Allocation Mechanisms of Non-Structural Carbohydrates of Robinia pseudoacacia L. Seedlings in Response to Drought and Waterlogging
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Non-Structural Carbohydrate Measurements
2.3. Soil Moisture and Seedling Biomass
2.4. Statistical Analysis
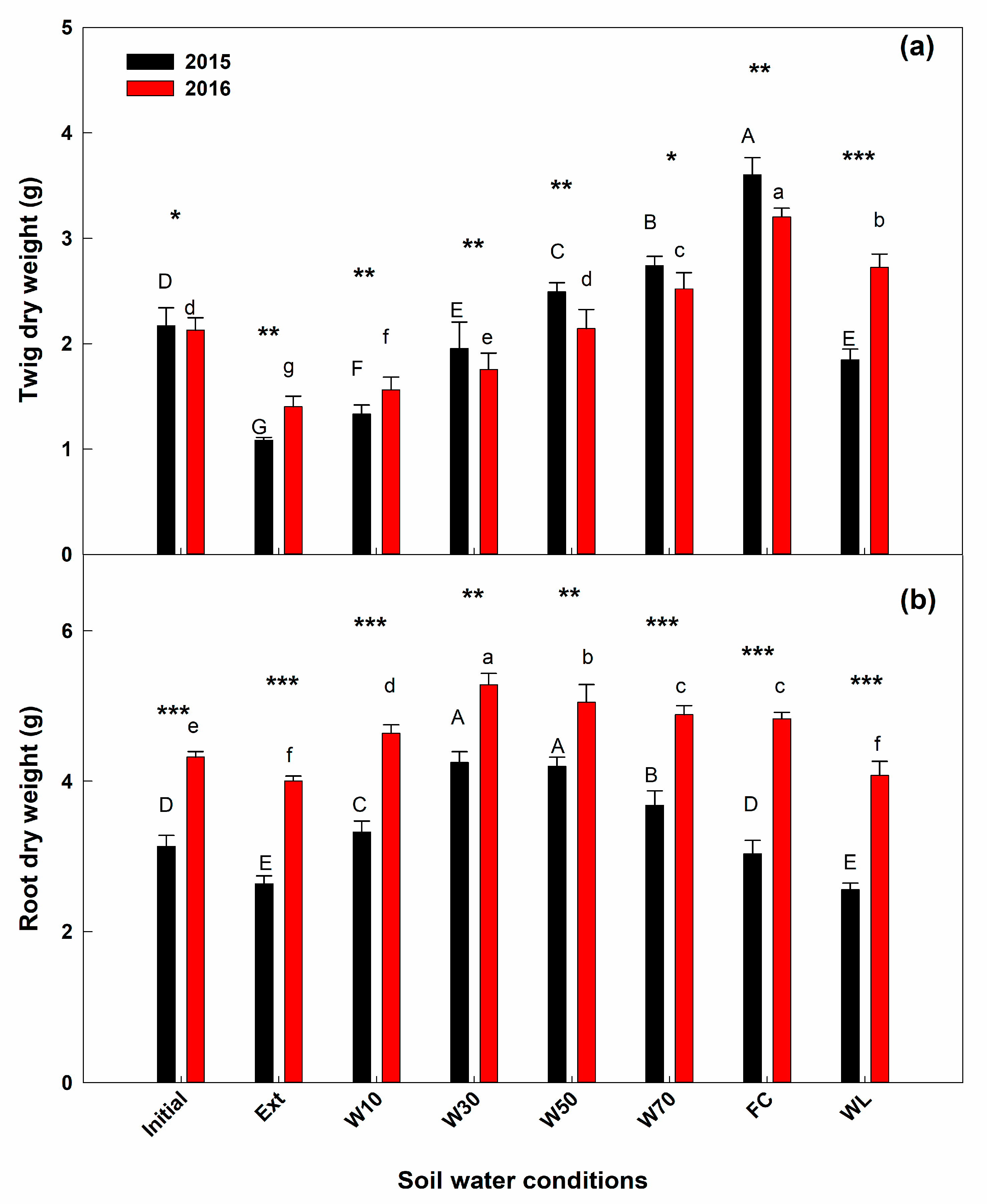
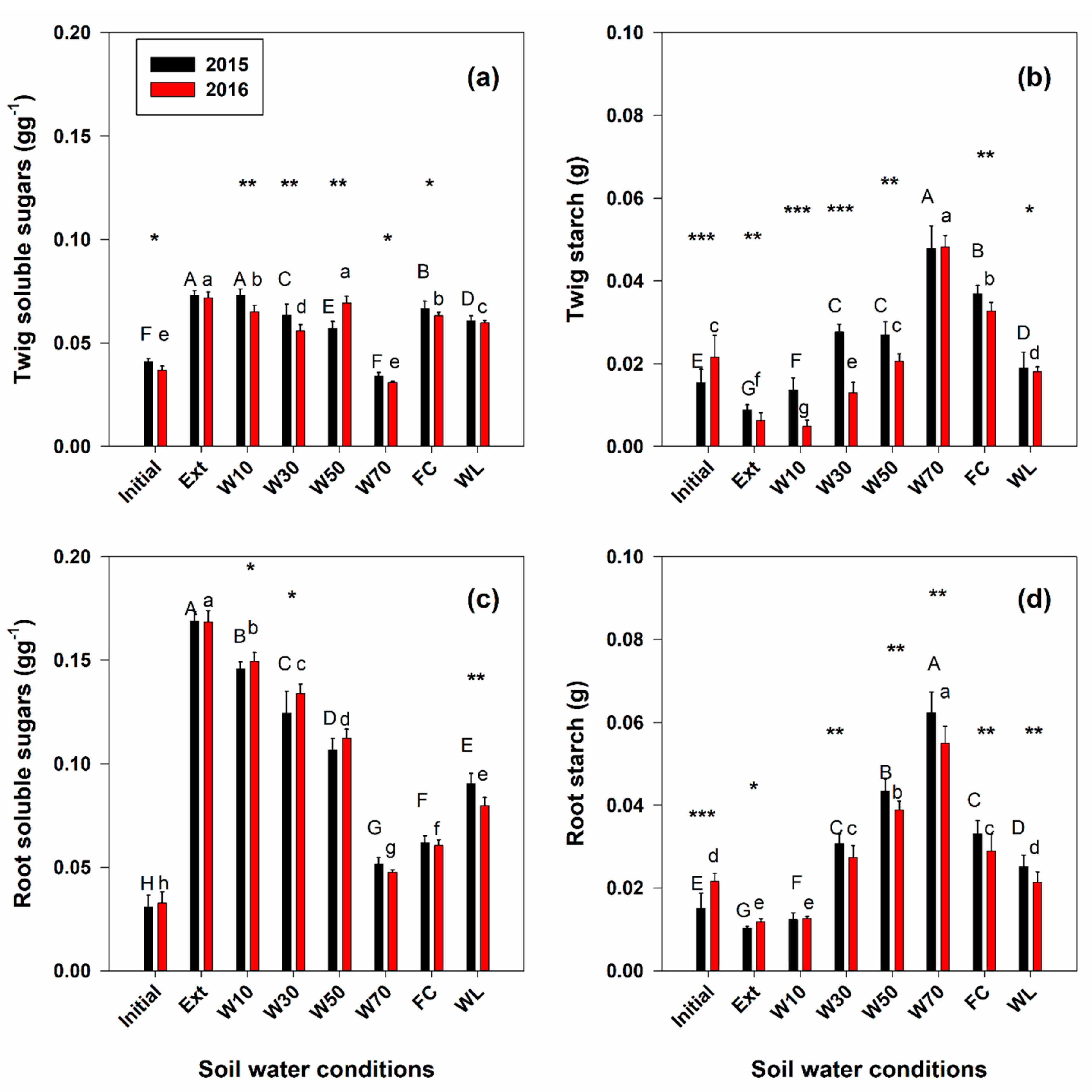
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.I.; Nicholls, N.; Easterling, D.; Goodess, C.M.; Kanae, S.; Kossin, J.; Luo, Y.; Marengo, J.; McInnes, K.; Rahimi, M.; et al. Changes in climate extremes and their impacts on the natural physical environment. In Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation: Special Report of the Intergovernmental Panel on Climate Change; Field, C., Barros, V., Stocker, T., Dahe, Q., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 109–230. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Peng, C.; Ma, Z.; Lei, X.; Zhu, Q.; Chen, H.; Wang, W.; Liu, S.; Li, W.; Fang, X.; Zhou, X. A drought-induced pervasive increase in tree mortality across Canada’s boreal forests. Nat. Clim. Chang. 2011, 1, 467–471. [Google Scholar] [CrossRef]
- Van Mantgem, P.J.; Stephenson, N.L.; Byrne, J.C.; Daniels, L.D.; Franklin, J.F.; Fule, P.Z.; Harmon, M.E.; Larson, A.J.; Smith, J.M.; Taylor, A.H.; et al. Widespread increase of tree mortality rates in the Western United States. Science 2009, 323, 521–524. [Google Scholar] [CrossRef]
- Greenwood, S.; Ruiz-Benito, P.; Martínez-Vilalta, J.; Lloret, F.; Kitzberger, T.; Allen, C.D.; Fensham, R.; Laughlin, D.C.; Kattge, J.; Bönisch, G.; et al. Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol. Lett. 2017, 20, 539–553. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Beerling, D.J.; Breshears, D.D.; Fisher, R.A.; Raffa, K.F.; Stitt, M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 2011, 26, 523–532. [Google Scholar] [CrossRef]
- Meir, P.; Mencuccini, M.; Dewar, R.C. Drought-related tree mortality: Addressing the gaps in understanding and prediction. New Phytol. 2015, 207, 28–33. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, A.P.; Mitchell, P.J.M.; Pinkard, E.A.; Tissue, D.T. Thirsty roots and hungry leaves: Unravelling the roles of carbon and water dynamics in tree mortality. New Phytol. 2013, 200, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Dietze, M.C.; Moorcroft, P.R. Tree mortality in the Eastern and Central United States: Patterns and drivers. Glob. Chang. Biol. 2011, 17, 3312–3326. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.S.; Trumbore, S.E. Contribution of new photosynthetic assimilates to respiration by perennial grasses and shrubs: Residence times and allocation patterns. New Phytol. 2007, 176, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Aguadé, D.; Poyatos, R.; Rosas, T.; Martínez-Vilalta, J. Comparative Drought Responses of Quercus ilex L. and Pinus sylvestris L. in a Montane Forest Undergoing a Vegetation Shift. Forests 2015, 6, 2505. [Google Scholar] [CrossRef]
