection in highly exposed uninfected kenyan sex workers. Aids 2007, 21, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Ball, T.B.; Ao, Z.; Kimani, J.; Yao, X.; Plummer, F.A. Reduced HIV-1 long terminal repeat transcription in subjects with protective interferon regulatory factor-1 genotype: A potential mechanism mediating resistance to infection by HIV-1. Scand. J. Infect. Dis. 2010, 42, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Fowke, K.R.; Kaul, R.; Rosenthal, K.L.; Oyugi, J.; Kimani, J.; Rutherford, W.J.; Nagelkerke, N.J.; Ball, T.B.; Bwayo, J.J.; Simonsen, J.N.; et al. HIV-1-specific cellular immune responses among HIV-1-resistant sex workers. Immunol. Cell Biol. 2000, 78, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.; Plummer, F.A.; Kimani, J.; Dong, T.; Kiama, P.; Rostron, T.; Njagi, E.; MacDonald, K.S.; Bwayo, J.J.; McMichael, A.J.; et al. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 2000, 164, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, L.R.; Nyanga, B.; Chege, D.; Izulla, P.; Kimani, M.; Huibner, S.; Gelmon, L.; Block, K.E.; Cicala, C.; Anzala, A.O.; et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J. Immunol. 2011, 187, 6032–6042. [Google Scholar] [CrossRef] [PubMed]
- McLaren, P.J.; Ball, T.B.; Wachihi, C.; Jaoko, W.; Kelvin, D.J.; Danesh, A.; Kimani, J.; Plummer, F.A.; Fowke, K.R. HIV-exposed seronegative commercial sex workers show a quiescent phenotype in the CD4+ T cell compartment and reduced expression of HIV-dependent host factors. J. Infect. Dis. 2010, 202, S339–S344. [Google Scholar] [CrossRef] [PubMed]
- Alimonti, J.B.; Koesters, S.A.; Kimani, J.; Matu, L.; Wachihi, C.; Plummer, F.A.; Fowke, K.R. CD4+ T cell responses in HIV-exposed seronegative women are qualitatively distinct from those in HIV-infected women. J. Infect. Dis. 2005, 191, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, V.; Fourcade, L.; Labbe, A.C.; Alary, M.; Guedou, F.; Poudrier, J.; Roger, M. Highly-exposed HIV-1 seronegative female commercial sex workers sustain in their genital mucosa increased frequencies of tolerogenic myeloid and regulatory T-cells. Sci. Rep. 2017, 7, 43857. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, S.I.; Abe, F.; Kanno, A.; Tanimura, N.; Mori Saitoh, Y.; Fukui, R.; Shibata, T.; Sato, K.; Ichinohe, T.; Hayashi, M.; et al. TLR7 mediated viral recognition results in focal type I interferon secretion by dendritic cells. Nat. Commun. 2017, 8, 1592. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.; Goujon, C.; Malim, M.H. HIV-1 and interferons: Who’s interfering with whom? Nat. Rev. Microbiol. 2015, 13, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Siewe, B.; Stapleton, J.T.; Martinson, J.; Keshavarzian, A.; Kazmi, N.; Demarais, P.M.; French, A.L.; Landay, A. Regulatory B cell frequency correlates with markers of HIV disease progression and attenuates anti-HIV CD8+ T cell function in vitro. J. Leukoc. Biol. 2013, 93, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Chagnon-Choquet, J.; Fontaine, J.; Poudrier, J.; Roger, M. IL-10 and lymphotoxin-alpha expression profiles within marginal zone-like B-cell populations are associated with control of HIV-1 disease progression. PLoS ONE 2014, 9, e101949. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ellis, G.; Pallant, C.; Lopes, A.R.; Khanna, P.; Peppa, D.; Chen, A.; Blair, P.; Dusheiko, G.; Gill, U.; et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J. Immunol. 2012, 189, 3925–3935. [Google Scholar] [CrossRef] [PubMed]
