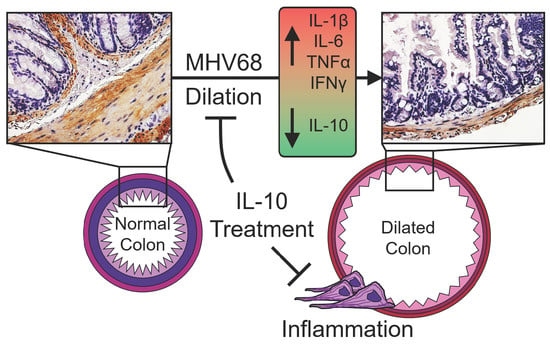
Mouse Gamma Herpesvirus MHV-68 Induces Severe Gastrointestinal (GI) Dilatation in Interferon Gamma Receptor-Deficient Mice (IFNγR−/−) That Is Blocked by Interleukin-10
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. MHV-68 Virus Passage and Preparation
2.3. MHV-68 Infection in Interferon Gamma Receptor-Deficient (IFNγR−/−) Mice
2.4. Histological and Morphometric Analysis
2.5. Western Blot
2.6. Cytokine Assays
2.7. Immunohistochemistry
2.8. Flow Cytometry
2.9. RT-PCR Analysis
2.10. Statistical Analysis
3. Results
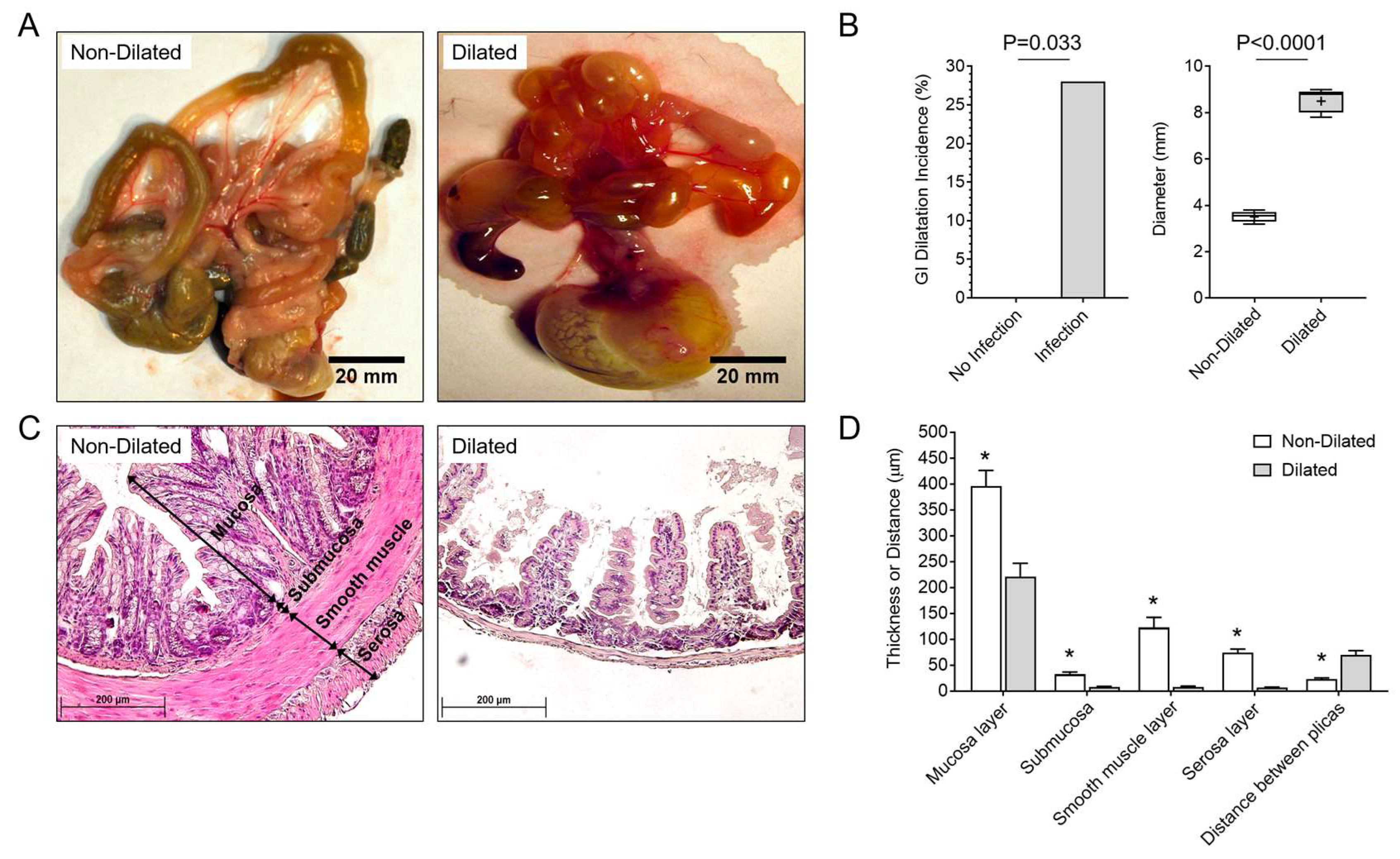
3.1. GI Dilatation in Herpesvirus (MHV-68)-Infected IFNγR−/− Mice
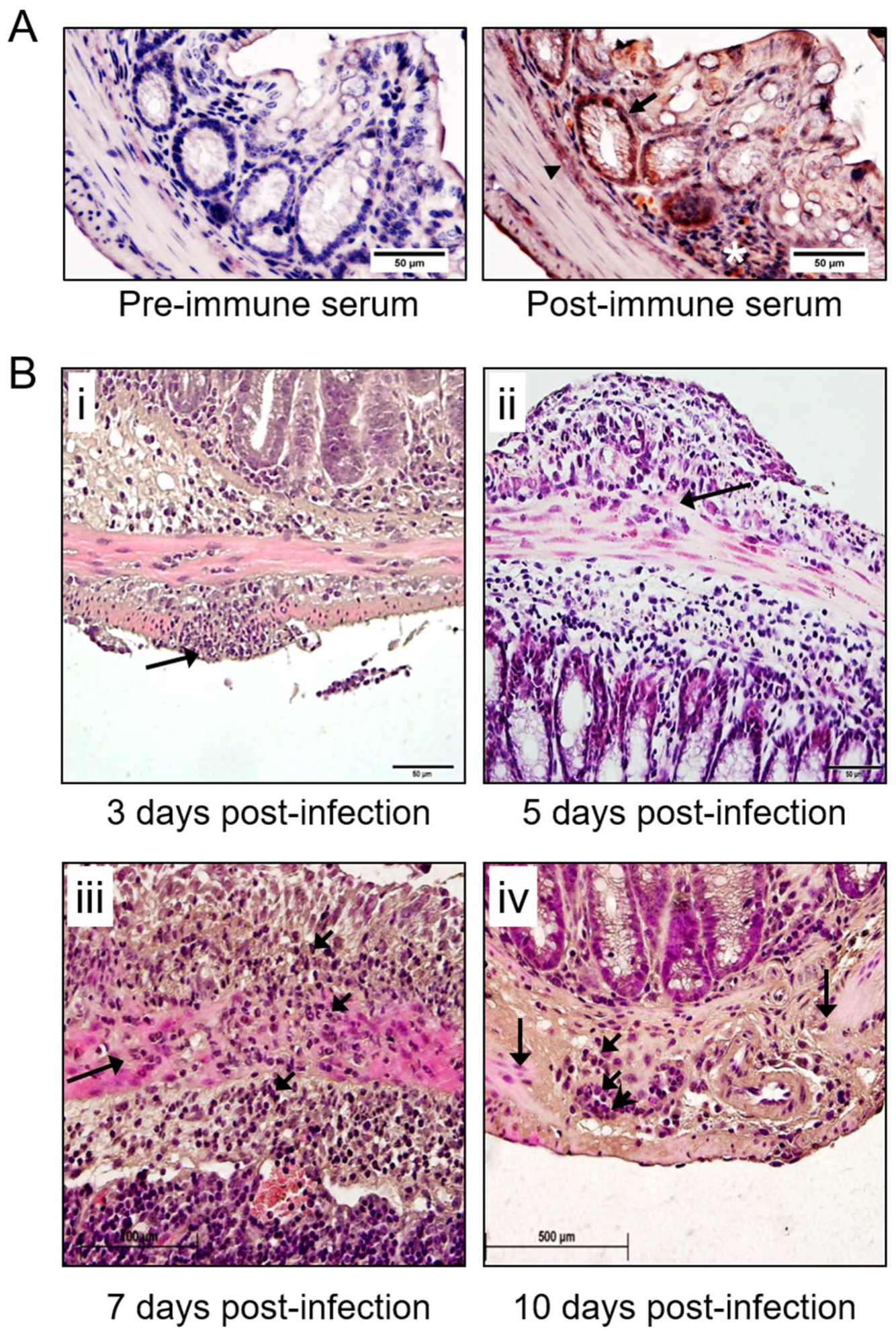
3.2. Detection of MHV-68 and Macrophage Infiltrates the Colon in Infected IFNγR−/− Mice
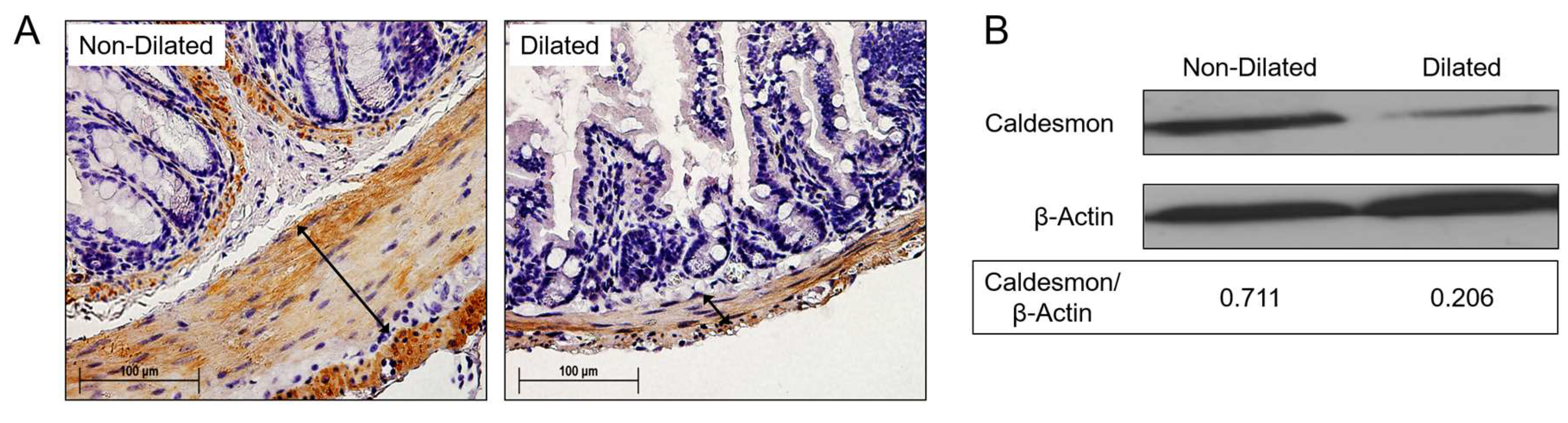
3.3. Degradation of SMC Is Associated with GI Tract Dilatation in MHV-68 Infections
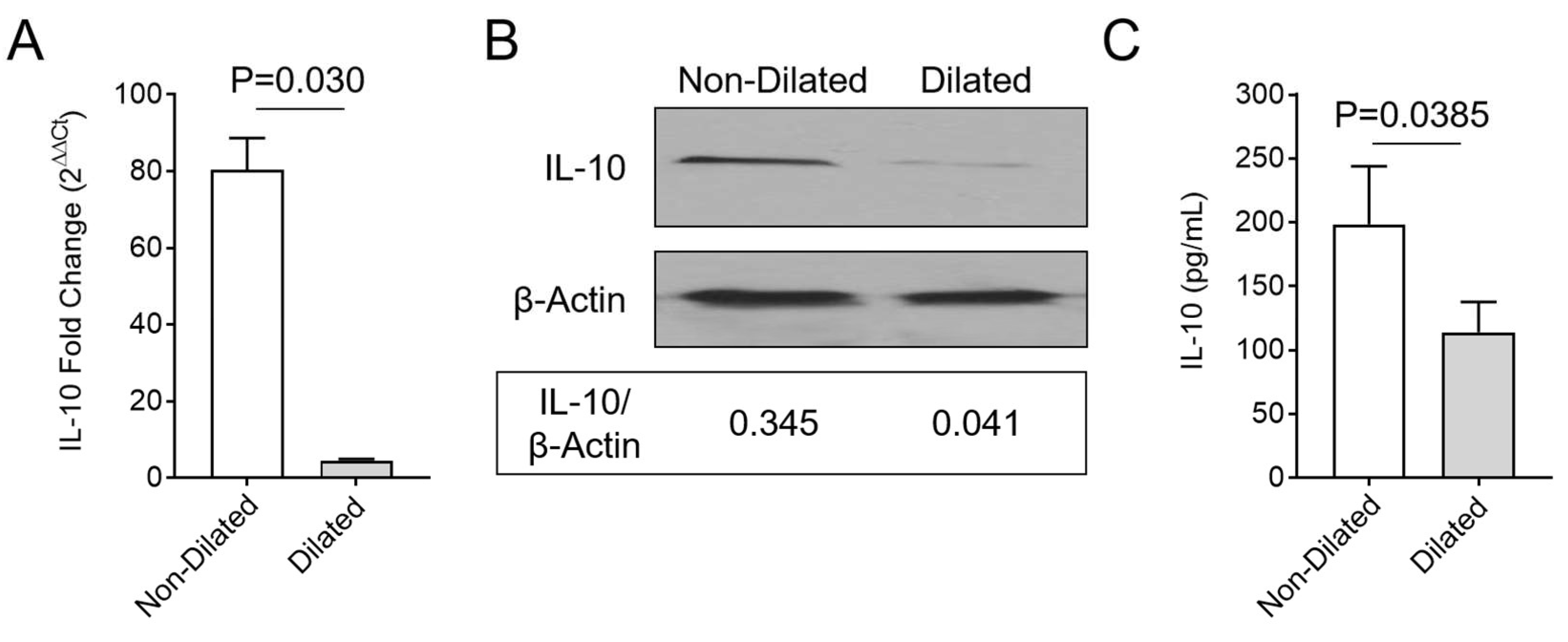
3.4. Enhanced Macrophage Invasion in Colon Samples is Accompanied by Decreased Systemic IL-10
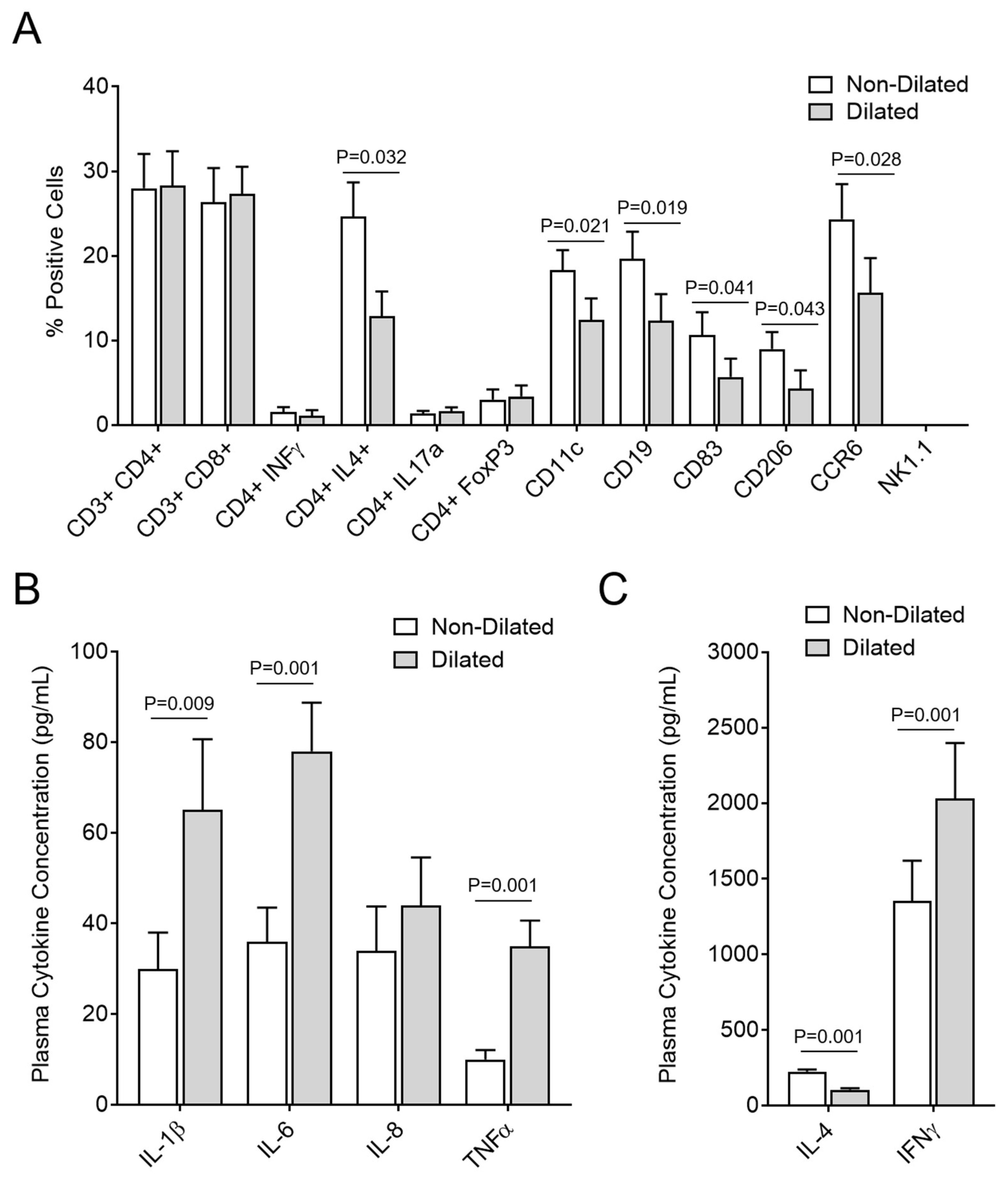
3.5. GI Dilatation Is Associated with Decreased Th2 Cells, B Cells, Monocytes, and Dendritic Cells in Spleen Cell Isolates and Significantly Modified Plasma Cytokines
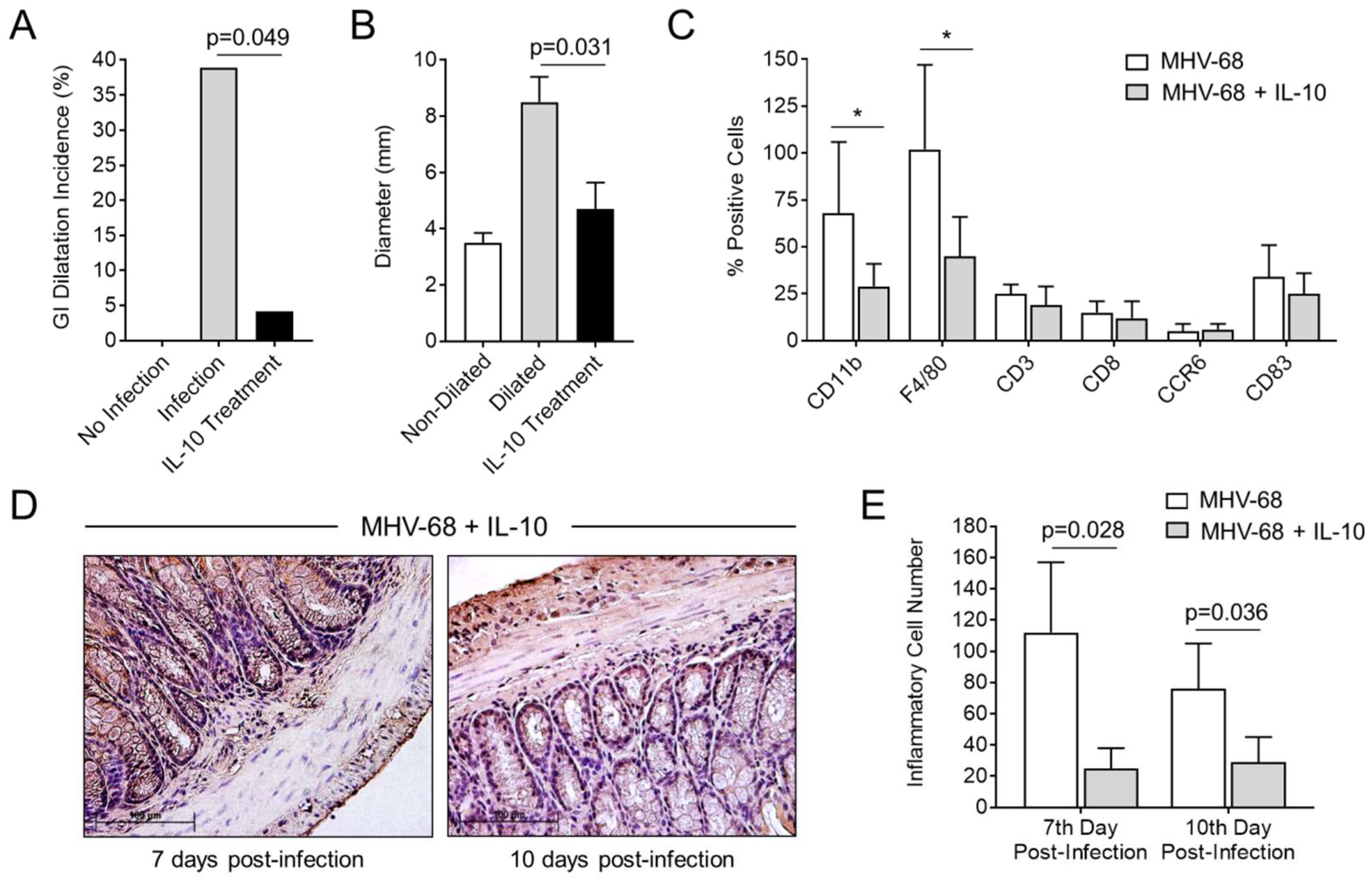
