Canine Influenza Virus is Mildly Restricted by Canine Tetherin Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Identification and Cloning of the Canine Tetherin Gene
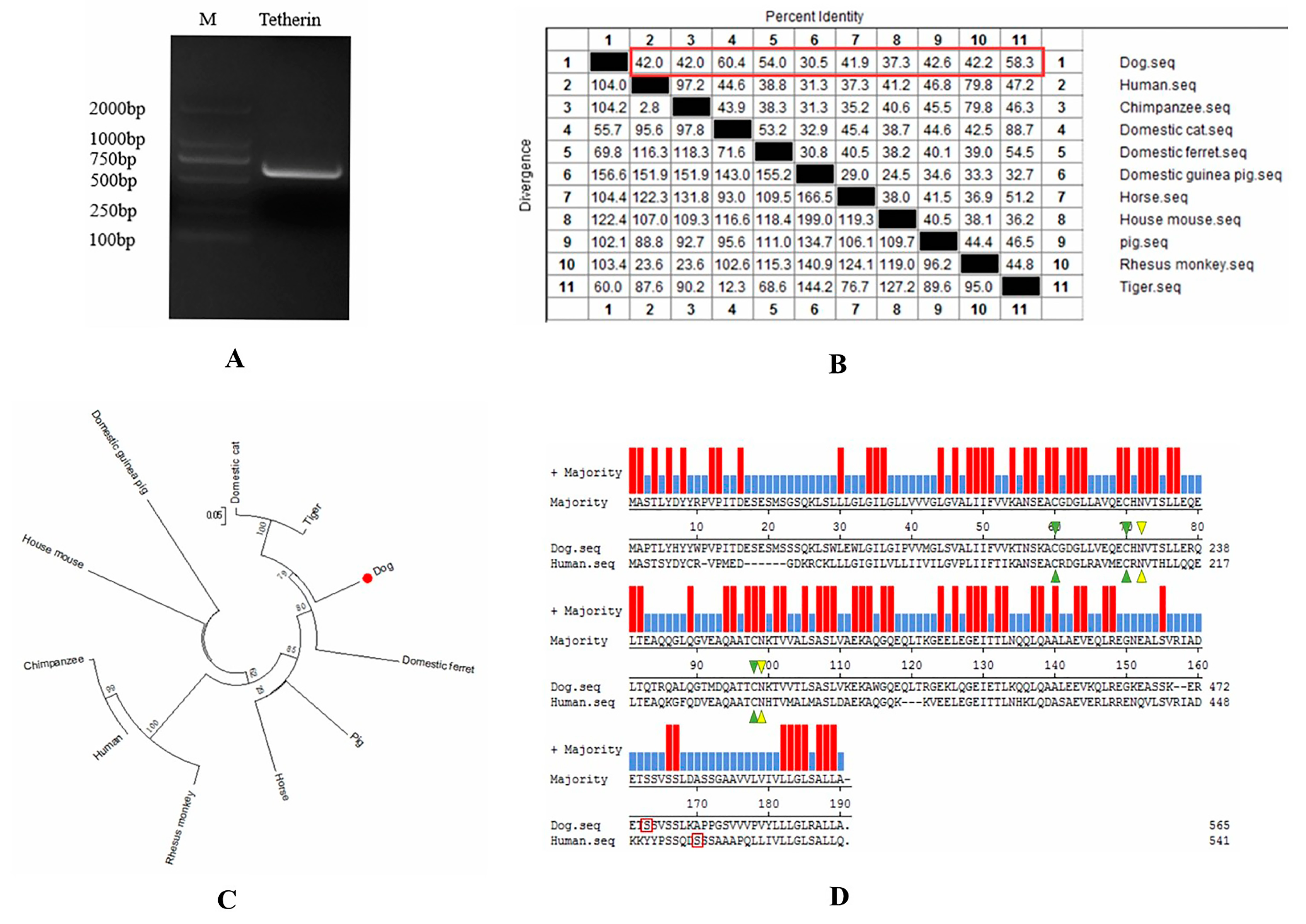
2.3. Bioinformatics Analysis
2.4. G418 Screening for Cell Lines with Stable Expression
2.5. CCK-8 Assay
2.6. Tetherin Interference Experiment
2.7. Indirect Immunofluorescence and Laser Scanning Confocal Microscopy
3. Results
3.1. Bioinformatic Analysis of Canine Tetherin
3.2. Analysis of Canine Tetherin Expression Profiles in Tissues
3.3. Canine Tetherin Protein Expression and Cellular Localization
3.4. The Expression of Canine Tetherin Is Inducible by IFN-α and CIV Infection
3.5. CCK-8 Assay
3.6. Tetherin-Abundant MDCK Cells Exhibit Decreased CIV Production
3.7. Interference with Tetherin Expression is Associated with Increased CIV Production
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| (EID50) | 50% egg infective dose |
| (aa) | Amino acid |
| (ABSL-3) | Animal biosafety level 3 |
| (DAPI) | 4′,6-Diamidino-2-phenylindole |
| (CIV) | Canine influenza virus |
| (CCK-8) | Cell Counting Kit-8 |
| (CDS) | Coding sequence |
| (Cy3) | Cyanine-3 |
| (DMEM) | Dulbecco’s modified Eagle’s medium |
| (FBS) | Foetal bovine serum |
| (FITC) | Fluorescein isothiocyanate |
| (GAPDH) | Glyceraldehyde-3-phosphate dehydrogenase gene |
| (HCV) | Hepatitis C virus |
| (HPAI) | Highly pathogenic avian influenza |
| (HEK) | Human embryonic kidney (cells) |
| (ISGs) | IFN-stimulated genes |
| (IAV) | Influenza A virus |
| (IFN) | Interferon |
| (IRF) | Interferon regulatory factor |
| (ISRE) | Interferon-stimulated response element |
| (KSHV) | Kaposi’s sarcoma-associated herpesvirus |
| (MDCK) | Madin–Darby canine kidney (cells) |
| (NF-AT) | Nuclear factor of activated T-cells |
| (ORF) | Open reading frame |
| (PBMC) | Peripheral blood mononuclear cells |
| (PFU) | Plaque-forming unit |
| (RTFQ PCR) | Real-time fluorescence quantitative PCR |
| (RT-PCR) | Reverse transcription-polymerase chain reaction |
| (STAT) | Signal transducer and activator of transcription |
| (siRNA) | Small interfering RNA |
| (SPF) | Specific-pathogen-free |
| (UTR) | Untranslated region |
| (VSV) | Vesicular stomatitis virus |
| (VLPs) | Virus-like particles |
References
- Neil, S.J.; Zang, T.; Bieniasz, P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 2008, 451, 425–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouvenet, N.; Neil, S.J.; Zhadina, M.; Zang, T.; Kratovac, Z.; Lee, Y.; McNatt, M.; Hatziioannou, T.; Bieniasz, P.D. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 2009, 83, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Kaletsky, R.L.; Francica, J.R.; Agrawal-Gamse, C.; Bates, P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. USA 2009, 106, 2886–2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakuma, T.; Noda, T.; Urata, S.; Kawaoka, Y.; Yasuda, J. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 2009, 83, 2382–2385. [Google Scholar] [CrossRef] [PubMed]