- De Waal Malefyt, R.; Haanen, J.; Spits, H.; Roncarolo, M.G.; te Velde, A.; Figdor, C.; Johnson, K.; Kastelein, R.; Yssel, H.; de Vries, J.E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 1991, 174, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Amodio, G.; Gregori, S. Human tolerogenic DC-10: Perspectives for clinical applications. Transplant. Res. 2012, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Amodio, G.; Comi, M.; Tomasoni, D.; Gianolini, M.E.; Rizzo, R.; LeMaoult, J.; Roncarolo, M.G.; Gregori, S. HLA-G expression levels influence the tolerogenic activity of human DC-10. Haematologica 2015, 100, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Card, C.M.; McLaren, P.J.; Wachihi, C.; Kimani, J.; Plummer, F.A.; Fowke, K.R. Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4+CD25+FOXP3+ regulatory T cells. J. Infect. Dis. 2009, 199, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Gagliani, N.; Magnani, C.F.; Huber, S.; Gianolini, M.E.; Pala, M.; Licona-Limon, P.; Guo, B.; Herbert, D.R.; Bulfone, A.; Trentini, F.; et al. Coexpression of CD49B and LAG-3 identifies human and mouse t regulatory type 1 cells. Nat. Med. 2013, 19, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Attanasio, J.; Wherry, E.J. Costimulatory and coinhibitory receptor pathways in infectious disease. Immunity 2016, 44, 1052–1068. [Google Scholar] [CrossRef] [PubMed]
- Day, T.A.; Kublin, J.G. Lessons learned from HIV vaccine clinical efficacy trials. Curr. HIV Res. 2013, 11, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Wills, S.; Hwang, K.K.; Liu, P.; Dennison, S.M.; Tay, M.Z.; Shen, X.; Pollara, J.; Lucas, J.T.; Parks, R.; Rerks-Ngarm, S.; et al. HIV-1-specific iga monoclonal antibodies from an HIV-1 vaccinee mediate galactosylceramide blocking and phagocytosis. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Excler, J.L.; Michael, N.L. Lessons from the RV144 Thai phase III HIV-1 vaccine trial and the search for correlates of protection. Annu. Rev. Med. 2015, 66, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.B.; Liao, H.X.; Moody, M.A.; Kepler, T.B.; Alam, S.M.; Gao, F.; Wiehe, K.; Trama, A.M.; Jones, K.; Zhang, R.; et al. HIV-1 vaccines. Diversion of HIV-1 vaccine-induced immunity by GP41-microbiota cross-reactive antibodies. Science 2015, 349, aab1253. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.; Ruprecht, R.M. Mucosal iga responses: Damaged in established HIV infection-yet, effective weapon against HIV transmission. Front. Immunol. 2017, 8, 1581. [Google Scholar] [CrossRef] [PubMed]
- Bomsel, M.; Tudor, D.; Drillet, A.S.; Alfsen, A.; Ganor, Y.; Roger, M.G.; Mouz, N.; Amacker, M.; Chalifour, A.; Diomede, L.; et al. Immunization with HIV-1 GP41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal shiv challenges. Immunity 2011, 34, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Sholukh, A.M.; Watkins, J.D.; Vyas, H.K.; Gupta, S.; Lakhashe, S.K.; Thorat, S.; Zhou, M.; Hemashettar, G.; Bachler, B.C.; Forthal, D.N.; et al. Defense-in-depth by mucosally administered anti-HIV dimeric IgA2 and systemic IgG1 mabs: Complete protection of rhesus monkeys from mucosal SHIV challenge. Vaccine 2015, 33, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.D.; Sholukh, A.M.; Mukhtar, M.M.; Siddappa, N.B.; Lakhashe, S.K.; Kim, M.; Reinherz, E.L.; Gupta, S.; Forthal, D.N.; Sattentau, Q.J.; et al. Anti-HIV iga isotypes: Differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission. Aids 2013, 27, F13–F20. [Google Scholar] [CrossRef] [PubMed]