- Lin, T.; Zheng, H.; Huang, Z.; Wang, J.; Zhu, J. Non-Structural Carbohydrate Dynamics in Leaves and Branches of Pinus massoniana (Lamb.) Following 3-Year Rainfall Exclusion. Forests 2018, 9, 315. [Google Scholar] [CrossRef]
- Chapin, F.S.; Shaver, G.R. Differences in carbon and nutrient fractions among arctic growth forms. Oecologia 1988, 77, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S.; Schulze, E.; Mooney, H.A. The ecology and economics of storage in plants. Ann. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Richardson, A.D.; Carbone, M.S.; Keenan, T.F.; Czimczik, C.I.; Hollinger, D.Y.; Murakami, P.; Schaberg, P.G.; Xu, X. Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytol. 2013, 197, 850–861. [Google Scholar] [CrossRef]
- Palacio, S.; Millard, P.; Maestro, M.; Montserrat-Martí, G. Non-structural carbohydrates and nitrogen dynamics in Mediterranean sub-shrubs: An analysis of the functional role of overwintering leaves. Plant Biol. 2007, 9, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.; Trumbore, S.E.; Allen, M.F. Evidence of old carbon used to grow new fine roots in a tropical forest. New Phytol. 2009, 182, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.S.; Czimczik, C.I.; Keenan, T.F.; Murakami, P.F.; Pederson, N.; Schaberg, P.G.; Xu, X.; Richardson, A.D. Age, allocation and availability of nonstructural carbon in mature red maple trees. New Phytol. 2013, 200, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Czimczik, C.; Trumbore, S.; Xu, X.; Carbone, M.; Richardson, A. Extraction of nonstructural carbon and cellulose from wood for radiocarbon analysis. Bio-protocol 2014, 4, e1169. [Google Scholar] [CrossRef]
- Trumbore, S.; Czimczik, C.I.; Sierra, C.A.; Muhr, J.; Xu, X. Non-structural carbon dynamics and allocation relate to growth rate and leaf habit in California oaks. Tree Physiol. 2015, 35, 1206–1222. [Google Scholar] [CrossRef] [PubMed]
- Hoch, G.; Richter, A.; Korner, C. Non-structural carbon compounds in temperate forest trees. Plant Cell Environ. 2003, 26, 1067–1081. [Google Scholar] [CrossRef]
- Gessler, A.; Treydte, K. The fate and age of carbon–insights into the storage and remobilization dynamics in trees. New Phytol. 2016, 209, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, N.; Gessler, A.; Kayler, Z.; Keel, S.G.; Badeck, F.; Barthel, M.; Boeckx, P.; Buchmann, N.; Brugnoli, E.; Esperschütz, J.; et al. Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: A review. Biogeosciences 2011, 8, 3457–3489. [Google Scholar] [CrossRef]
