Can Future CO2 Concentrations Mitigate the Negative Effects of High Temperature and Longer Droughts on Forest Growth?
Abstract
1. Introduction
2. Materials and Methods
2.1. Site-Scale Experiment
2.2. Analysis
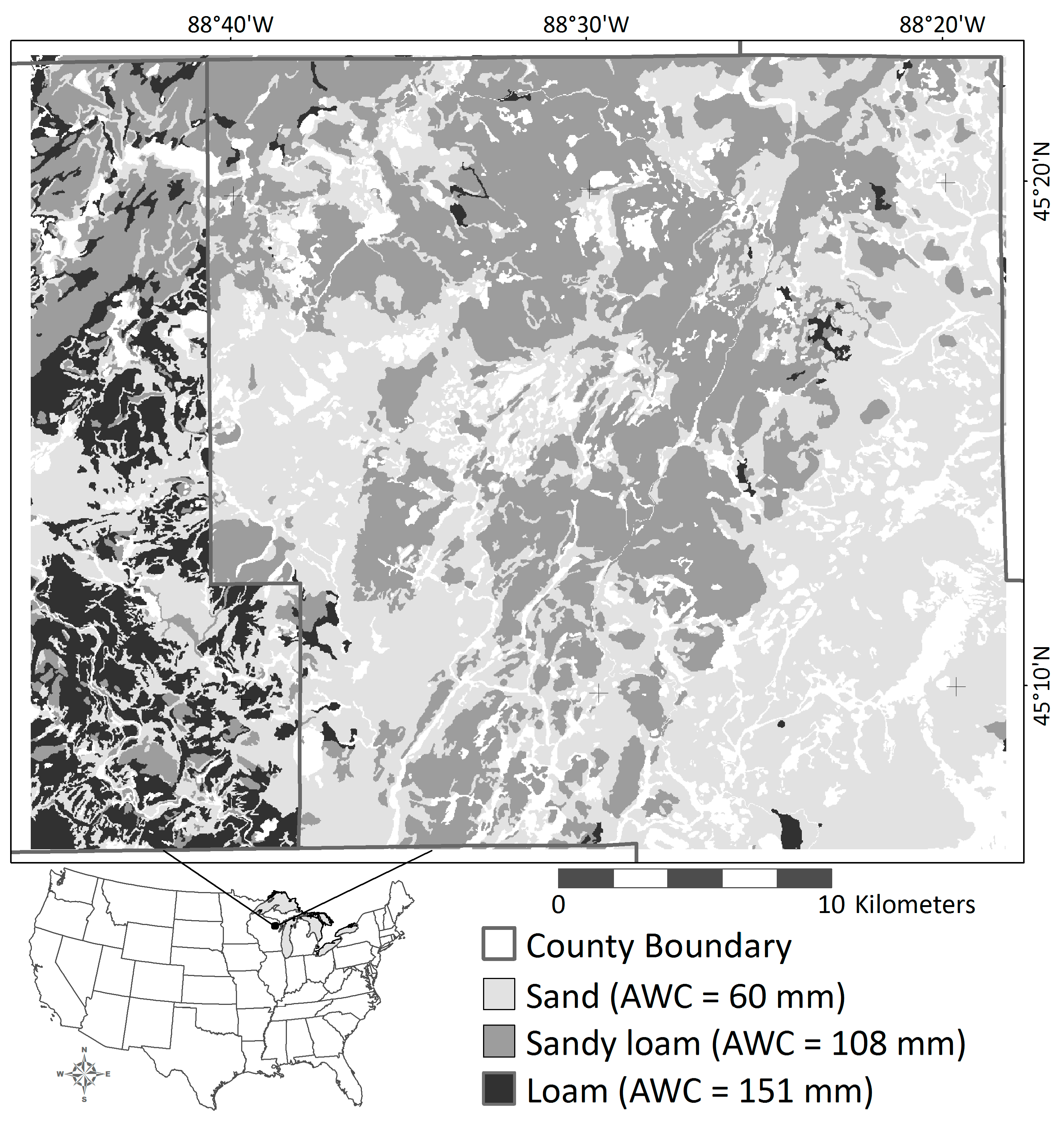
2.3. Landscape-Scale