A Built-In CpG Adjuvant in RSV F Protein DNA Vaccine Drives a Th1 Polarized and Enhanced Protective Immune Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Human CpG-Modified Plasmids
2.2. Virus Preparation
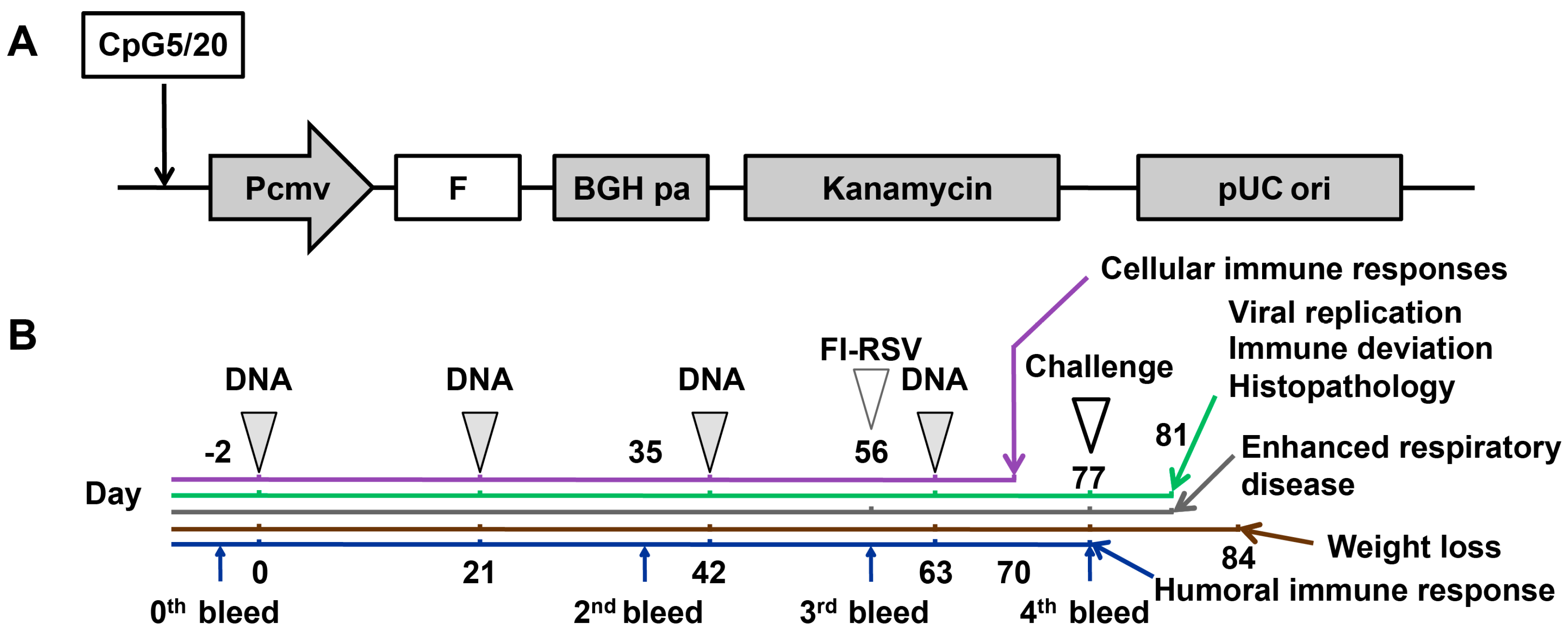
2.3. Vaccination and Challenge of Mice
2.4. Antibody Titer Measurement and Virus Neutralization Assay
2.5. Enzyme-Linked Immunospot (ELISPOT) Assays
2.6. Quantification of RSV Titers and Cytokines in Lungs with Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) and RT-PCR, Respectively
2.7. Lung Histopathology of the BALB/c Mice Challenged with RSV Following Immunization
2.8. Statistical Analyses
2.9. Ethical Approval
3. Results
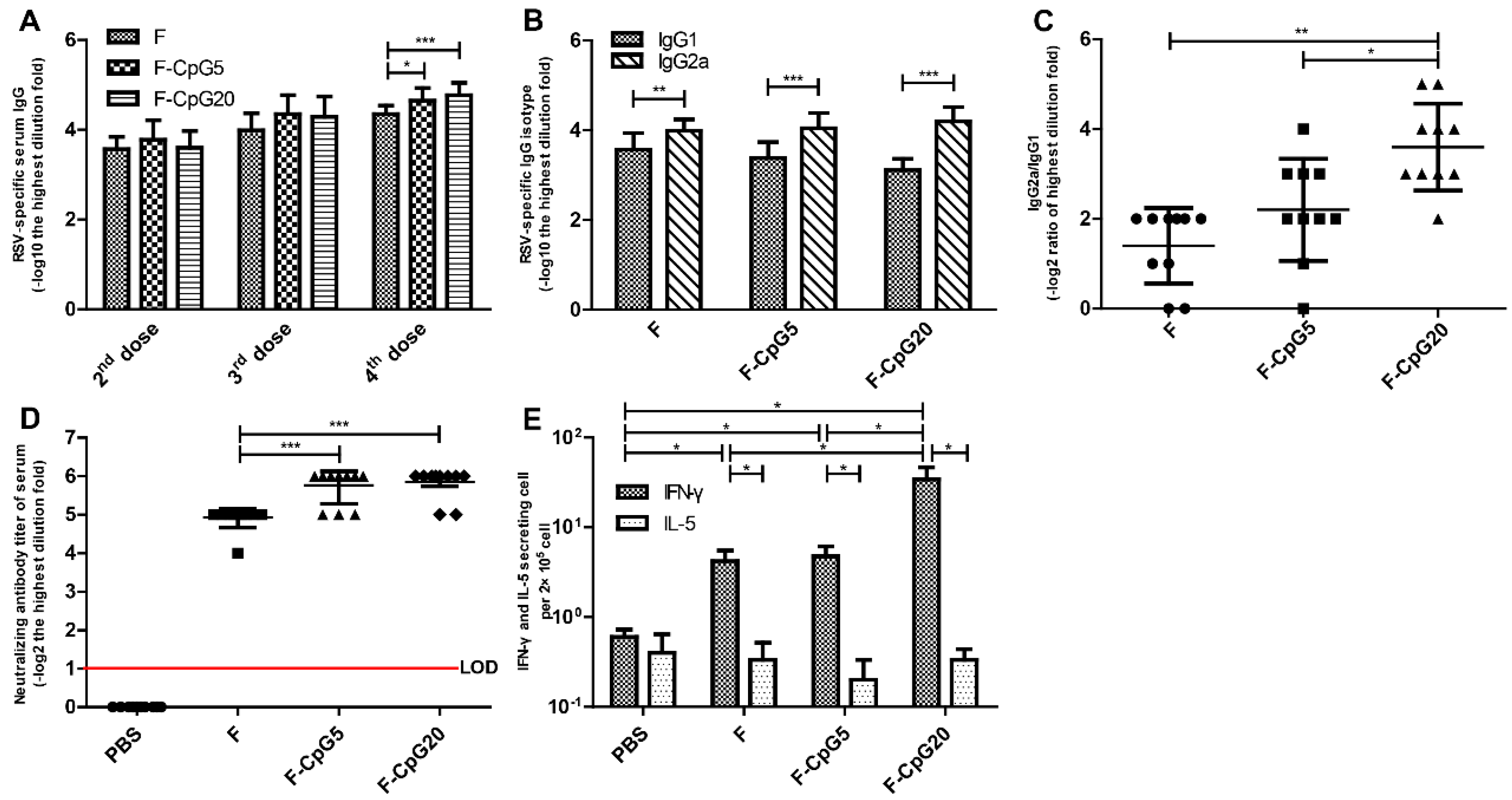
3.1. RSV-Specific Immune Responses
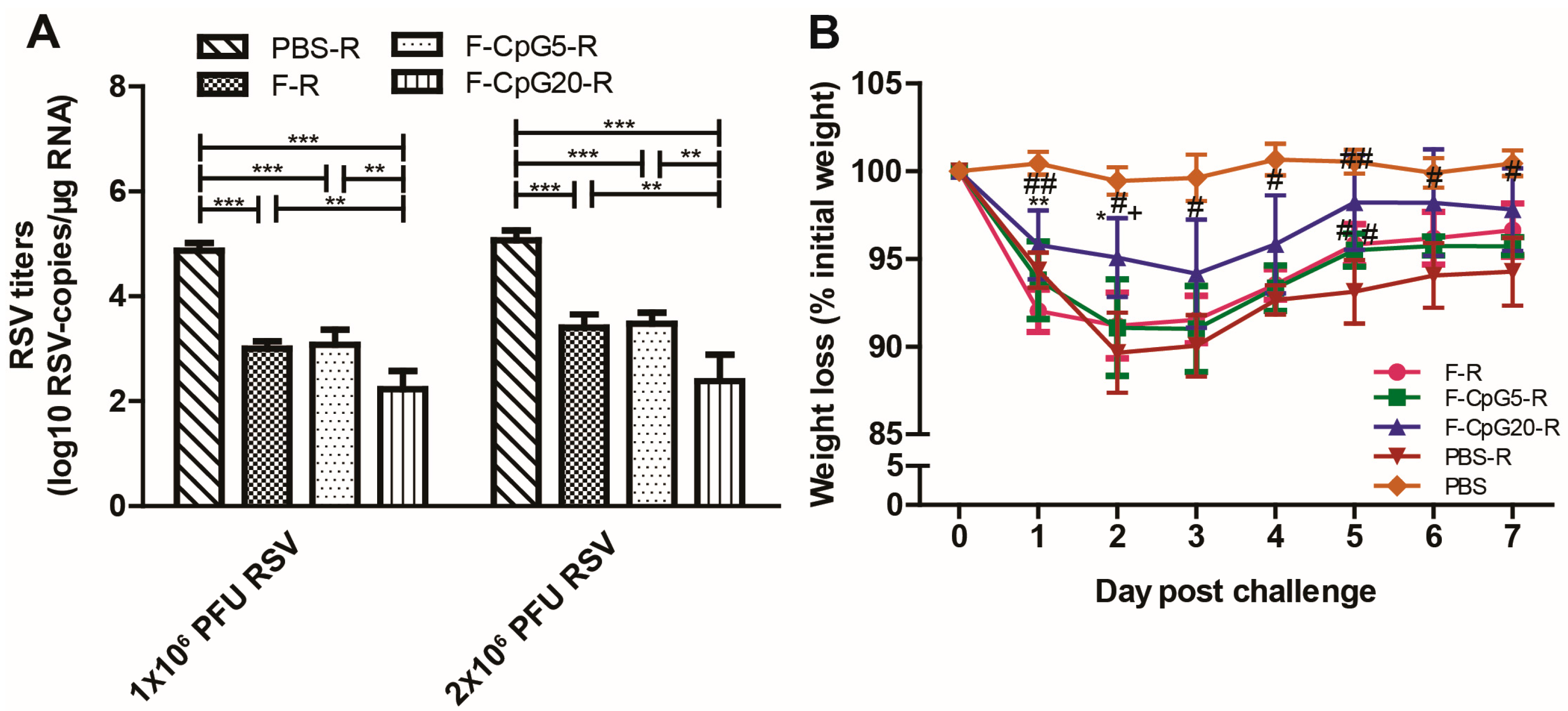
3.2. Protection against RSV Infection
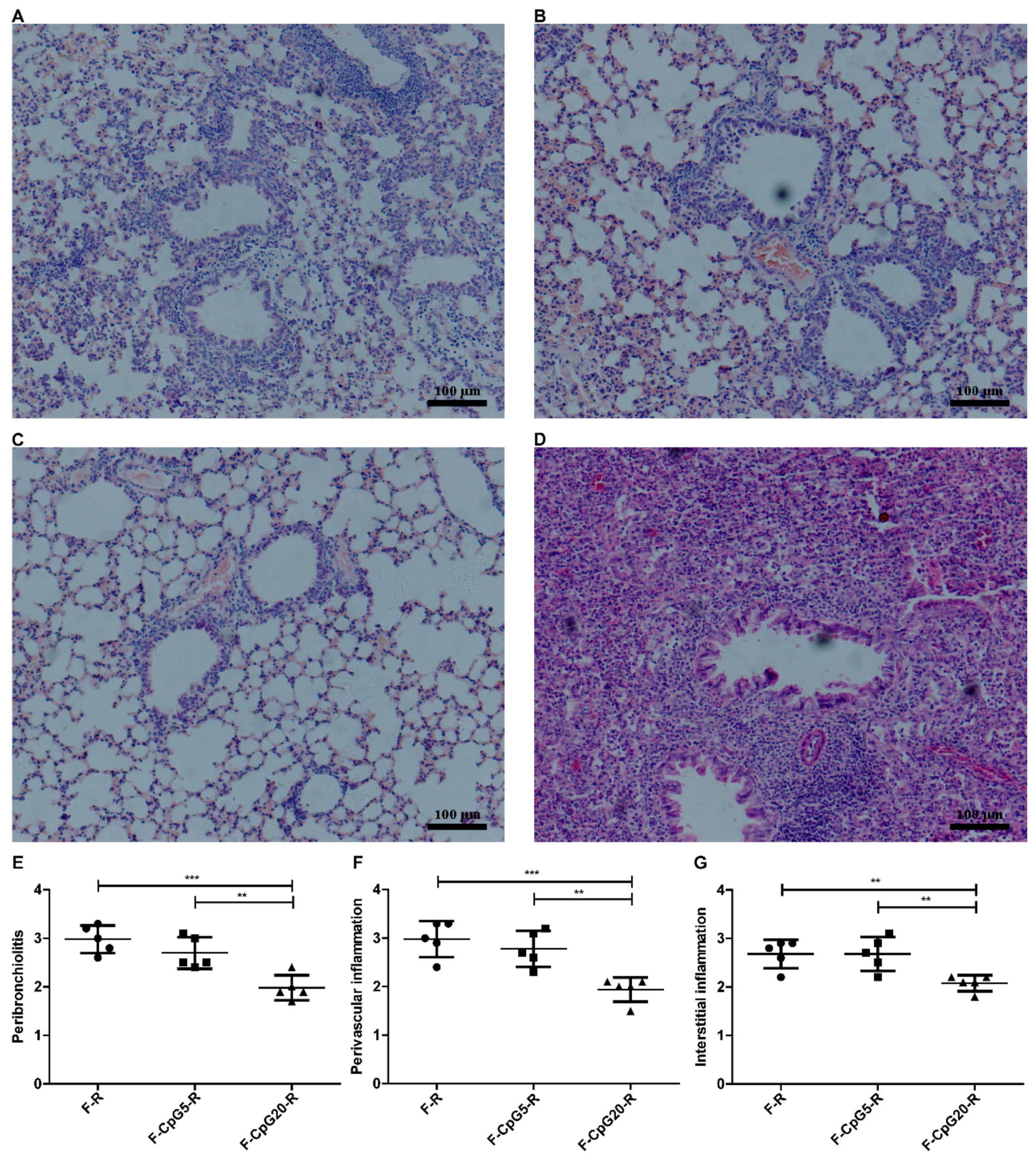
3.3. Pulmonary Pathology after RSV Challenge
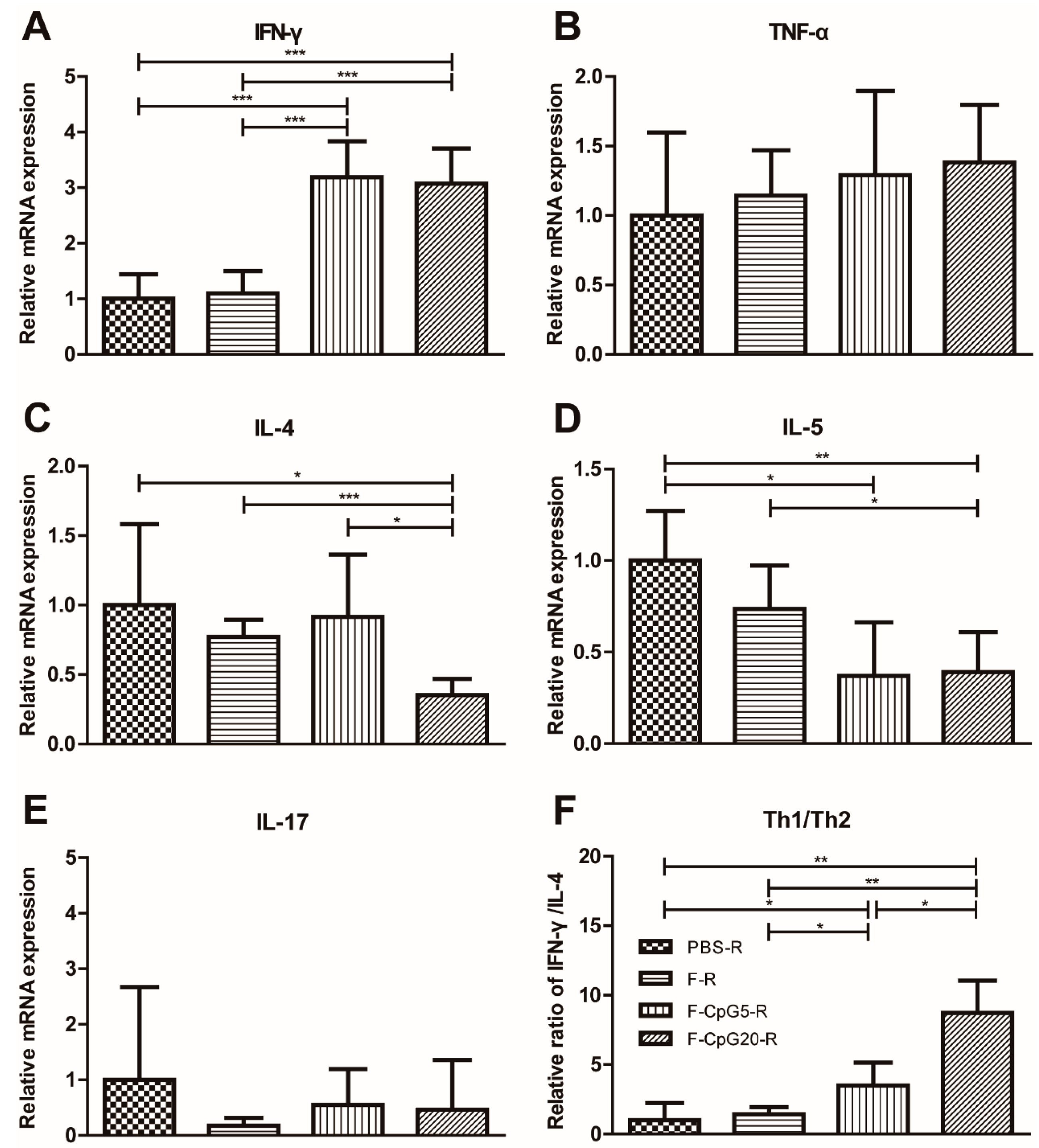
3.4. Pulmonary Cytokine mRNA Levels after Challenge
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shay, D.K.; Holman, R.C.; Roosevelt, G.E.; Clarke, M.J.; Anderson, L.J. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979–1997. J. Infect. Dis. 2001, 183, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Fearns, R.; Graham, B.S. Respiratory syncytial virus: Virology, reverse genetics, and pathogenesis of disease. Curr. Top. Microbiol. Immunol. 2013, 372, 3–38. [Google Scholar] [PubMed]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Blount, R.E., Jr.; Morris, J.A.; Savage, R.E. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc. Soc. Exp. Biol. Med. 1956, 92, 544–549. [Google Scholar] [PubMed]