3.6. Treatment with IL-10 Significantly Reduces GI Inflammation and Dilatation in MHV-68-Infected IFNγR−/− Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tibbetts, S.A.; Loh, J.; van Berkel, V.; McClellan, J.S.; Jacoby, M.A.; Kapadia, S.B.; Speck, S.H.; Virgin, H.W. Establishment and Maintenance of Gammaherpesvirus Latency Are Independent of Infective Dose and Route of Infection. J. Virol. 2003, 77, 7696–7701. [Google Scholar] [CrossRef] [PubMed]
- Tripp, R.A.; Hamilton-Easton, A.M.; Cardin, R.D.; Nguyen, P.; Behm, F.G.; Woodland, D.L.; Doherty, P.C.; Blackman, M.A. Pathogenesis of an infectious mononucleosis-like disease induced by a murine gamma-herpesvirus: Role for a viral superantigen? J. Exp. Med. 1997, 185, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Canny, S.P.; Goel, G.; Reese, T.A.; Zhang, X.; Xavier, R.; Virgin, H.W. Latent Gammaherpesvirus 68 Infection Induces Distinct Transcriptional Changes in Different Organs. J. Virol. 2014, 88, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R.; Racca, F.; Paolucci, S.; Campanini, G.; Pozzi, L.; Betti, E.; Riboni, R.; Vanoli, A.; Baldanti, F.; Corazza, G.R. Human cytomegalovirus and Epstein-Barr virus infection in inflammatory bowel disease: Need for mucosal viral load measurement. World J. Gastroenterol. 2015, 21, 1915. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Pozzetto, B.; Roblin, X. Cytomegalovirus and ulcerative colitis: Place of antiviral therapy. World J. Gastroenterol. 