The Influence of Individual-Specific Plant Parameters and Species Composition on the Allergenic Potential of Urban Green Spaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. IUGZA—Urban Green Zone Allergenicity Index
2.3. IISA—Index of Individual-Specific Allergenic Potential of Green Spaces
2.4. Parameters Used for IUGZA and IISA
2.5. Planting Scenarios
2.6. Shannon Index (HS)
3. Results
3.1. Plant Characteristics and Current Allergenic Potential of the Park
3.2. Planting Scenarios and Associated Biodiversity
4. Discussion
4.1. Comparison of Indices and Conceptual Remarks
4.2. Planting Scenarios and Recommendations for Plantings in Urban Green Areas
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bergmann, K.-C.; Heinrich, J.; Niemann, H. Current status of allergy prevalence in Germany: Position paper of the Environmental Medicine Commission of the Robert Koch Institute. Allergo J. Int. 2016, 25, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Bachert, C.; Bauer, C.-P.; Czech, W. Weißbuch Allergie in Deutschland, 3rd ed.; Springer Medizin: München, Germany, 2010; ISBN 978-3-89935-245-0. (In German) [Google Scholar]
- Langen, U.; Schmitz, R.; Steppuhn, H. Häufigkeit allergischer Erkrankungen in Deutschland: Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundh. Gesundheitsforsch. Gesundheitssch. 2013, 56, 698–706. (In German) [Google Scholar] [CrossRef] [PubMed]
- Bundesministerium für Umwelt, Naturschutz, Bau und Reaktorsicherheit. Grün in der Stadt—Für eine Lebenswerte Zukunft. Grünbuch Stadtgrün; BMUB: Berlin, Germany, 2015. (In German) [Google Scholar]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; ISBN 1-59726-040-1. [Google Scholar]
- Lyytimäki, J.; Sipilä, M. Hopping on one leg—The challenge of ecosystem disservices for urban green management. Urban For. Urban Green. 2009, 8, 309–315. [Google Scholar] [CrossRef]
- Baumschulen Gebr. van den Berk. Van den Berk über Bäume, 2nd ed.; Baumschule Van den Berk: Sint-Oedenrode, Germany, 2004; ISBN 90-807408-6-1. (In German) [Google Scholar]
- Seyfang, V. Studie zum Wissenschaftlichen Erkenntnisstand über das Allergiepotential von Pollenflug der Gehölze im Öffentlichen Grün der Städte und Gemeinden und Mögliche Minderungsstrategien; Hochschule Ostwestfalen-Lippe: Lemgo, Germany, 2008. (In German) [Google Scholar]
- Huntington, L. Das Gartenbuch für Allergiker. Die Schönsten Pflanzen, die Besten Arbeitsweisen; VGS: Köln, Germany, 1999; ISBN 3802513851. (In German) [Google Scholar]
- Ogren, T.L. Allergy-Free Gardening. The Revolutionary Guide to Healthy Landscaping; Ten Speed Press: Berkeley, CA, USA, 2000; ISBN 1580081665. [Google Scholar]
- Bergmann, K.-C.; Zuberbier, T.; Ausgustin, J.; Mücke, H.-G.; Straff, W. Klimawandel und Pollenallergie. Städte und Kommunen sollten bei der Bepflanzung des öffentlichen Raums Rücksicht auf Pollenallergiker nehmen. Allergo J. 2012, 21, 103–108. (In German) [Google Scholar] [CrossRef]