- Chanock, R.M. Recovery of a new type of myxovirus from infants with croup. Ann. N. Y. Acad. Sci. 1957, 67, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S.; Anderson, L.J. Challenges and opportunities for respiratory syncytial virus vaccines. Curr. Top. Microbiol. Immunol. 2013, 372, 391–404. [Google Scholar] [PubMed]
- Chin, J.; Magoffin, R.L.; Shearer, L.A.; Schieble, J.H.; Lennette, E.H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 1969, 89, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Fulginiti, V.A.; Eller, J.J.; Sieber, O.F.; Joyner, J.W.; Minamitani, M.; Meiklejohn, G. Respiratory virus immunization: A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am. J. Epidemiol. 1969, 89, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Kapikian, A.Z.; Mitchell, R.H.; Chanock, R.M.; Shvedoff, R.A.; Stewart, C.E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 1969, 89, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.R.; Walsh, E.E. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J. Clin. Microbiol. 1988, 26, 1595–1597. [Google Scholar] [PubMed]
- Polack, F.P.; Teng, M.N.; Collins, P.L.; Prince, G.A.; Exner, M.; Regele, H.; Lirman, D.D.; Rabold, R.; Hoffman, S.J.; Karp, C.L.; et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J. Exp. Med. 2002, 196, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Killikelly, A.M.; Kanekiyo, M.; Graham, B.S. Pre-fusion F is absent on the surface of formalin-inactivated respiratory syncytial virus. Sci. Rep. 2016, 6, 34108. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S.; Henderson, G.S.; Tang, Y.W.; Lu, X.; Neuzil, K.M.; Colley, D.G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J. Immunol. 1993, 151, 2032–2040. [Google Scholar] [PubMed]
- Waris, M.E.; Tsou, C.; Erdman, D.D.; Zaki, S.R.; Anderson, L.J. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J. Virol. 1996, 70, 2852–2860. [Google Scholar] [PubMed]