2016, 22, 2030–2045. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.S.; Lee, W.Y.; Yu, W.L. Coexisting cytomegalovirus infection in immunocompetent patients with Clostridium difficile colitis. J. Microbiol. Immunol. Infect. 2016, 49, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Autenrieth, D.M.; Baumgart, D.C. Toxic megacolon. Inflamm. Bowel Dis. 2012, 18, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.L.; Valdes-Dapena, A.; Stein, G.N.; Bockus, H.L. Toxic megacolon in ulcerative colitis. Gastroenterology 1959, 37, 239–255. [Google Scholar] [PubMed]
- Caprilli, R.; Vernia, P.; Colaneri, O.; Frieri, G. Risk factors in toxic megacolon. Dig. Dis. Sci. 1980, 25, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Virgin, H.W.; Latreille, P.; Wamsley, P.; Hallsworth, K.; Weck, K.E.; Dal Canto, A.J.; Speck, S.H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 1997, 71, 5894–5904. [Google Scholar] [PubMed]
- Sunil-Chandra, N.P.; Arno, J.; Fazakerley, J.; Nash, A.A. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am. J. Pathol. 1994, 145, 818–826. [Google Scholar] [PubMed]
- Weck, K.E.; Dal Canto, A.J.; Gould, J.D.; O’Guin, A.K.; Roth, K.A.; Saffitz, J.E.; Speck, S.H.; Virgin, H.W. Murine gamma-herpesvirus 68 causes severe large-vessel arteritis in mice lacking interferon-gamma responsiveness: A new model for virus-induced vascular disease. Nat. Med. 