- Sandifer, P.A.; Sutton-Grier, A.E.; Ward, B.P. Exploring connections among nature, biodiversity, ecosystem services, and human health and well-being: Opportunities to enhance health and biodiversity conservation. Ecosyst. Serv. 2015, 12, 1–15. [Google Scholar] [CrossRef]
- Hanski, I.; von Hertzen, L.; Fyhrquist, N.; Koskinen, K.; Torppa, K.; Laatikainen, T.; Karisola, P.; Auvinen, P.; Paulin, L.; Mäkelä, M.J.; et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl. Acad. Sci. USA 2012, 109, 8334–8339. [Google Scholar] [CrossRef] [PubMed]
- Cariñanos, P.; Casares-Porcel, M. Urban green zones and related pollen allergy: A review. Some guidelines for designing spaces with low allergy impact. Landsc. Urban Plan. 2011, 101, 205–214. [Google Scholar] [CrossRef]
- Hruska, K. Assessment of urban allergophytes using an allergen index. Aerobiologia 2003, 19, 107–111. [Google Scholar] [CrossRef]
- Ciferri, E.; Torrisi, M.; Staffolani, L.; Hruska, K. Ecological study of the urban allergenic flora of central Italy. J. Mediter. Ecol. 2006, 7, 15–21. [Google Scholar]
- Cariñanos, P.; Casares-Porcel, M.; Quesada-Rubio, J.-M. Estimating the allergenic potential of urban green spaces: A case-study in Granada, Spain. Landsc. Urban Plan. 2014, 123, 134–144. [Google Scholar] [CrossRef]
- Friedman, J. The Ecology and Evolution of Wind Pollination. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2009. [Google Scholar]
- Givnish, T.J. Ecological constraints on the evolution of breeding systems in seed plants: Dioecy and dispersal in Gymnosperms. Evolution 1980, 34, 959. [Google Scholar] [CrossRef] [PubMed]
- Besl, M. Der Hofgarten vor der Sommerresidenz Eichstätt. Genese und Vergleiche. Master’s Thesis, Catholic University of Eichstätt-Ingolstadt, Eichstätt, Germany, 2011. (In German). [Google Scholar]
- Byng, J.W.; Chase, M.W.; Christenhusz, M.J.; Fay, M.F.; Judd, W.S.; Mabberley, D.J.; Sennikov, A.N.; Soltis, D.E.; Soltis, P.S.; Stevens, P.F.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Pütz, N. Studienhilfe Botanik; Vechtaer fachdidaktische Forschungen und Berichte No. 17; Institut für Didaktik der Naturwissenschaften, der Mathematik und des Sachunterrichts: Vechta, Germany, 2008. (In German) [Google Scholar]
- Umweltforschungszentrum Leipzig Halle. BiolFlor: Datenbank Biologisch-Ökologischer Merkmale der Flora von Deutschland. Available online: http://www2.ufz.de/biolflor/index.jsp (accessed on 20 February 2017). (In German).
- Gurk, C.; Hepp, C. Baumkunde.de: Online-Datenbank für Bäume und Sträucher. Available online: http://www.baumkunde.de/ (accessed on 20 February 2017). (In German).
- Jäger, E.J.; Müller, G.K.; Ritz, C.; Welk, E.; Wesche, K. Rothmaler—Exkursionsflora von Deutschland. Gefäßpflanzen: Atlasband, 11th ed.; Springer Spektrum: München, Germany, 2007. (In German) [Google Scholar]
- Stiftung Deutscher Polleninformationsdienst. Pollenflugkalender für Deutschland: Pollenflug Gesamtdeutscher Raum 2007–2011. Available online: http://www.pollenstiftung.de/pollenvorhersage/pollenflug-kalender/ (accessed on 7 March 2017). (In German).