- Knudson, C.J.; Hartwig, S.M.; Meyerholz, D.K.; Varga, S.M. RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS Pathog. 2015, 11, e1004757. [Google Scholar] [CrossRef] [PubMed]
- Hallak, L.K.; Collins, P.L.; Knudson, W.; Peeples, M.E. Iduronic acid-containing glycosaminoglycans on target cells are required for efficient respiratory syncytial virus infection. Virology 2000, 271, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Techaarpornkul, S.; Barretto, N.; Peeples, M.E. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. J. Virol. 2001, 75, 6825–6834. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Dormitzer, P.R.; Nokes, D.J.; Rappuoli, R.; Roca, A.; Graham, B.S. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine 2013, 31, B209–B215. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, J.B.; Wahren, B.; Liu, M.A. Gene-based vaccines: Recent technical and clinical advances. Trends Mol. Med. 2006, 12, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Mahendran, M.; Gupta, P.K.; Rai, A. DNA vaccines and their applications in veterinary practice: Current perspectives. Vet. Res. Commun. 2008, 32, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M.; Ye, A.K.; Conover, J.; Klinman, D.M. CpG motif in bacteria- DNA rapidly induce B-cell, T-cell, and natural-killer-cell cytokine production. Arthritis Rheum. 1995, 38, 271. [Google Scholar]
- Krieg, A.M.; Yi, A.K.; Matson, S.; Waldschmidt, T.J.; Bishop, G.A.; Teasdale, R.; Koretzky, G.A.; Klinman, D.M. CpG motif in bacterial-DNA trigger direct B-cell activation. Nature 1995, 374, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Gursel, M.; Verthelyi, D.; Gursel, I.; Ishii, K.J.; Klinman, D.M. Differential and competitive activation of human immune cells by distinct classes of CpG oligodeoxynucleotide. J. Leukocyte Biol. 2002, 71, 813–820. [Google Scholar] [PubMed]