1997, 3, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Dal Canto, A.J.; Swanson, P.E.; O’Guin, A.K.; Speck, S.H.; Virgin, H.W. IFN-γ action in the media of the great elastic arteries, a novel immunoprivileged site. J. Clin. Investig. 2001, 107. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, D.; Abbott, J.; Liu, L.; Bartee, M.Y.; Long, M.; Davids, J.; Williams, J.; Feldmann, H.; Strong, J.; et al. Myxomavirus-derived serpin prolongs survival and reduces inflammation and hemorrhage in an unrelated lethal mouse viral infection. Antimicrob. Agents Chemother. 2013, 57, 4114–4127. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.A.; Petty, C.C.; Bost, K.L. Infection with murine gammaherpesvirus 68 exacerbates inflammatory bowel disease in IL-10-deficient mice. Inflamm. Res. 2009, 58, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, P.G.; Efstathiou, S. Immune Mechanisms in Murine Gammaherpesvirus-68 Infection. Viral Immunol. 2005, 18, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Tarakanova, V.L.; Suarez, F.; Tibbetts, S.A.; Jacoby, M.A.; Weck, K.E.; Hess, J.L.; Speck, S.H.; Virgin, H.W. Murine Gammaherpesvirus 68 Infection Is Associated with Lymphoproliferative Disease and Lymphoma in BALB 2 Microglobulin-Deficient Mice. J. Virol. 2005, 79, 14668–14679. [Google Scholar] [CrossRef] [PubMed]