- Palacio, S.; Hoch, G.; Sala, A.; Körner, C.; Millard, P. Does carbon storage limit tree growth? New Phytol. 2014, 201, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Keel, S.G.; Siegwolf, R.T.W.; Körner, C. Canopy CO2 enrichment permits tracing the fate of recently assimilated carbon in a mature deciduous forest. New Phytol. 2006, 172, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Keel, S.G.; Siegwolf, R.T.; Jaggi, M.; Korner, C. Rapid mixing between old and new C pools in the canopy of mature forest trees. Plant Cell Environ. 2007, 30, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; McDowell, N.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- McDowell, N.G.; Fisher, R.A.; Xu, C.; Domec, J.C.; Hölttä, T.; Mackay, D.S.; Sperry, J.S.; Boutz, A.; Dickman, L.; Gehres, N.; et al. Evaluating theories of drought-induced vegetation mortality using a multimodel-experiment framework. New Phytol. 2013, 200, 304–321. [Google Scholar] [CrossRef] [PubMed]
- Sala, A. Lack of direct evidence for the carbon-starvation hypothesis to explain drought-induced mortality in trees. Proc. Natl. Acad. Sci. USA 2009, 106, E68. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants Without Soil. California Agricultural Experiment Station Circular No. 347; California Agricultural Experiment Station: Oakland, CA, USA, 1950. [Google Scholar]
- Kirkham, M.B. Chapter 10–Field Capacity, Wilting Point, Available Water, and the Nonlimiting Water Range. In Principles of Soil and Plant Water Relations, 2nd ed.; Kirkham, M.B., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 153–170. [Google Scholar]
- Wilson, R.; Cataldo, A.; Andersen, C.P. Determination of total nonstructural carbohydrates in tree species by high-performance anion-exchange chromatography with pulsed amperometric detection. Can. J. For. Res. 1995, 25, 2022–2028. [Google Scholar] [CrossRef]
- Raessler, M.; Wissuwa, B.; Breul, A.; Unger, W.; Grimm, T. Chromatographic analysis of major non-structural carbohydrates in several wood species—An analytical approach for higher accuracy of data. Anal. Methods 2010, 2, 532. [Google Scholar] [CrossRef]
- Kagan, I.A.; Kirch, B.H.; Thatcher, C.D.; Teutsch, C.D.; Pleasant, R.S. Chromatographic profiles of nonstructural carbohydrates contributing to the colorimetrically determined fructan, ethanol-soluble, and water-soluble carbohydrate contents of five grasses. Anim. Feed Sci. Technol. 2014, 188, 53–63. [Google Scholar] [CrossRef]
- Quentin, A.G.; Pinkard, E.A.; Ryan, M.G.; Tissue, D.T.; Baggett, L.S.; Adams, H.D.; Maillard, P.; Marchand, J.; Landhausser, S.M.; Lacointe, A.; et al. Non-structural carbohydrates in woody plants compared among laboratories. Tree Physiol. 2015, 35, 1146–1165. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Das, K.R. A brief review of tests for normality. Am. J. Theor. Appl. Stat. 2016, 5, 5–12. [Google Scholar] [CrossRef]