- Hartmann, G.; Battiany, J.; Poeck, H.; Wagner, M.; Kerkmann, M.; Lubenow, N.; Rothenfusser, S.; Endres, S. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-α induction in plasmacytoid dendritic cells. Eur. J. Immunol. 2003, 33, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Klinman, D.M.; Currie, D.; Gursel, I.; Verthelyi, D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol. Rev. 2004, 199, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, X.; Yang, C.; Yu, S.; Xu, H. Three CpG oligodeoxynucleotide classes differentially enhance antigen-specific humoral and cellular immune responses in mice. Vaccine 2011, 29, 5778–5784. [Google Scholar] [CrossRef] [PubMed]
- Verthelyi, D.; Ishii, K.J.; Gursel, M.; Takeshita, F.; Klinman, D.M. Human peripheral blood cells differentially recognize and respond to two distinct CpG motif. J. Immunol. 2001, 166, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- Krug, A.; Rothenfusser, S.; Hornung, V.; Jahrsdorfer, B.; Blackwell, S.; Ballas, Z.K.; Endres, S.; Krieg, A.M.; Hartmann, G. Identification of CpG oligonucleotide sequences with high induction of IFN-α/β in plasmacytoid dendritic cells. Eur. J. Immunol. 2001, 31, 2154–2163. [Google Scholar] [CrossRef]
- Marshall, J.D.; Fearon, K.; Abbate, C.; Subramanian, S.; Yee, P.; Gregorio, J.; Coffman, R.L.; van Nest, G. Identification of a novel CpG DNA class and motif that optimally stimulate B cell and plasmacytoid dendritic cell functions. J. Leukocyte Biol. 2003, 73, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Samulowitz, U.; Weber, M.; Weeratna, R.; Uhlmann, E.; Noll, B.; Krieg, A.M.; Vollmer, J. A novel class of immune-stimulatory CpG oligodeoxynucleotides unifies high potency in type I interferon induction with preferred structural properties. Oligonucleotides 2010, 20, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, J.; Klinman, D.M. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 2014, 32, 6377–6389. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, J.; Weeratna, R.; Payette, P.; Jurk, M.; Schetter, C.; Laucht, M.; Wader, T.; Tluk, S.; Liu, M.; Davis, H.L.; et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur. J. Immunol. 2004, 34, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Roman, M.; Tighe, H.; Lee, D.; Corr, M.; Nguyen, M.D.; Silverman, G.J.; Lotz, M.; Carson, D.A.; Raz, E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science 1996, 273, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Klinman, D.M.; Yamshchikov, G.; Ishigatsubo, Y. Contribution of CpG motif to the immunogenicity of DNA vaccines. J. Immunol. 1997, 158, 3635–3639. [Google Scholar] [PubMed]
- Krieg, A.M.; Wu, T.; Weeratna, R.; Efler, S.M.; Love-Homan, L.; Yang, L.; Yi, A.-K.; Short, D.; Davis, H.L. Sequence motif in adenoviral DNA block immune activation by stimulatory CpG motif. Proc. Natl. Acad. Sci. USA 1998, 95, 12631–12636. [Google Scholar] [CrossRef] [PubMed]
- Coban, C.; Ishii, K.J.; Gursel, M.; Klinman, D.M.; Kumar, N. Effect of plasmid backbone modification by different human CpG motif on the immunogenicity of DNA vaccine vectors. J. Leukocyte Biol. 2005, 78, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Z.; Li, N.; Ma, Y.; Wang, S.; Yu, W.Y.; Sun, Z.W. Three types of human CpG motif differentially modulate and augment immunogenicity of nonviral and viral replicon DNA vaccines as built-in adjuvants. Eur. J. Immunol. 2013, 43, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; He, J.S.; Fu, Y.H.; Zheng, X.X.; Fang, X. Research on the methods for titrating respiratory syncytial virus. Chin. J. Exp. Clin. Virol. 2010, 24, 147–149. [Google Scholar]