Experiment
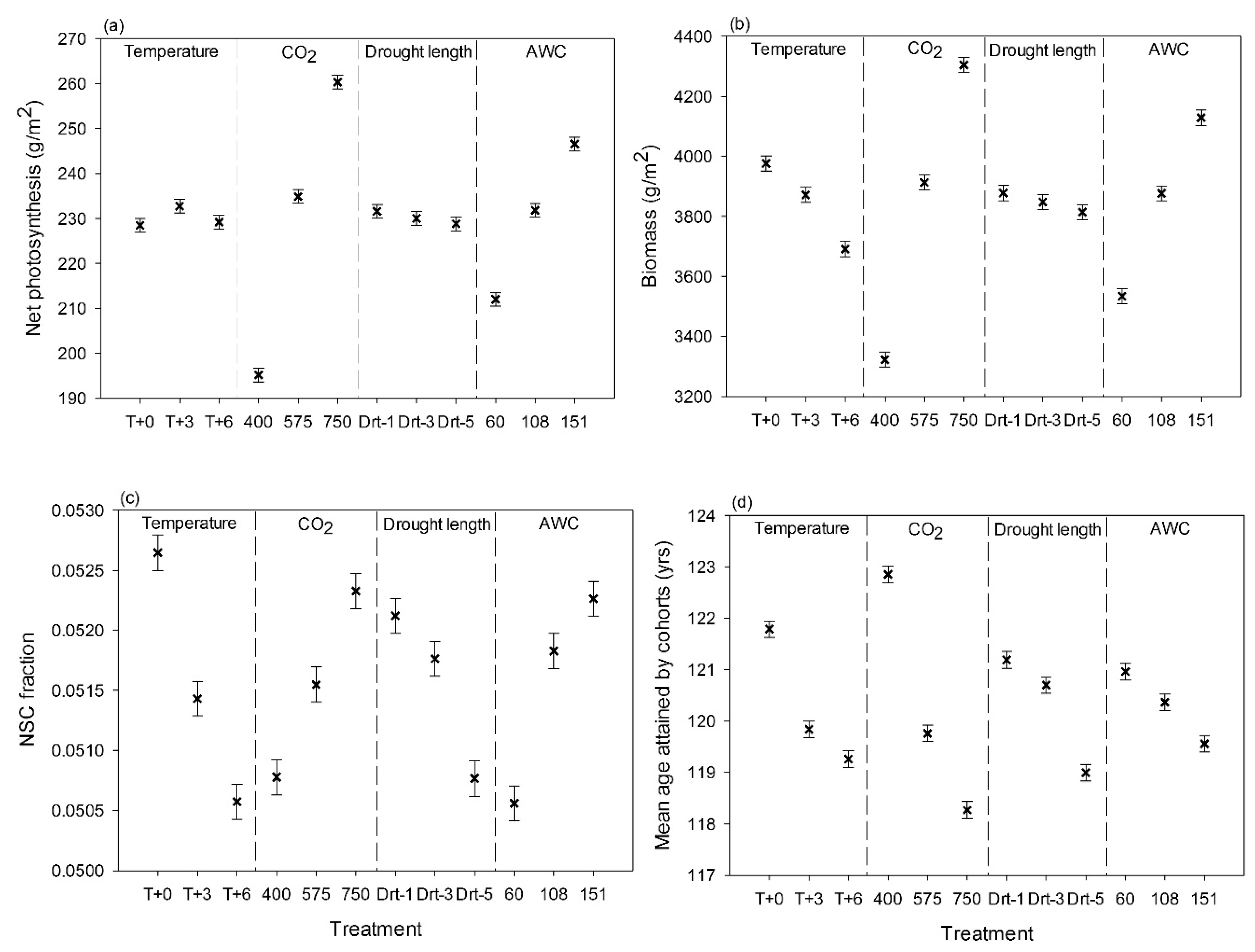
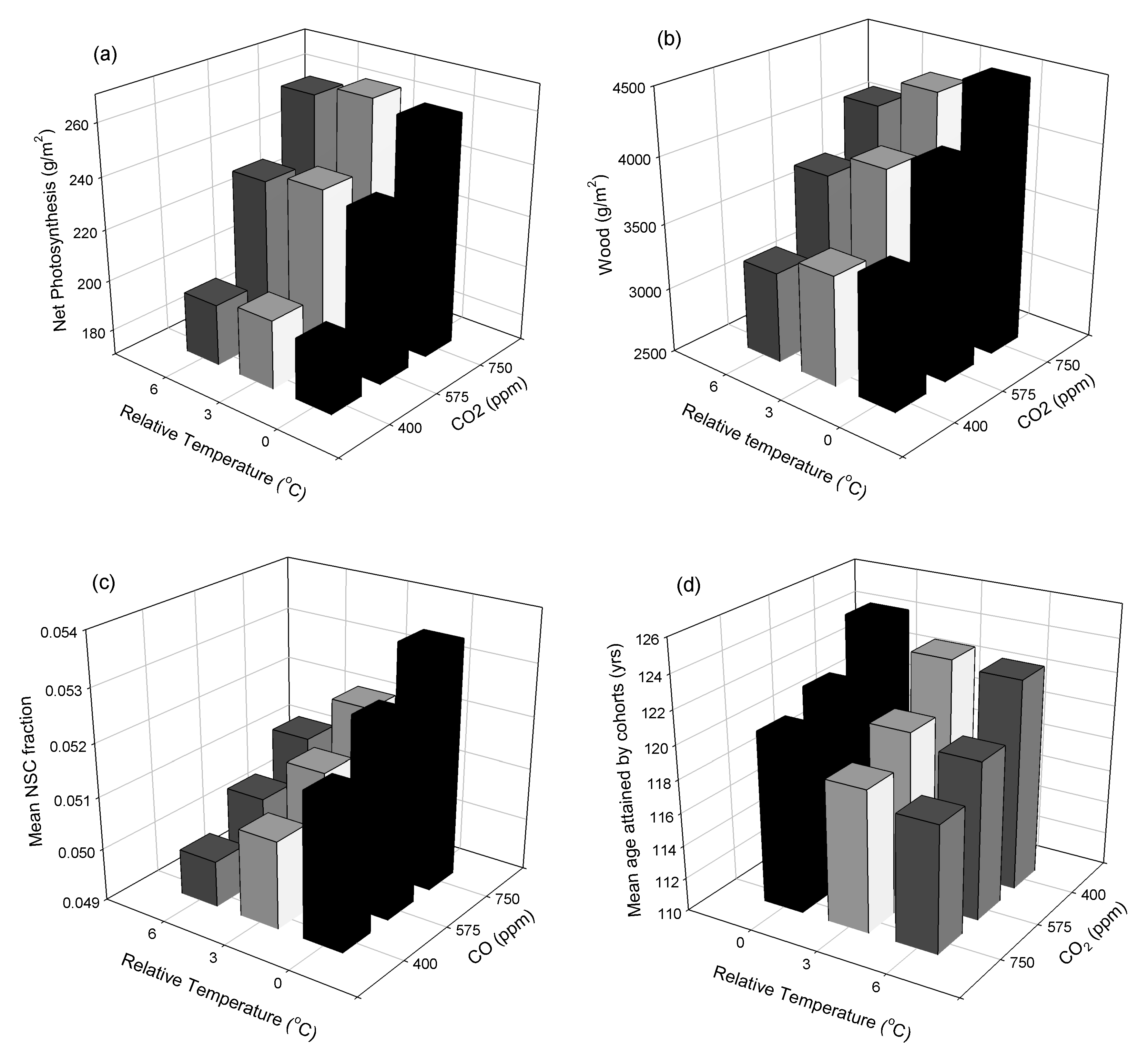