- Fekety, R.; Shah, A.B. Diagnosis and treatment of Clostridium difficile colitis. JAMA 1993, 269, 71–75. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A.; Barrat, F.J.; Castro, A.G.; Vicari, A.; Hawrylowicz, C. Strategies for use of IL-10 or its antagonists in human disease. Immunol. Rev. 2008, 223, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wong, K.; Ouyang, W.; Rutz, S. Targeting IL-10 Family Cytokines for the Treatment of Human Diseases. Cold Spring Harb. Perspect. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.H.; Papadakis, K.A. Inflammatory bowel disease: Updates on molecular targets for biologics. Gut Liver 2017, 11, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Sellon, R.K.; Tonkonogy, S.; Schultz, M.; Dieleman, L.A.; Grenther, W.; Balish, E.; Rennick, D.M.; Sartor, R.B. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 1998, 66, 5224–5231. [Google Scholar] [PubMed]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Schwager, K.; Kaspar, M.; Bootz, F.; Marcolongo, R.; Paresce, E.; Neri, D.; Trachsel, E. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res. Ther. 2009, 11. [Google Scholar] [CrossRef] [PubMed]
- Galeazzi, M.; Baldi, C.; Schwager, K.; Neri, D.; Giovannoni, L.; Selvi, E. A phase IB clinical trial with dekavil (F8-IL10), an anti-inflammatory immunocytokine for the treatment of rheumatoid arthritis, used in combination with methotrexate. Ann. Rheum. Dis. 2013, 71, 378. [Google Scholar] [CrossRef]