- Lammoglia, M.A.; Garcez, N.; Cabrera, A.; López, R.D.; Rentería, I.D.C.D.; Rojas-Ronquillo, R. Behavior affected by routine oxytocin injection in crossbred cows in the tropics. Rev. Bras. Zootec. 2016, 45, 478–482. [Google Scholar] [CrossRef]
- Minucci, J.M.; Miniat, C.F.; Teskey, R.O.; Wurzburger, N. Tolerance or avoidance: Drought frequency determines the response of an N2-fixing tree. New Phytol. 2017, 215, 434–442. [Google Scholar] [CrossRef]
- Salkind, N. Encyclopedia of Research Design; Sage: Los Angeles, CA, USA, 2010. [Google Scholar]
- El-Juhany, L.I. Carbon partitioning into structural and nonstructural compounds in Leucaena leucocephala trees as affected by water stress. J. Adv. Agric. Res. 2003, 8, 277–286. [Google Scholar]
- Botelho, M.R.; Heuvel, J.E.V. Preliminary assessment of the impact of current flooding practices on nonstructural carbohydrate concentrations of cranberry. HortTechnology 2006, 16, 277–285. [Google Scholar]
- Heuvel, J.E.V.; Goffinet, M.C. The effects of flood initiation timing and water temperature during flooding on nonstructural carbohydrate concentration and anatomy of cranberry. HortScience 2008, 43, 338–345. [Google Scholar]
- Kozlowski, T.T. Responses of woody plants to flooding and salinity. Tree Physiol. 1997, 17, 490. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S.G. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 2002, 68, 270–334. [Google Scholar] [CrossRef]
- Carpenter, L.T.; Pezeshki, S.R.; Shields, F.D. Responses of nonstructural carbohydrates to shoot removal and soil moisture treatments in Salix nigra. Trees 2008, 22, 737–748. [Google Scholar] [CrossRef]
- West, A.G.; Hultine, K.R.; Sperry, J.S.; Bush, S.E.; Ehleringer, J.R. Transpiration and hydraulic strategies in a piñon–juniper woodland. Ecol. Appl. 2008, 18, 911–927. [Google Scholar] [CrossRef] [PubMed]
- Plaut, J.A.; Yepez, E.A.; Hill, J.; Pangle, R.; Sperry, J.S.; Pockman, W.T.; McDowell, N.G. Hydraulic limits preceding mortality in a piñon-juniper woodland under experimental drought. Plant Cell Environ. 2012, 35, 1601–1617. [Google Scholar] [CrossRef] [PubMed]
- Wiley, E.; Rogers, B.J.; Hodgkinson, R.; Landhäusser, S.M. Nonstructural carbohydrate dynamics of lodgepole pine dying from mountain pine beetle attack. New Phytol. 2016, 209, 550–562. [Google Scholar] [CrossRef]
- McDowell, N.G.; Ryan, M.G.; Zeppel, M.J.B.; Tissue, D.T. Feature: Improving our knowledge of drought-induced forest mortality through experiments, observations, and modeling. New Phytol. 2013, 200, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.C.; McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.J. Larger trees suffer most during drought in forests worldwide. Nat. Plants 2015, 1, 15139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cao, Y.; Chen, Y.; Liu, G. Non-structural carbohydrate dynamics in Robinia pseudoacacia saplings under three levels of continuous drought stress. Trees 2015, 29, 1837–1849. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees–from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.D.; Germino, M.J.; Breshears, D.D.; Barron-Gafford, G.A.; Guardiola-Claramonte, M.; Zou, C.B.; Huxman, T.E. Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol. 2013, 197, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Chang. 2014, 4, 710–714. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Burslem, D.F.R.P.; Caduff, A.; Tay, J.; Hector, A. Contrasting nonstructural carbohydrate dynamics of tropical tree seedlings under water deficit and variability. New Phytol. 2015, 205, 1083–1094. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Anderegg, L.D.L. Hydraulic and carbohydrate changes in experimental drought-induced mortality of saplings in two conifer species. Tree Physiol. 2013, 33, 252–260. [Google Scholar] [CrossRef]
- Rosas, T.; Galiano, L.; Ogaya, R.; Peñuelas, J.; Martínez-Vilalta, J. Dynamics of non-structural carbohydrates in three Mediterranean woody species following long-term experimental drought. Front. Plant Sci. 2013, 4, 400. [Google Scholar] [CrossRef]
- Ramel, F.; Sulmon, C.; Gouesbet, G.; Couée, I. Natural variation reveals relationships between pre-stress carbohydrate nutritional status and subsequent responses to xenobiotic and oxidative stress in Arabidopsis thaliana. Ann. Bot. 2009, 104, 1323–1337. [Google Scholar] [CrossRef]
- Sulpice, R.; Pyl, E.-T.; Ishihara, H.; Trenkamp, S.; Steinfath, M.; Witucka-Wall, H.; Gibon, Y.; Usadel, B.; Poree, F.; Piques, M.C.; et al. Starch as a major integrator in the regulation of plant growth. Proc. Natl. Acad. Sci. USA 2009, 106, 10348–10353. [Google Scholar] [CrossRef] [PubMed]
- Rudack, K.; Seddig, S.; Sprenger, H.; Köhl, K.; Uptmoor, R.; Ordon, F. Drought stress-induced changes in starch yield and physiological traits in potato. J. Agron. Crop Sci. 2017, 203, 494–505. [Google Scholar] [CrossRef]
- Kühn, C. Sucrose Transporters and Plant Development. In Transporters and Pumps in Plant Signaling. Signaling and Communication in Plants; Geisler, M., Venema, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 7. [Google Scholar]
- Watanabe, K.; Nakabaru, M.; Taira, E.; Ueno, M.; Kawamitsu, Y. Relationships between nutrients and sucrose concentrations in sugarcane juice and use of juice analysis for nutrient diagnosis in Japan. Plant Prod. Sci. 2016, 19, 215–222. [Google Scholar] [CrossRef]