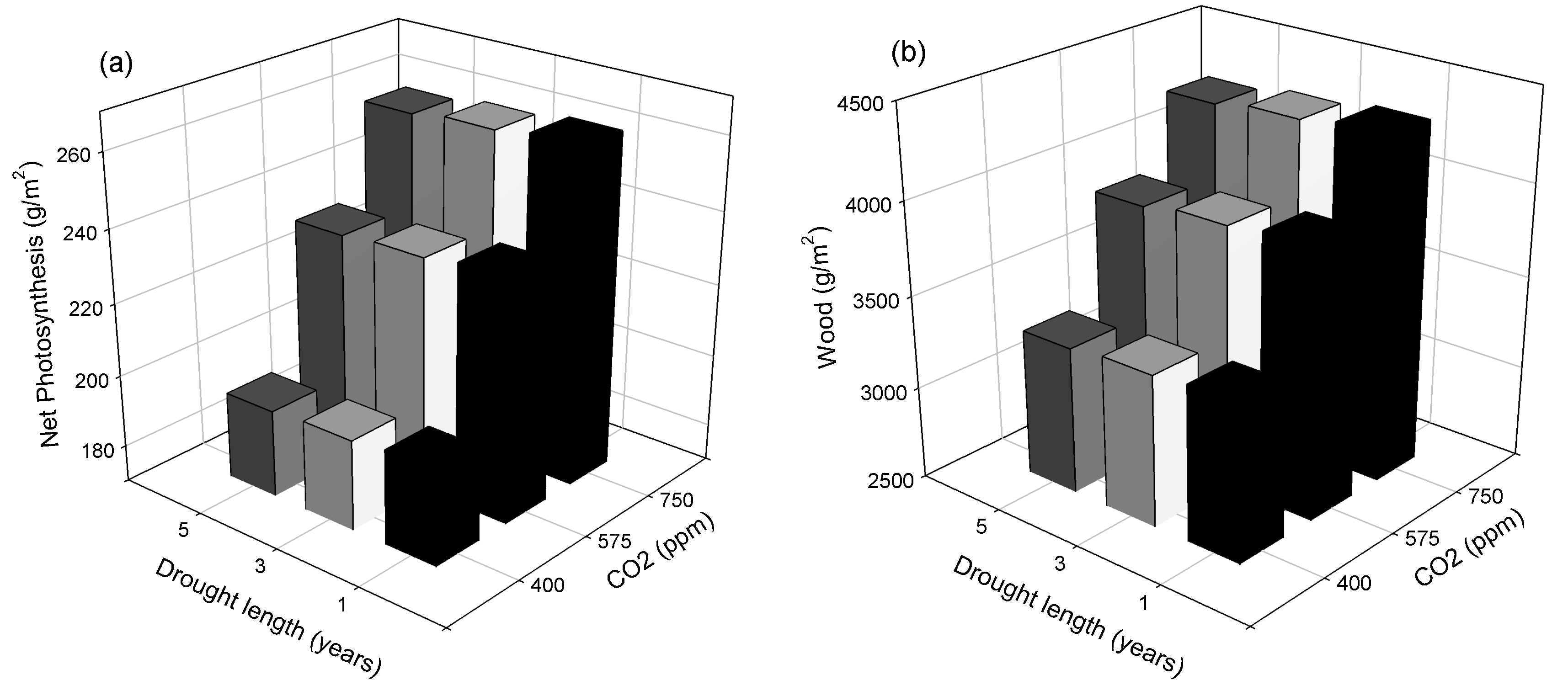
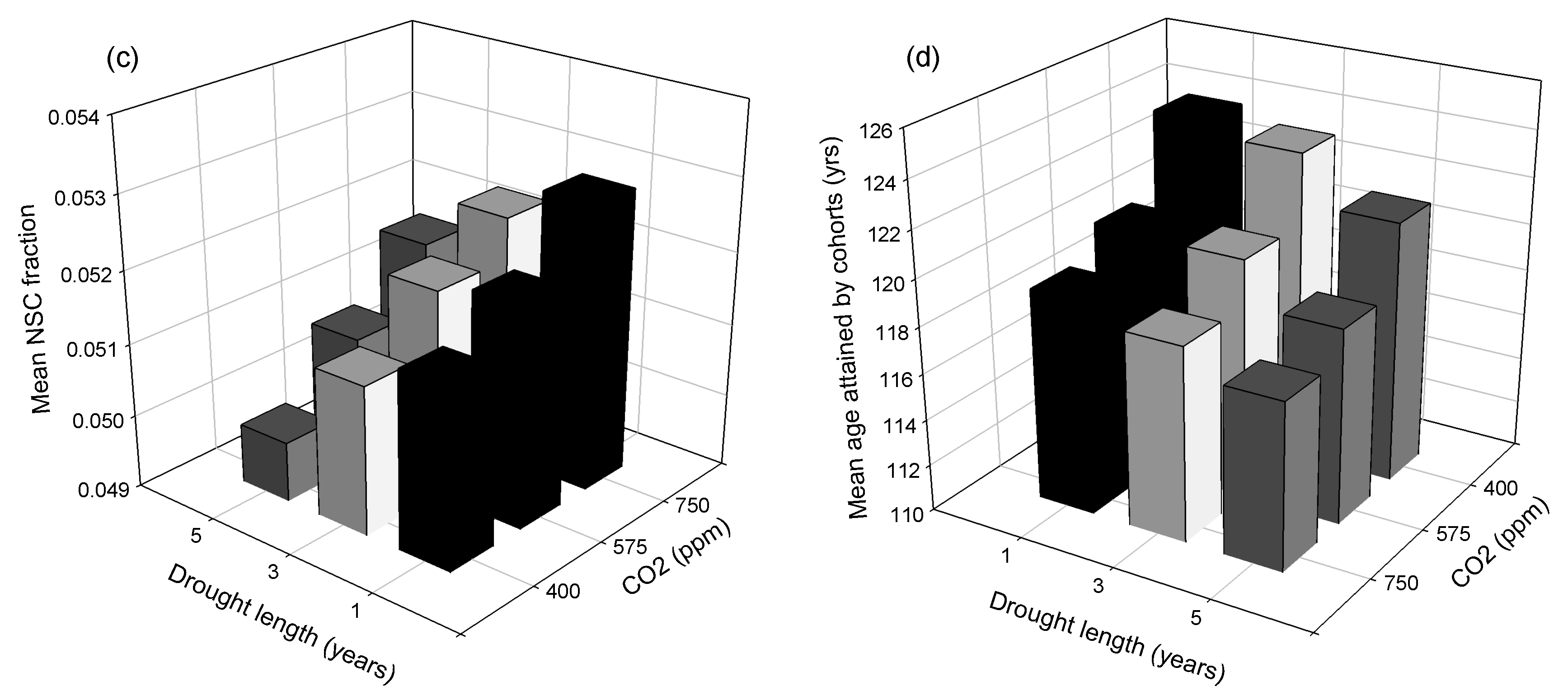