- Jiao, Y.Y.; Fu, Y.H.; Yan, Y.F.; Hua, Y.; Ma, Y.; Zhang, X.J.; Song, J.D.; Peng, X.L.; Huang, J.; Hong, T.; et al. A single intranasal administration of virus-like particle vaccine induces an efficient protection for mice against human respiratory syncytial virus. Antivir. Res. 2017, 144, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Jiao, Y.Y.; He, J.S.; Giang, G.Y.; Zhang, W.; Yan, Y.F.; Ma, Y.; Hua, Y.; Zhang, Y.; Peng, X.-L.; et al. Sublingual administration of a helper-dependent adenoviral vector expressing the codon-optimized soluble fusion glycoprotein of human respiratory syncytial virus elicits protective immunity in mice. Antivir. Res. 2014, 105, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Van Elden, L.J.; van Loon, A.M.; van der Beek, A.; Hendriksen, K.A.; Hoepelman, A.I.; van Kraaij, M.G.; Schipper, P.; Nijhuis, M. Applicability of a real-time quantitative PCR assay for diagnosis of respiratory syncytial virus infection in immunocompromised adults. J. Clin. Microbiol. 2003, 41, 4378–4381. [Google Scholar] [CrossRef] [PubMed]
- Kohlmann, R.; Schwannecke, S.; Tippler, B.; Ternette, N.; Temchura, V.V.; Tenbusch, M.; Uberla, K.; Grunwald, T. Protective efficacy and immunogenicity of an adenoviral vector vaccine encoding the codon-optimized F protein of respiratory syncytial virus. J. Virol. 2009, 83, 12601–12610. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Choi, E.J.; Lee, K.S.; Kim, H.R.; Na, B.R.; Kwon, M.S.; Jeong, G.-S.; Choi, H.G.; Choi, E.Y.; Jun, C.-D. Oral administration of p-hydroxycinnamic acid attenuates atopic dermatitis by downregulating Th1 and Th2 cytokine production and keratinocyte activation. PLoS ONE 2016, 11, e0150952. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.; Jung, C.K.; Shackel, I.; Williams, P.M. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal. Biochem. 1999, 270, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Zakrajsek, B.A.; Mills, A.G.; Gorn, V.; Singer, M.J.; Reed, M.W. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: Comparison of endpoint and real-time methods. Anal. Biochem. 2000, 285, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sambhara, S.; Li, C.X.; Ewasyshyn, M.; Parrington, M.; Caterini, J.; James, O.; Cates, G.; Du, R.-P.; Klein, M. Protection against respiratory syncytial virus infection by DNA immunization. J. Exp. Med. 1998, 188, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sambhara, S.; Li, C.X.; Ettorre, L.; Switzer, I.; Cates, G.; James, O.; Parrington, M.; Oomen, R.; Du, R.-P.; et al. Plasmid DNA encoding the respiratory syncytial virus G protein is a promising vaccine candidate. Virology 2000, 269, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.R.F.; Schultheis, K.; Morrow, M.P.; Kraynyak, K.A.; McCoy, J.R.; Yim, K.C.; Muthumani, K.; Humeau, L.; Weiner, D.B.; Sardesai, N.Y.; et al. Development of an intradermal DNA vaccine delivery strategy to achieve single-dose immunity against respiratory syncytial virus. Vaccine 2017, 35, 2840–2847. [Google Scholar] [CrossRef] [PubMed]
- Hancock, G.E.; Heers, K.M.; Smith, J.D.; Scheuer, C.A.; Ibraghimov, A.R.; Pryharski, K.S. CpG containing oligodeoxynucleotides are potent adjuvants for parenteral vaccination with the fusion (F) protein of respiratory syncytial virus (RSV). Vaccine 2001, 19, 4874–4882. [Google Scholar] [CrossRef]