- Mitchell, A. Die Wald- und Parkbäume Europas. Ein Bestimmungsbuch für Dendrologen und Naturfreunde, 2nd ed.; Parey: Hamburg, Germany, 1979; ISBN 3490059182. (In German) [Google Scholar]
- Bartels, H. Gehölzkunde; Ulmer: Stuttgart, Germany, 1993. (In German) [Google Scholar]
- Schütt, P.; Weisgerber, H.; Schuck, H.J.; Lang, U.M.; Stimm, B.; Roloff, A. Enzyklopädie der Laubbäume. Die Große Enzyklopädie mit über 800 Farbfotos unter Mitwirkung von 30 Experten; Nikol: Hamburg, Germany, 2006; ISBN 3937872396. (In German) [Google Scholar]
- Schütt, P.; Schuck, H.J.; Stimm, B. Lexikon der Bauch- und Strauchbaumarten. Das Standardwerk der Forstbotanik, 3rd ed.; Nikol: Hamburg, Germany, 2014. (In German) [Google Scholar]
- Stinglwagner, G.K.F.; Haseder, I.E.; Erlbeck, R. Das Kosmos Wald- und Forstlexikon, 2nd ed.; Kosmos: Stuttgart, Germany, 2005; ISBN 3440103757. (In German) [Google Scholar]
- Kunick, W. Biotopkartierung—Landschaftsökologische Grundlagen; Teil 3; Stadt Köln: Köln, Germany, 1983. (In German) [Google Scholar]
- Wittig, R. Siedlungsvegetation. 40 Tabellen; Ulmer: Stuttgart (Hohenheim), Germany, 2002; ISBN 9783800136933. (In German) [Google Scholar]
- Roloff, A.; Bonn, S.; Gillner, S. Konsequenzen des Klimawandels—Vorstellung der Klima-Arten-Matrix (KLAM) zur Auswahl geeigneter Baumarten. Stadt+Grün 2009, 57, 53–60. [Google Scholar]
- Munk, K. Ökologie—Evolution; Thieme: Stuttgart, Germany, 2009; ISBN 3131448814. (In German) [Google Scholar]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tuscon, AZ, USA, 2010. [Google Scholar]
- Maya Manzano, J.M.; Tormo Molina, R.; Fernández Rodríguez, S.; Silva Palacios, I.; Gonzalo Garijo, Á. Distribution of ornamental urban trees and their influence on airborne pollen in the SW of Iberian Peninsula. Landsc. Urban Plan. 2017, 157, 434–446. [Google Scholar] [CrossRef]