- Batraville, L.A.; Richard, J.; Veillette, M.; Labbe, A.C.; Alary, M.; Guedou, F.; Kaufmann, D.E.; Poudrier, J.; Finzi, A.; Roger, M. Short communication: Anti-HIV-1 envelope immunoglobulin Gs in blood and cervicovaginal samples of beninese commercial sex workers. AIDS Res. Hum. Retroviruses 2014, 30, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Levinson, P.; Guthrie, B.L.; Lohman-Payne, B.; Bosire, R.; Liu, A.Y.; Hirbod, T.; Kiarie, J.; Overbaugh, J.; John-Stewart, G.; et al. Cervicovaginal HIV-1-neutralizing immunoglobulin a detected among HIV-1-exposed seronegative female partners in HIV-1-discordant couples. Aids 2012, 26, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Seaton, K.E.; Ballweber, L.; Lan, A.; Donathan, M.; Hughes, S.; Vojtech, L.; Moody, M.A.; Liao, H.X.; Haynes, B.F.; Galloway, C.G.; et al. HIV-1 specific iga detected in vaginal secretions of HIV uninfected women participating in a microbicide trial in southern Africa are primarily directed toward gp120 and gp140 specificities. PLoS ONE 2014, 9, e101863. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.; Trabattoni, D.; Bwayo, J.J.; Arienti, D.; Zagliani, A.; Mwangi, F.M.; Kariuki, C.; Ngugi, E.N.; MacDonald, K.S.; Ball, T.B.; et al. HIV-1-specific mucosal iga in a cohort of HIV-1-resistant kenyan sex workers. Aids 1999, 13, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Broliden, K.; Hinkula, J.; Devito, C.; Kiama, P.; Kimani, J.; Trabbatoni, D.; Bwayo, J.J.; Clerici, M.; Plummer, F.; Kaul, R. Functional HIV-1 specific iga antibodies in HIV-1 exposed, persistently igg seronegative female sex workers. Immunol. Lett. 2001, 79, 29–36. [Google Scholar] [CrossRef]
- Kaul, R.; Plummer, F.; Clerici, M.; Bomsel, M.; Lopalco, L.; Broliden, K. Mucosal iga in exposed, uninfected subjects: Evidence for a role in protection against HIV infection. Aids 2001, 15, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Belec, L.; Ghys, P.D.; Hocini, H.; Nkengasong, J.N.; Tranchot-Diallo, J.; Diallo, M.O.; Ettiegne-Traore, V.; Maurice, C.; Becquart, P.; Matta, M.; et al. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative african women. J. Infect. Dis. 2001, 184, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Hocini, H.; Bomsel, M. Infectious human immunodeficiency virus can rapidly penetrate a tight human epithelial barrier by transcytosis in a process impaired by mucosal immunoglobulins. J. Infect. Dis. 1999, 179, S448–S453. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.; Rowland-Jones, S.L.; Kimani, J.; Dong, T.; Yang, H.B.; Kiama, P.; Rostron, T.; Njagi, E.; Bwayo, J.J.; MacDonald, K.S.; et al. Late seroconversion in HIV-resistant nairobi prostitutes despite pre-existing HIV-specific CD8+ responses. J. Clin. Investig. 2001, 107, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Slack, E.; Balmer, M.L.; Macpherson, A.J. B cells as a critical node in the microbiota-host immune system network. Immunol. Rev. 2014, 260, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, R.G.; Breden, F.; Plummer, F.; Berry, J.D. Molecular characterization of the cervical and systemic B-cell repertoire: Unique, yet overlapping, immune compartments of an HIV-1 resistant individual. MAbs 2011, 3, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A.; Cols, M.; Puga, I. Marginal zone B cells: Virtues of innate-like antibody-producing lymphocytes. Nat. Rev. Immunol. 2013, 13, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Victora, G.D.; Nussenzweig, M.C. Germinal centers. Annu. Rev. Immunol. 2012, 30, 429–457. [Google Scholar] [CrossRef] [PubMed]
- Bikos, V.; Karypidou, M.; Stalika, E.; Baliakas, P.; Xochelli, A.; Sutton, L.A.; Papadopoulos, G.; Agathangelidis, A.; Papadopoulou, E.; Davis, Z.; et al. An immunogenetic signature of ongoing antigen interactions in splenic marginal zone lymphoma expressing IGHV1-2*04 receptors. Clin. Cancer Res. 2016, 22, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Georgiev, I.; Wu, X.; Yang, Z.Y.; Dai, K.; Finzi, A.; Kwon, Y.D.; Scheid, J.F.; Shi, W.; Xu, L.; et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 2010, 329, 811–817. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Qiao, X.; Klasse, P.J.; Chiu, A.; Chadburn, A.; Knowles, D.M.; Moore, J.P.; Cerutti, A. HIV-1 envelope triggers polyclonal ig class switch recombination through a CD40-independent mechanism involving baff and C-type lectin receptors. J. Immunol. 2006, 176, 3931–3941. [Google Scholar] [CrossRef] [PubMed]