- Tayyari, F.; Sutton, T.C.; Manson, H.E.; Hegele, R.G. CpG-oligodeoxynucleotides inhibit RSV-enhanced allergic sensitisation in guinea pigs. Eur. Respir. J. 2005, 25, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Garlapati, S.; Garg, R.; Brownlie, R.; Latimer, L.; Simko, E.; Hancock, R.E.; Babiuk, L.A.; Gerdts, V.; Potter, A.; van Drunen Littel-van den Hurk, S. Enhanced immune responses and protection by vaccination with respiratory syncytial virus fusion protein formulated with CpG oligodeoxynucleotide and innate defense regulator peptide in polyphosphazene microparticles. Vaccine 2012, 30, 5206–5214. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Jiao, Y.-Y.; Yu, Y.-Z.; Jiang, N.; Hua, Y.; Zhang, X.-J.; Fu, Y.-H.; Peng, X.-L.; Zheng, Y.-P.; Anderson, L.J.; et al. A Built-In CpG Adjuvant in RSV F Protein DNA Vaccine Drives a Th1 Polarized and Enhanced Protective Immune Response. Viruses 2018, 10, 38. https://doi.org/10.3390/v10010038
Ma Y, Jiao Y-Y, Yu Y-Z, Jiang N, Hua Y, Zhang X-J, Fu Y-H, Peng X-L, Zheng Y-P, Anderson LJ, et al. A Built-In CpG Adjuvant in RSV F Protein DNA Vaccine Drives a Th1 Polarized and Enhanced Protective Immune Response. Viruses. 2018; 10(1):38. https://doi.org/10.3390/v10010038
Chicago/Turabian StyleMa, Yao, Yue-Ying Jiao, Yun-Zhou Yu, Nan Jiang, Ying Hua, Xiu-Juan Zhang, Yuan-Hui Fu, Xiang-Lei Peng, Yan-Peng Zheng, Larry J. Anderson, and et al. 2018. "A Built-In CpG Adjuvant in RSV F Protein DNA Vaccine Drives a Th1 Polarized and Enhanced Protective Immune Response" Viruses 10, no. 1: 38. https://doi.org/10.3390/v10010038
APA StyleMa, Y., Jiao, Y.-Y., Yu, Y.-Z., Jiang, N., Hua, Y., Zhang, X.-J., Fu, Y.-H., Peng, X.-L., Zheng, Y.-P., Anderson, L. J., & He, J.-S. (2018). A Built-In CpG Adjuvant in RSV F Protein DNA Vaccine Drives a Th1 Polarized and Enhanced Protective Immune Response. Viruses, 10(1), 38. https://doi.org/10.3390/v10010038