- Radoshitzky, S.R.; Dong, L.; Chi, X.; Clester, J.C.; Retterer, C.; Spurgers, K.; Kuhn, J.H.; Sandwick, S.; Ruthel, G.; Kota, K.; et al. Infectious Lassa virus, but not filoviruses, is restricted by BST-2/tetherin. J. Virol. 2010, 84, 10569–10580. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.S.; Irie, T.; Yoshida, A.; Kawabata, R.; Kadoi, T.; Sakaguchi, T. Inhibition of virus-like particle release of Sendai virus and Nipah virus, but not that of mumps virus, by tetherin/CD317/BST-2. Hiroshima J. Med. Sci. 2012, 61, 59–67. [Google Scholar] [PubMed]
- Mansouri, M.; Viswanathan, K.; Douglas, J.L.; Hines, J.; Gustin, J.; Moses, A.V.; Fruh, K. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2009, 83, 9672–9681. [Google Scholar] [CrossRef] [PubMed]
- Pardieu, C.; Vigan, R.; Wilson, S.J.; Calvi, A.; Zang, T.; Bieniasz, P.; Kellam, P.; Towers, G.J.; Neil, S.J. The RING-CH ligase K5 antagonizes restriction of KSHV and HIV-1 particle release by mediating ubiquitin-dependent endosomal degradation of tetherin. PLOS Pathog. 2010, 6, e1000843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidner, J.M.; Jiang, D.; Pan, X.B.; Chang, J.; Block, T.M.; Guo, J.T. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J. Virol. 2010, 84, 12646–12657. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.B.; Qu, X.W.; Jiang, D.; Zhao, X.L.; Han, J.C.; Wei, L. BST2/Tetherin inhibits hepatitis C virus production in human hepatoma cells. Antiviral Res. 2013, 98, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.B.; Han, J.C.; Cong, X.; Wei, L. BST2/tetherin inhibits dengue virus release from human hepatoma cells. PLoS ONE 2012, 7, e51033. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shi, Z.; Jiao, P.; Zhang, G.; Zhong, Z.; Tian, W.; Long, L.P.; Cai, Z.; Zhu, X.; Liao, M.; et al. Avian-origin H3N2 canine influenza A viruses in Southern China. Infect. Genet. Evol. 2010, 10, 1286–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, D.; Lee, C.; Kang, B.; Jung, K.; Oh, T.; Kim, H.; Park, B.; Oh, J. Experimental infection of dogs with avian-origin canine influenza A virus (H3N2). Emerg. Infect. Dis. 2009, 15, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, R.; Zhang, C.; Wang, S.; He, W.; Zhang, J.; Liu, J.; Cai, Y.; Zhou, J.; Su, S. Genetic and evolutionary analysis of emerging H3N2 canine influenza virus. Emerg. Microbes Infect. 2018. [Google Scholar] [CrossRef] [PubMed]
- New Virus Strain Causes Midwest Dog Flu Outbreak. Available online: http://news.cornell.edu/stories/2015/04/new-virus-strain-causes-midwest-dog-flu-outbreak (accessed on 16 August 2017).