- Weill, J.C.; Weller, S.; Reynaud, C.A. Human marginal zone B cells. Annu. Rev. Immunol. 2009, 27, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Dosenovic, P.; Soldemo, M.; Scholz, J.L.; O’Dell, S.; Grasset, E.K.; Pelletier, N.; Karlsson, M.C.; Mascola, J.R.; Wyatt, R.T.; Cancro, M.P.; et al. Blys-mediated modulation of naive B cell subsets impacts HIV env-induced antibody responses. J. Immunol. 2012, 188, 6018–6026. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Lapointe, R.; Malaspina, A.; Ostrowski, M.; Cole, C.E.; Chun, T.W.; Adelsberger, J.; Baseler, M.; Hwu, P.; Fauci, A.S. CD40-mediated induction of CD4 and CXCR4 on B lymphocytes correlates with restricted susceptibility to human immunodeficiency virus type 1 infection: Potential role of b lymphocytes as a viral reservoir. J. Virol. 1999, 73, 7972–7980. [Google Scholar] [PubMed]
- Moir, S.; Fauci, A.S. Insights into B cells and HIV-specific B-cell responses in HIV-infected individuals. Immunol. Rev. 2013, 254, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, J.; Chagnon-Choquet, J.; Valcke, H.S.; Poudrier, J.; Roger, M. High expression levels of B lymphocyte stimulator (blys) by dendritic cells correlate with hiv-related B-cell disease progression in humans. Blood 2011, 117, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.I.; Jacobson, L.P.; Margolick, J.B.; Breen, E.C.; Macatangay, B.; Penugonda, S.; Martinez-Maza, O.; Bream, J.H. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. Aids 2015, 29, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Chagnon-Choquet, J.; Gauvin, J.; Roger, J.; Fontaine, J.; Poudrier, J.; Roger, M. HIV nef promotes expression of B-lymphocyte stimulator by blood dendritic cells during HIV infection in humans. J. Infect. Dis. 2015, 211, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.M.; Ouellet, M.; Tremblay, M.J. HIV-1-triggered release of type I IFN by plasmacytoid dendritic cells induces BAFF production in monocytes. J. Immunol. 2015, 194, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Sjostrand, M.; Johansson, A.; Aqrawi, L.; Olsson, T.; Wahren-Herlenius, M.; Espinosa, A. The expression of baff is controlled by irf transcription factors. J. Immunol. 2016, 196, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Chu, V.T.; Enghard, P.; Riemekasten, G.; Berek, C. In vitro and in vivo activation induces BAFF and april expression in B cells. J. Immunol. 2007, 179, 5947–5957. [Google Scholar] [CrossRef] [PubMed]
- Sabourin-Poirier, C.; Fourcade, L.; Chagnon-Choquet, J.; Labbe, A.C.; Alary, M.; Guedou, F.; Poudrier, J.; Roger, M. Blood B lymphocyte stimulator (BLYS)/BAFF levels may reflect natural immunity to HIV in highly exposed uninfected Beninese commercial sex workers. Sci. Rep. 2016, 6, 32318. [Google Scholar] [CrossRef] [PubMed]
- Varin, M.M.; Le Pottier, L.; Youinou, P.; Saulep, D.; Mackay, F.; Pers, J.O. B-cell tolerance breakdown in Sjogren’s syndrome: Focus on BAFF. Autoimmun. Rev. 2010, 9, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Borhis, G.; Richard, Y. Subversion of the B-cell compartment during parasitic, bacterial, and viral infections. BMC Immunol. 2015, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Gauvin, J.; Chagnon-Choquet, J.; Poudrier, J.; Roger, M. Fluctuations in blood marginal zone B-cell frequencies may reflect migratory patterns associated with HIV-1 disease progression status. PLoS ONE 2016, 11, e0155868. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).