- Cariñanos, P.; Adinolfi, C.; de Guardia, C.L.D.; de Linares, C.; Casares-Porcel, M. Characterization of allergen emission sources in urban areas. J. Environ. Qual. 2016, 45, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Burbach, G.J.; Heinzerling, L.M.; Edenharter, G.; Bachert, C.; Bindslev-Jensen, C.; Bonini, S.; Bousquet, J.; Bousquet-Rouanet, L.; Bousquet, P.J.; Bresciani, M.; et al. GA(2)LEN skin test study II: Clinical relevance of inhalant allergen sensitizations in Europe. Allergy 2009, 64, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Stanley, R.G.; Linskens, H.F. Pollen. Biologie, Biochemie, Gewinnung und Verwendung, Lizenzausg; Freund: Greifenberg, Germany, 1985; ISBN 9783924733001. [Google Scholar]
- Makra, L.; Matyasovszky, I.; Tusnády, G.; Wang, Y.; Csépe, Z.; Bozóki, Z.; Nyúl, L.G.; Erostyák, J.; Bodnár, K.; Sümeghy, Z.; et al. Biogeographical estimates of allergenic pollen transport over regional scales: Common ragweed and Szeged, Hungary as a test case. Agric. For. Meteorol. 2016, 221, 94–110. [Google Scholar] [CrossRef]
- Emberlin, J.; Norris-Hill, J. Spatial variation of pollen deposition in North London. Grana 1991, 30, 190–195. [Google Scholar] [CrossRef]
- Jochner, S.C.; Beck, I.; Behrendt, H.; Traidl-Hoffmann, C.; Menzel, A. Effects of extreme spring temperatures on urban phenology and pollen production: A case study in Munich and Ingolstadt. Clim. Res. 2011, 49, 101–112. [Google Scholar] [CrossRef]
- Vara, A.; Fernández-González, M.; Aira, M.J.; Rodríguez-Rajo, F.J. Fraxinus pollen and allergen concentrations in Ourense (South-western Europe). Environ. Res. 2016, 147, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Chiesura, A. The role of urban parks for the sustainable city. Landsc. Urban Plan. 2004, 68, 129–138. [Google Scholar] [CrossRef]