- Songserm, T.; Amonsin, A.; Jam-on, R.; Sae-Heng, N.; Pariyothorn, N.; Payungporn, S.; Theamboonlers, A.; Chutinimitkul, S.; Thanawongnuwech, R.; Poovorawan, Y. Fatal avian influenza A H5N1 in a dog. Emerg. Infect. Dis. 2006, 12, 1744–1747. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Deng, G.; Li, Z.; Tian, G.; Li, Y.; Jiao, P.; Zhang, L.; Liu, Z.; Webster, R.G.; Yu, K. The evolution of H5N1 influenza viruses in ducks in southern China. Proc. Natl. Acad. Sci. USA 2004, 101, 10452–10457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amonsin, A.; Payungporn, S.; Theamboonlers, A.; Thanawongnuwech, R.; Suradhat, S.; Pariyothorn, N.; Tantilertcharoen, R.; Damrongwantanapokin, S.; Buranathai, C.; Chaisingh, A.; et al. Genetic characterization of H5N1 influenza A viruses isolated from zoo tigers in Thailand. Virology 2006, 344, 480–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keawcharoen, J.; Oraveerakul, K.; Kuiken, T.; Fouchier, R.A.; Amonsin, A.; Payungporn, S.; Noppornpanth, S.; Wattanodorn, S.; Theambooniers, A.; Tantilertcharoen, R.; et al. Avian influenza H5N1 in tigers and leopards. Emerg. Infect. Dis. 2004, 10, 2189–2191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yang, H.; Chen, W.; Cao, W.; Zhong, G.; Jiao, P.; Deng, G.; Yu, K.; Yang, C.; Bu, Z.; et al. A naturally occurring deletion in its NS gene contributes to the attenuation of an H5N1 swine influenza virus in chickens. J. Virol. 2008, 82, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhong, G.; Wang, G.; Deng, G.; Li, Y.; Shi, J.; Zhang, Z.; Guan, Y.; Jiang, Y.; Bu, Z.; et al. Dogs are highly susceptible to H5N1 avian influenza virus. Virology 2010, 405, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, R.A.; Garcia-Sastre, A. Influenza A viruses: New research developments. Nat. Rev. Microbiol. 2011, 9, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Bruce, E.A.; Abbink, T.E.; Wise, H.M.; Rollason, R.; Galao, R.P.; Banting, G.; Neil, S.J.; Digard, P. Release of filamentous and spherical influenza A virus is not restricted by tetherin. J. Gen. Virol. 2012, 93, 963–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, R.; Leser, G.P.; Lamb, R.A. Influenza virus is not restricted by tetherin whereas influenza VLP production is restricted by tetherin. Virology 2011, 417, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, M.; Bertram, S.; Gnirss, K.; Nehlmeier, I.; Gawanbacht, A.; Kirchhoff, F.; Ehrhardt, C.; Ludwig, S.; Kiene, M.; Moldenhauer, A.S.; et al. Influenza A virus does not encode a tetherin antagonist with Vpu-like activity and induces IFN-dependent tetherin expression in infected cells. PLoS ONE 2012, 7, e43337. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Grado, V.H.; Hai, R.; Fernandes, F.; Belicha-Villanueva, A.; Carter, C.; Yondola, M.A. Modulation of an ectodomain motif in the influenza A virus neuraminidase alters tetherin sensitivity and results in virus attenuation in vivo. J. Mol. Biol. 2014, 426, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Mangeat, B.; Cavagliotti, L.; Lehmann, M.; Gers-Huber, G.; Kaur, I.; Thomas, Y.; Kaiser, L.; Piguet, V. Influenza virus partially counteracts restriction imposed by tetherin/BST-2. J. Biol. Chem. 2012, 287, 22015–22029. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, M.; Hoffmann, H.H.; Scull, M.A.; Gilmore, R.H.; Bell, K.L.; Ciancanelli, M.; Wilson, S.J.; Crotta, S.; Yu, Y.; Flatley, B.; et al. A serpin shapes the extracellular environment to prevent influenza A virus maturation. Cell 2015, 160, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Gnirss, K.; Zmora, P.; Blazejewska, P.; Winkler, M.; Lins, A.; Nehlmeier, I.; Gartner, S.; Moldenhauer, A.S.; Hofmann-Winkler, H.; Wolff, T.; et al. Tetherin Sensitivity of Influenza A Viruses Is Strain Specific: Role of Hemagglutinin and Neuraminidase. J. Virol. 2015, 89, 9178–9188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, A.; Miguet, N.; Natrajan, G.; Usami, Y.; Yamanaka, H.; Renesto, P.; Hartlieb, B.; McCarthy, A.A.; Simorre, J.P.; Gottlinger, H.; et al. Structural basis of HIV-1 tethering to membranes by the BST-2/tetherin ectodomain. Cell Host Microbe 2010, 7, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Blasius, A.L.; Giurisato, E.; Cella, M.; Schreiber, R.D.; Shaw, A.S.; Colonna, M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 2006, 177, 3260–3265. [Google Scholar] [CrossRef] [PubMed]
- Julkunen, I.; Sareneva, T.; Pirhonen, J.; Ronni, T.; Melen, K.; Matikainen, S. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 2001, 12, 171–180. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition in the innate immune response. Biochem. J. 2009, 420, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotter, D.; Sauter, D.; Kirchhoff, F. Emerging role of the host restriction factor tetherin in viral immune sensing. J. Mol. Biol. 2013, 425, 4956–4964. [Google Scholar] [CrossRef] [PubMed]
- Yi, E.; Oh, J.; Giao, N.Q.; Oh, S.; Park, S.H. Enhanced production of enveloped viruses in BST-2-deficient cell lines. Biotechnol. Bioeng. 2017, 114, 2289–2297. [Google Scholar] [CrossRef] [PubMed]
- Liberatore, R.A.; Bieniasz, P.D. Tetherin is a key effector of the antiretroviral activity of type I interferon in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 18097–18101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkmann, C.; Nehlmeier, I.; Walendy-Gnirss, K.; Nehls, J.; Gonzalez, H.M.; Hoffmann, M.; Qiu, X.; Takada, A.; Schindler, M.; Pohlmann, S. The Tetherin Antagonism of the Ebola Virus Glycoprotein Requires an Intact Receptor-Binding Domain and Can Be Blocked by GP1-Specific Antibodies. J. Virol. 2016, 90, 11075–11086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Yin, L.; Mei, S.; Li, J.; Xu, F.; Sun, H.; Liu, X.; Cen, S.; Liang, C.; Li, A.; et al. BST-2 restricts IAV release and is countered by the viral M2 protein. Biochem. J. 2017, 474, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Neil, S.J. The antiviral activities of tetherin. Curr. Top. Microbiol. Immunol. 2013, 371, 67–104. [Google Scholar] [PubMed]