- Peacock, J.W.; Bost, K.L. Murine gammaherpesvirus-68-induced interleukin-10 increases viral burden, but limits virus-induced splenomegaly and leukocytosis. Immunology 2001, 104, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Pritchard, K.; Moody, C.J. Caldesmon: A calmodulin-binding actin-regulatory protein. Cell Calcium 1986, 7, 309–327. [Google Scholar] [CrossRef]
- Storsteen, K.A.; Kernohan, J.W.; Bargen, J.A. The myenteric plexus in chronic ulcerative colitis. Surg. Gynecol. Obstet. 1953, 97, 335–343. [Google Scholar] [PubMed]
- Collins, S.M.; Hurst, S.M.; Main, C.; Stanley, E.; Khan, I.; Blennerhassett, P.; Swain, M. Effect of inflammation of enteric nerves. Cytokine-induced changes in neurotransmitter content and release. Ann. N. Y. Acad. Sci. 1992, 664, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Geboes, K.; Collins, S. Structural abnormalities of the nervous system in Crohn’s disease and ulcerative colitis. Neurogastroenterol. Motil. 1998, 10, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.N.; Bazzocchi, G.; Chan, S.; Akashi, K.; Villanueva-Meyer, J.; Yanni, G.; Mena, I.; Snape, W.J. Colonic motility and transit in health and ulcerative colitis. Gastroenterology 1991, 101, 1289–1297. [Google Scholar] [CrossRef]
- Latella, G.; Papi, C. Crucial steps in the natural history of inflammatory bowel disease. World J. Gastroenterol. 2012, 18, 3790–3799. [Google Scholar] [CrossRef] [PubMed]