- García, M.; Moneo, I.; Audicana, M.T.; del Pozo, M.D.; Muñoz, D.; Fernández, E.; Díez, J.; Etxenagusia, M.A.; Ansotegui, I.J.; Fernández de Corres, L. The use of IgE immunoblotting as a diagnostic tool in Anisakis simplex allergy. J. Allergy Clin. Immunol. 1997, 99, 497–501. [Google Scholar] [CrossRef]
- Trigo, I.F.; Davies, T.D.; Bigg, G.R. Objective climatology of cyclones in the Mediterranean region. J. Clim. 1999, 12, 1685–1696. [Google Scholar] [CrossRef]
- Beggs, P.J. Impacts of climate change on aeroallergens: Past and future. Clin. Exp. Allergy 2004, 34, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
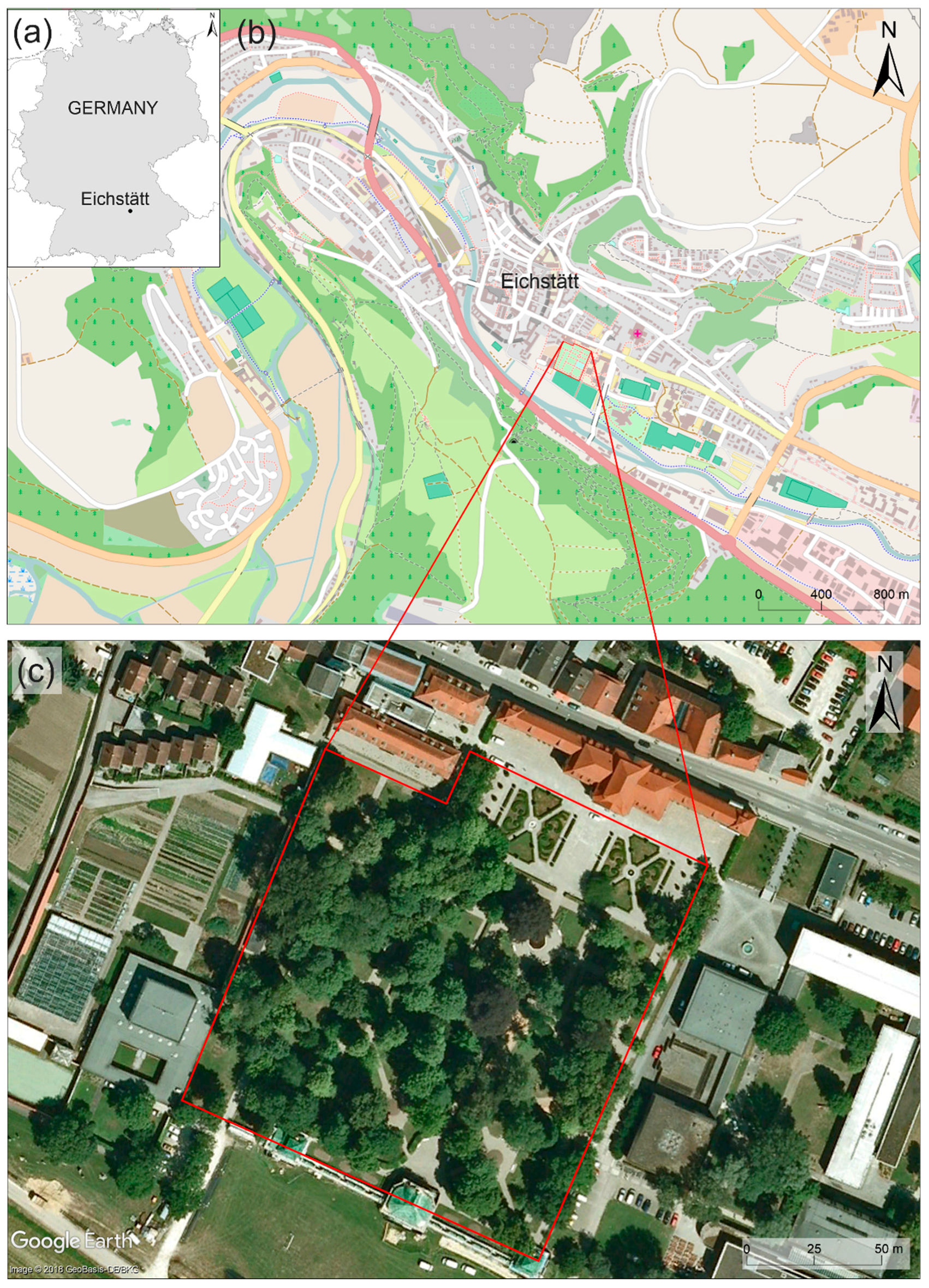
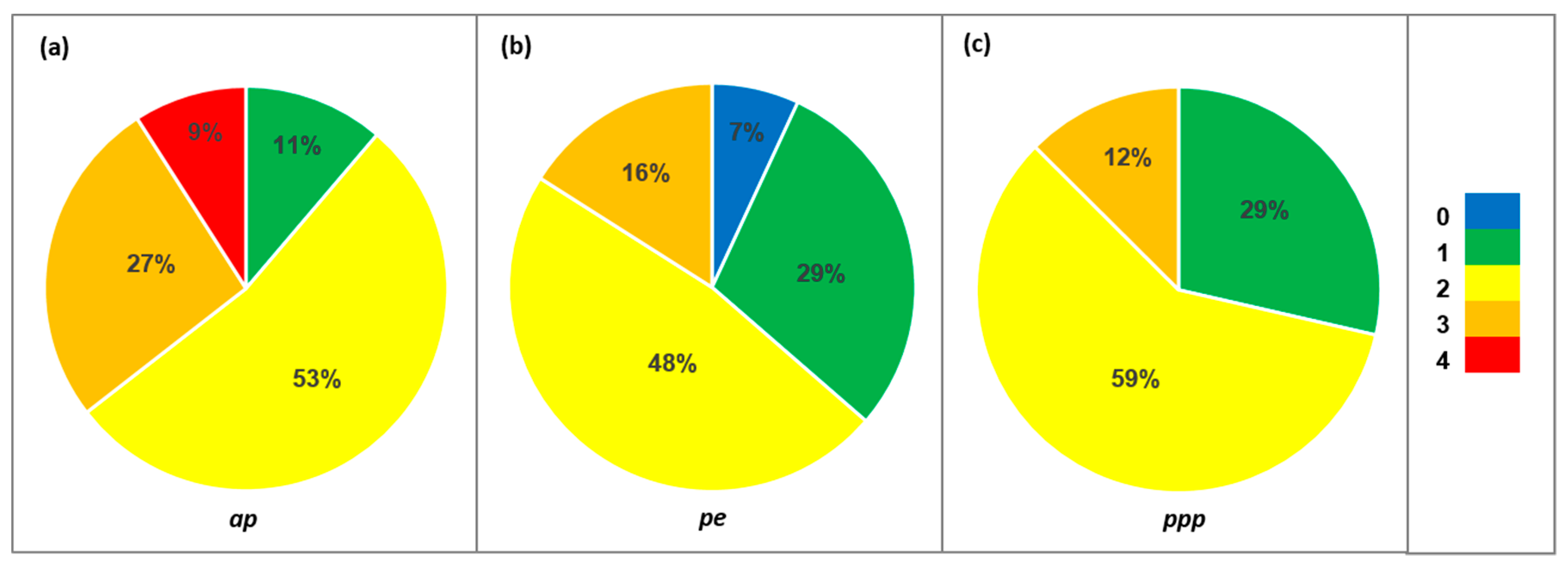
| Species | N | Family | ap | pe | ppp | Species | N | Family | ap | pe | ppp |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acer griseum (Franch.) Pax 1902 | 1 | Sapindaceae | 2 | 2 | 1 | Liquidambar styraciflua L. | 1 | Altingiaceae | 2 | 3 | 1 |
| Acer monspessulanum L. | 1 | Sapindaceae | 2 | 1 | 2 | Liriodendron tulipifera L. | 1 | Magnoliaceae | 1 | 1 | 2 |
| Acer negundo L. | 1 | Sapindaceae | 2 | 2 | 1 | Magnolia × soulangeana Soul.-Bod. | 3 | Magnoliaceae | 2 | 1 | 2 |
| Acer pensylvanicum L. 1753 | 1 | Sapindaceae | 2 | 2 | 1 | Magnolia kobus DC. | 1 | Magnoliaceae | 2 | 1 | 2 |
| Acer platanoides L. | 19 | Sapindaceae | 3 | 2 | 2 | Magnolia stellata (Siebold & Zucc.) Maxim. | 3 | Magnoliaceae | 2 | 1 | 2 |
| Acer pseudoplatanus L. 1753 | 24 | Sapindaceae | 3 | 2 | 1 | Morus alba L. 1753 | 1 | Moraceae | 2 | 1 | 1 |
| Acer rubrum L. 1753 | 2 | Sapindaceae | 1 | 2 | 2 | Nothofagus Antarctica (G. Forster) Oerst. | 1 | Nothofagaceae | 4 | 3 | 2 |