- Chardon, F.; Bedu, M.; Calenge, F.; Klemens, P.A.; Spinner, L.; Clement, G.; Chietera, G.; Leran, S.; Ferrand, M.; Lacombe, B.; et al. Leaf fructose content is controlled by the vacuolar transporter SWEET17 in Arabidopsis. Curr. Biol. 2013, 23, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Adams, H.D.; Guardiola-Claramonte, M.; Barron-Gafford, G.A.; Villegas, J.C.; Breshears, D.D.; Zou, C.B.; Troch, P.A.; Huxman, T.E. Reply to Sala: Temperature sensitivity in drought-induced tree mortality hastens the need to further resolve a physiological model of death. Proc. Natl. Acad. Sci. USA 2009, 106, E69. [Google Scholar] [CrossRef]
- Epron, D.; Bahn, M.; Derrien, D.; Lattanzi, F.A.; Pumpanen, J.; Gessler, A.; Hogberg, P.; Maillard, P.; Dannoura, M.; Gerant, D.; et al. Pulse-labelling trees to study carbon allocation dynamics: A review of methods, current knowledge and future prospects. Tree Physiol. 2012, 32, 776–798. [Google Scholar] [CrossRef] [PubMed]
- Epron, D.; Nouvellon, Y.; Ryan, M.G. Introduction to the invited issue on carbon allocation of trees and forests. Tree Physiol. 2012, 32, 639–643. [Google Scholar] [CrossRef]
- Adams, H.D.; Williams, A.P.; Xu, C.; Rauscher, S.A.; Jiang, X.; McDowell, N.G. Empirical and process-based approaches to climate-induced forest mortality models. Front. Plant Sci. 2013, 4, 438. [Google Scholar] [CrossRef] [PubMed]
- Doughty, C.E.; Metcalfe, D.B.; Girardin, C.A.J.; Amézquita, F.F.; Cabrera, D.G.; Huasco, W.H.; Silva-Espejo, J.E.; Araujo-Murakami, A.; da Costa, M.C.; Rocha, W.; et al. Drought impact on forest carbon dynamics and fluxes in Amazonia. Nature 2015, 519, 78–82. [Google Scholar] [CrossRef]
- Nardini, A.; Casolo, V.; Dal Borgo, A.; Savi, T.; Stenni, B.; Bertoncin, P.; Zini, L.; McDowell, N.G. Rooting depth, water relations and non-structural carbohydrate dynamics in three woody angiosperms differentially affected by an extreme summer drought. Plant Cell Environ. 2016, 39, 618–627. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Peng, C.; Harrison, S.P.; Wei, H.; Wang, H.; Zhu, Q.; Wang, M. Allocation Mechanisms of Non-Structural Carbohydrates of Robinia pseudoacacia L. Seedlings in Response to Drought and Waterlogging. Forests 2018, 9, 754. https://doi.org/10.3390/f9120754
Yang B, Peng C, Harrison SP, Wei H, Wang H, Zhu Q, Wang M. Allocation Mechanisms of Non-Structural Carbohydrates of Robinia pseudoacacia L. Seedlings in Response to Drought and Waterlogging. Forests. 2018; 9(12):754. https://doi.org/10.3390/f9120754
Chicago/Turabian StyleYang, Bin, Changhui Peng, Sandy P. Harrison, Hua Wei, Han Wang, Qiuan Zhu, and Meng Wang. 2018. "Allocation Mechanisms of Non-Structural Carbohydrates of Robinia pseudoacacia L. Seedlings in Response to Drought and Waterlogging" Forests 9, no. 12: 754. https://doi.org/10.3390/f9120754
APA StyleYang, B., Peng, C., Harrison, S. P., Wei, H., Wang, H., Zhu, Q., & Wang, M. (2018). Allocation Mechanisms of Non-Structural Carbohydrates of Robinia pseudoacacia L. Seedlings in Response to Drought and Waterlogging. Forests, 9(12), 754. https://doi.org/10.3390/f9120754