- Latella, G.; Vernia, P.; Viscido, A.; Frieri, G.; Cadau, G.; Cocco, A.; Cossu, A.; Tomei, E.; Caprilli, R. GI distension in severe ulcerative colitis. Am. J. Gastroenterol. 2002, 97, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Khoury-Hanold, W.; Yordy, B.; Kong, P.; Kong, Y.; Ge, W.; Szigeti-Buck, K.; Ralevski, A.; Horvath, T.L.; Iwasaki, A. Viral Spread to Enteric Neurons Links Genital HSV-1 Infection to Toxic Megacolon and Lethality. Cell Host Microbe 2016, 19, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Rangaswamy, U.S.; Speck, S.H. Murine Gammaherpesvirus M2 Protein Induction of IRF4 via the NFAT Pathway Leads to IL-10 Expression in B Cells. PLoS Pathog. 2014, 10. [Google Scholar] [CrossRef] [PubMed]

| Number IFNγR−/− Mice No MHV-68 Infection | Time to Follow Up–Number of MHV-68 Infected IFNγR−/− Mice | |||||
|---|---|---|---|---|---|---|
| Treatment | 15 days | 3 days | 5 days | 7 days | 10 days | 15 days |
| Saline | 15 | 6 | 6 | 6 | 6 | 25 |
| IL-10 | 0 | 6 | 6 | 6 | 6 | 25 |
| Total numbers MHV-68 infected mice | 0 | 12 | 12 | 12 | 12 | 50 |
| Total number mice ± MHV-68 infection | Total—15 mice− MHV-68 infection | Total—98 mice + MHV-68 infection | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Bartee, M.Y.; Yaron, J.R.; Liu, L.; Zhang, L.; Zheng, D.; Hogue, I.B.; Bullard, W.L.; Tibbetts, S.; Lucas, A.R. Mouse