| Aesculus × carnea Zeyh. | 2 | Sapindaceae | 2 | 2 | 2 | Ostrya carpinifolia Scop. | 1 | Betulaceae | 4 | 3 | 3 |
| Aesculus hippocastanum L. | 18 | Sapindaceae | 2 | 2 | 2 | Paeonia × suffruticosa Andrews | 1 | Paeoniaceae | 1 | 1 | 2 |
| Ailanthus altissima (Mill.) Swingle | 1 | Simaroubaceae | 3 | 2 | 1 | Paulownia tomentosa (Thunb.) Steud. | 1 | Paulowniaceae | 2 | 1 | 2 |
| Berberis vulgaris L. | 1 | Berberidaceae | 1 | 1 | 3 | Philadelphus coronarius L. | 4 | Hydrangeaceae | 1 | 1 | 3 |
| Betula pendula Roth | 2 | Betulaceae | 4 | 3 | 2 | Picea omorika (Pančić) Purk. | 1 | Pinaceae | 1 | 3 | 1 |
| Buxus sempervirens L. | 24 | Buxaceae | 2 | 1 | 2 | Picea pungens Engelm. | 1 | Pinaceae | 1 | 3 | 3 |
| Carpinus betulus L. | 1 | Betulaceae | 4 | 3 | 2 | Platanus × hispanica (Aiton) Willd. | 1 | Platanaceae | 3 | 3 | 1 |
| Castanea sativa (Mill.) | 2 | Fagaceae | 2 | 3 | 1 | Potentilla fruticosa (L.) Rydb. | 1 | Rosaceae | 1 | 1 | 3 |
| Catalpa bignonioides Walter | 1 | Bignoniaceae | 3 | 1 | 2 | Prunus padus L. | 2 | Rosaceae | 2 | 1 | 2 |
| Celtis australis L. | 1 | Cannabaceae | 3 | 3 | 1 | Prunus sargentii ‘Accolade’ Rehder | 3 | Rosaceae | 2 | 1 | 2 |
| Cercidiphyllum japonicum Siebold & Zucc. | 1 | Cercidiphyllaceae | 2 | 1 | 2 | Prunus serrulata ‘Kanzan’ LINDL. | 1 | Rosaceae | 2 | 1 | 2 |
| Cercis siliquastrum L. | 1 | Fabaceae | 2 | 1 | 3 | Prunus tomentosa Thunb. | 1 | Rosaceae | 1 | 1 | 2 |
| Chaenomeles japonica (Thunb.) Lindl. ex Spach | 1 | Rosaceae | 1 | 1 | 2 | Pterocarya fraxinifolia (Lam.) Spach | 1 | Juglandaceae | 2 | 3 | 2 |
| Chamaecyparis lawsoniana (A. Murray) Parl. | 1 | Cupressaceae | 3 | 3 | 2 | Quercus petraea (Matt.) Liebl. | 1 | Fagaceae | 4 | 3 | 1 |
| Chamaecyparis nootkatensis D.Don 1824 | 1 | Cupressaceae | 3 | 3 | 2 | Quercus robur L. | 4 | Fagaceae | 4 | 3 | 1 |