- Xia, C.; Vijayan, M.; Pritzl, C.J.; Fuchs, S.Y.; McDermott, A.B.; Hahm, B. Hemagglutinin of Influenza A Virus Antagonizes Type I Interferon (IFN) Responses by Inducing Degradation of Type I IFN Receptor 1. J. Virol. 2015, 90, 2403–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, B.G.; Randall, R.E.; Ortin, J.; Jackson, D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 2008, 89, 2359–2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klemm, C.; Boergeling, Y.; Ludwig, S.; Ehrhardt, C. Immunomodulatory Nonstructural Proteins of Influenza A Viruses. Trends Microbiol. 2018, 26, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Huang, S.; Fu, C.; Wang, L.; Zheng, Y.; Zhou, P.; Li, S. Identification of the IFN-β response in H3N2 canine influenza virus infection. J. Gen. Virol. 2016, 97, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Yamamoto, S.P.; Misawa, N.; Yoshida, T.; Miyazawa, T.; Koyanagi, Y. Comparative study on the effect of human BST-2/Tetherin on HIV-1 release in cells of various species. Retrovirology 2009, 6, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupzig, S.; Korolchuk, V.; Rollason, R.; Sugden, A.; Wilde, A.; Banting, G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 2003, 4, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Rossman, J.S.; Lamb, R.A. Influenza virus assembly and budding. Virology 2011, 411, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, R.; Igarashi, M.; Takada, A. Influenza A Virus M2 Protein: Roles from Ingress to Egress. Int. J. Mol. Sci. 2017, 18, 2649. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wang, J.J.; Qi, M.; Yoon, J.J.; Chen, X.; Wen, X.; Hammonds, J.; Ding, L.; Spearman, P. Tetherin/BST-2 is essential for the formation of the intracellular virus-containing compartment in HIV-infected macrophages. Cell Host Microbe 2012, 12, 360–372. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Hao, X.; Zheng, Q.; Lin, X.; Zhang, X.; Zeng, W.; Ding, S.; Zhou, P.; Li, S. Canine Influenza Virus is Mildly Restricted by Canine Tetherin Protein. Viruses 2018, 10, 565. https://doi.org/10.3390/v10100565
Zheng Y, Hao X, Zheng Q, Lin X, Zhang X, Zeng W, Ding S, Zhou P, Li S. Canine Influenza Virus is Mildly Restricted by Canine Tetherin Protein. Viruses. 2018; 10(10):565. https://doi.org/10.3390/v10100565
Chicago/Turabian StyleZheng, Yun, Xiangqi Hao, Qingxu Zheng, Xi Lin, Xin Zhang, Weijie Zeng, Shiyue Ding, Pei Zhou, and Shoujun Li. 2018. "Canine Influenza Virus is Mildly Restricted by Canine Tetherin Protein" Viruses 10, no. 10: 565. https://doi.org/10.3390/v10100565
APA StyleZheng, Y., Hao, X., Zheng, Q., Lin, X., Zhang, X., Zeng, W., Ding, S., Zhou, P., & Li, S. (2018). Canine Influenza Virus is Mildly Restricted by Canine Tetherin Protein. Viruses, 10(10), 565. https://doi.org/10.3390/v10100565