Gamma Herpesvirus MHV-68 Induces Severe Gastrointestinal (GI) Dilatation in Interferon Gamma Receptor-Deficient Mice (IFNγR−/−) That Is Blocked by Interleukin-10. Viruses 2018, 10, 518. https://doi.org/10.3390/v10100518
Chen H, Bartee MY, Yaron JR, Liu L, Zhang L, Zheng D, Hogue IB, Bullard WL, Tibbetts S, Lucas AR. Mouse Gamma Herpesvirus MHV-68 Induces Severe Gastrointestinal (GI) Dilatation in Interferon Gamma Receptor-Deficient Mice (IFNγR−/−) That Is Blocked by Interleukin-10. Viruses. 2018; 10(10):518. https://doi.org/10.3390/v10100518
Chicago/Turabian StyleChen, Hao, Mee Yong Bartee, Jordan R. Yaron, Liying Liu, Liqiang Zhang, Donghang Zheng, Ian B. Hogue, Whitney L. Bullard, Scott Tibbetts, and Alexandra R. Lucas. 2018. "Mouse Gamma Herpesvirus MHV-68 Induces Severe Gastrointestinal (GI) Dilatation in Interferon Gamma Receptor-Deficient Mice (IFNγR−/−) That Is Blocked by Interleukin-10" Viruses 10, no. 10: 518. https://doi.org/10.3390/v10100518
APA StyleChen, H., Bartee, M. Y., Yaron, J. R., Liu, L., Zhang, L., Zheng, D., Hogue, I. B., Bullard, W. L., Tibbetts, S., & Lucas, A. R. (2018). Mouse Gamma Herpesvirus MHV-68 Induces Severe Gastrointestinal (GI) Dilatation in Interferon Gamma Receptor-Deficient Mice (IFNγR−/−) That Is Blocked by Interleukin-10. Viruses, 10(10), 518. https://doi.org/10.3390/v10100518