| Cornus mas L. | 4 | Cornaceae | 2 | 1 | 3 | Ribes alpinum L. | 2 | Grossulariaceae | 1 | 0 | 2 |
| Corylus avellana L. | 1 | Betulaceae | 4 | 3 | 3 | Sequoiadendron giganteum (Lindl.) J.Buchh. | 2 | Cupressaceae | 2 | 3 | 2 |
| Corylus colurna L. | 1 | Betulaceae | 4 | 3 | 2 | Sorbus aria (L.) Crantz | 1 | Rosaceae | 1 | 1 | 1 |
| Crataegus monogyna Jacq. | 1 | Rosaceae | 1 | 1 | 2 | Sorbus aucuparia L. | 2 | Rosaceae | 1 | 1 | 2 |
| Deutzia scabra Thunb | 1 | Hydrangeaceae | 1 | 1 | 2 | Sorbus domestica L. | 1 | Rosaceae | 1 | 1 | 1 |
| Fagus sylvatica L. | 3 | Fagaceae | 4 | 3 | 2 | Sorbus torminalis (L.) Crantz | 1 | Rosaceae | 1 | 1 | 2 |
| Fraxinus excelsior L. | 6 | Oleaceae | 4 | 3 | 2 | Spiraea x arguta Zabel | 1 | Rosaceae | 2 | 1 | 2 |
| Ginkgo biloba L. | 3 | Ginkgoaceae | 2 | 3 | 1 | Styphnolobium japonicum (L.) Schott | 1 | Fabaceae | 2 | 2 | 2 |
| Gleditsia triacanthos L. | 1 | Fabaceae | 1 | 0 | 2 | Taxus baccata L. | 12 | Taxaceae | 3 | 0 | 3 |
| Hedera helix ‘Arborescens‘ L. | 2 | Araliaceae | 2 | 1 | 3 | Tilia cordata Mill. | 15 | Malvaceae | 2 | 2 | 2 |
| Ilex aquifolium L. | 1 | Aquifoliaceae | 2 | 0 | 2 | Tilia platyphyllos Scop. | 21 | Malvaceae | 2 | 2 | 1 |
| Kolkwitzia amabilis Graebn. Christenh. | 1 | Caprifoliaceae | 1 | 1 | 2 | Tilia tomentosa Moench | 4 | Malvaceae | 2 | 2 | 2 |
| Larix decidua (Mill.) | 1 | Pinaceae | 1 | 3 | 3 | Sum | 231 |
| Parameters | Values for IUGZA and IISA | |
|---|---|---|
| Allergenic potential (ap) | 0 = non-allergenic (OPALS 1) | |
| 1 = low allergenicity (OPALS 2–4) | ||
| 2 = moderate allergenicity (OPALS 5–7) | ||
| 3 = high allergenicity (OPALS 8–10) | ||
| 4 = main local allergens | ||
| Type of pollen emissions (pe) | 0 = only female-sex individuals | |
| 1 = entomophilous | ||
| 2 = ampiphilous | ||
| 3 = anemophilous | ||
| Principal pollination period (ppp) | 1 = 1–4 weeks | |
| 2 = 5–8 weeks | ||
| 3 ≥ 9 weeks | ||
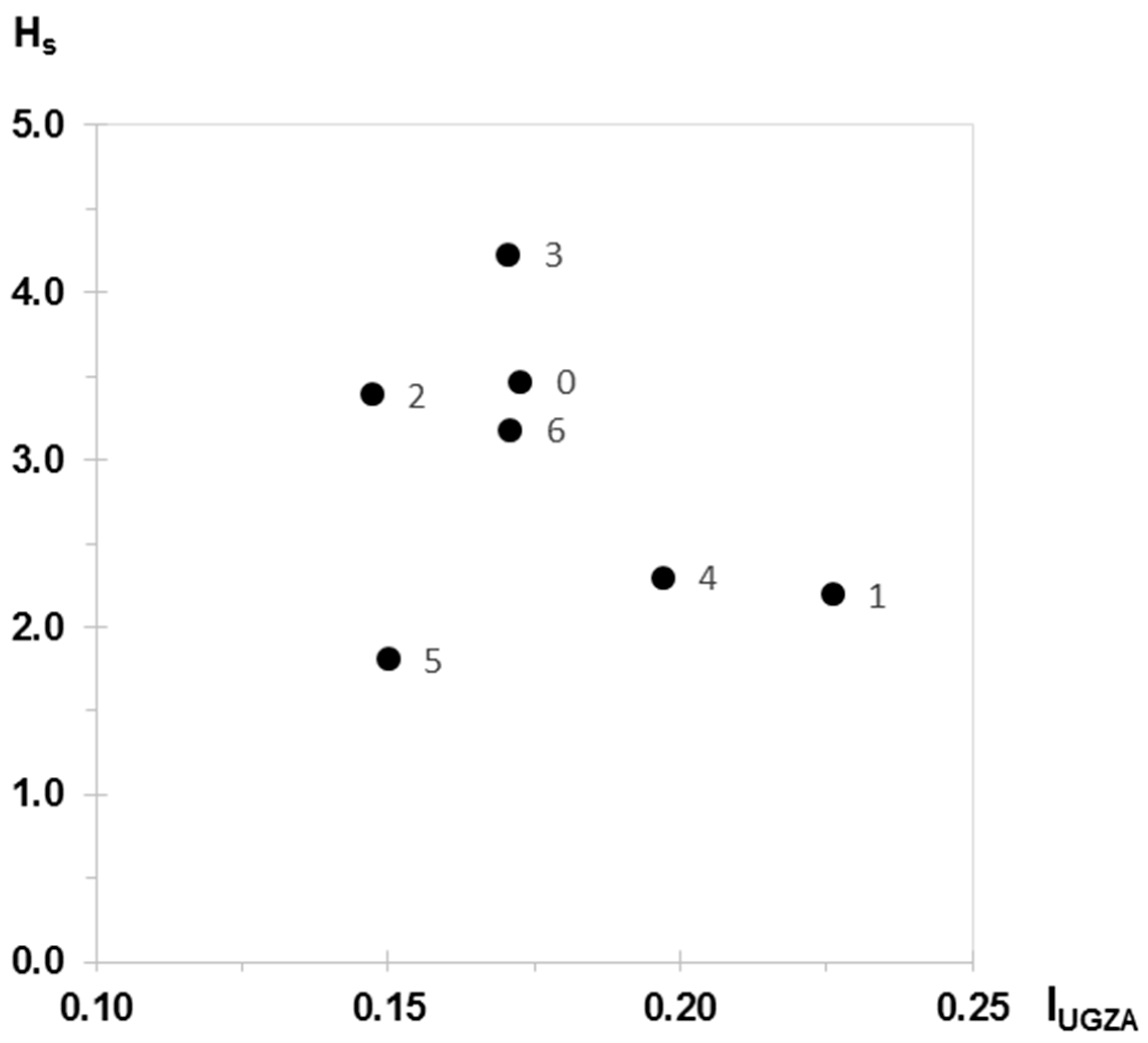
| IUGZA | IISA | |
| Crown height (H) | Mean height attained at reproductive maturity: 2, 6, 10, 14 m or exceptionally 18 m | Individual-specific measurements [m] |
| Plant surface (S) | Small-diameter: <4 m, medium-diameter: 4–6 m, large-diameter: >6 m | Individual-specific measurements using 4 radii [m] |
| IUGZA | Hs | |
|---|---|---|
| Current state (0) | 0.173 | 3.47 |
| Scenario 1 | 0.226 | 2.20 |
| Scenario 2 | 0.147 | 3.39 |
| Scenario 3 | 0.170 | 4.23 |
| Scenario 4 | 0.197 | 2.30 |
| Scenario 5 | 0.150 | 1.81 |
| Scenario 6 | 0.171 | 3.18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jochner-Oette, S.; Stitz, T.; Jetschni, J.; Cariñanos, P. The Influence of Individual-Specific Plant Parameters and Species Composition on the Allergenic Potential of Urban Green Spaces. Forests 2018, 9, 284. https://doi.org/10.3390/f9060284
Jochner-Oette S, Stitz T, Jetschni J, Cariñanos P. The Influence of Individual-Specific Plant Parameters and Species Composition on the Allergenic Potential of Urban Green Spaces. Forests. 2018; 9(6):284. https://doi.org/10.3390/f9060284
Chicago/Turabian StyleJochner-Oette, Susanne, Theresa Stitz, Johanna Jetschni, and Paloma Cariñanos. 2018. "The Influence of Individual-Specific Plant Parameters and Species Composition on the Allergenic Potential of Urban Green Spaces" Forests 9, no. 6: 284. https://doi.org/10.3390/f9060284
APA StyleJochner-Oette, S., Stitz, T., Jetschni, J., & Cariñanos, P. (2018). The Influence of Individual-Specific Plant Parameters and Species Composition on the Allergenic Potential of Urban Green Spaces. Forests, 9(6), 284. https://doi.org/10.3390/f9060284