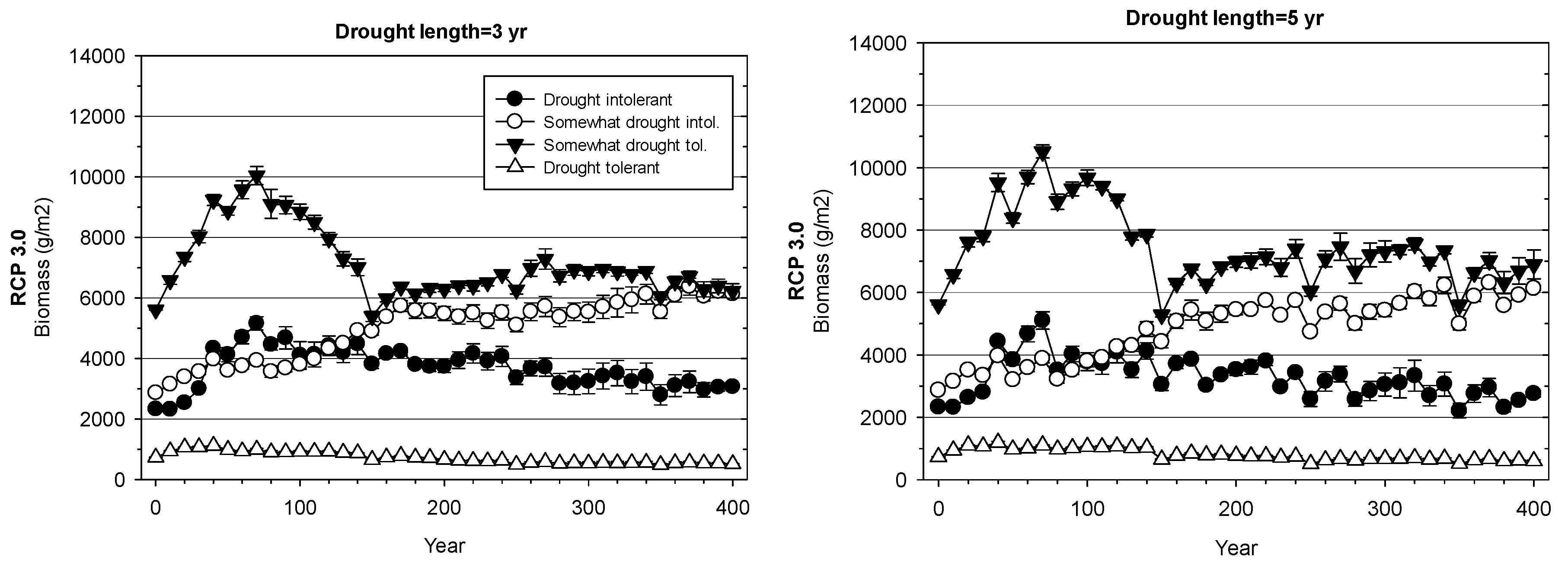
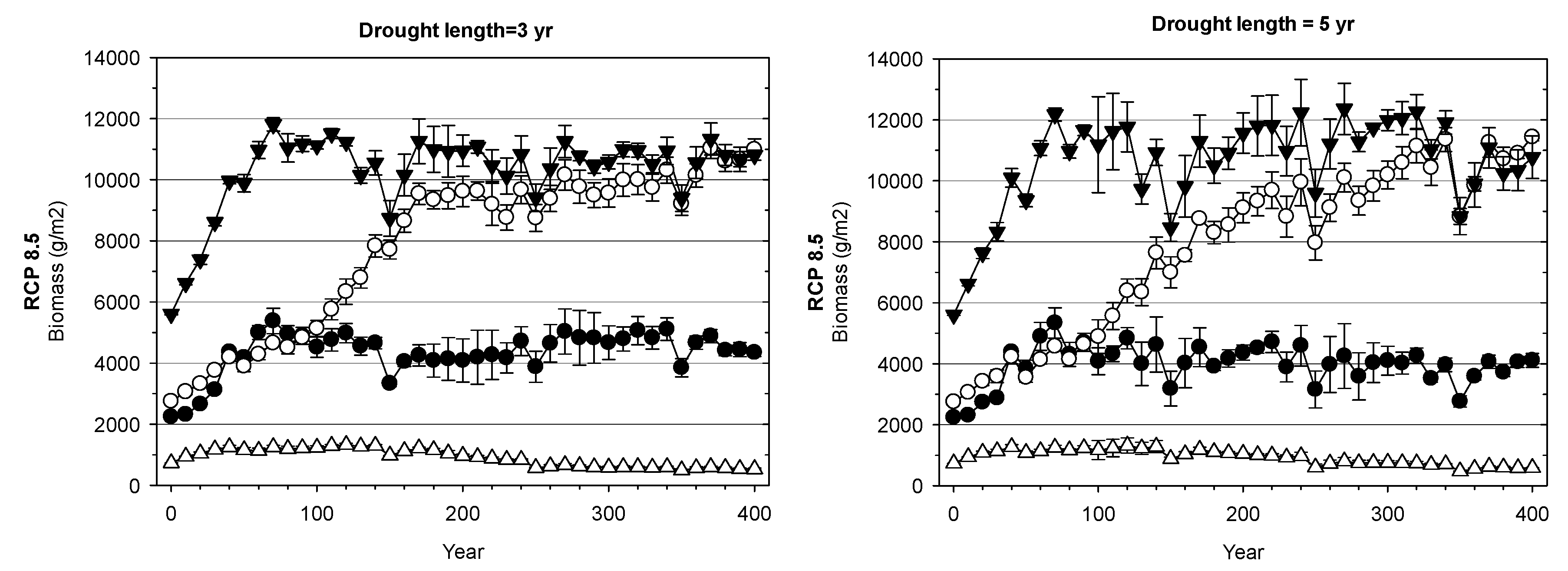
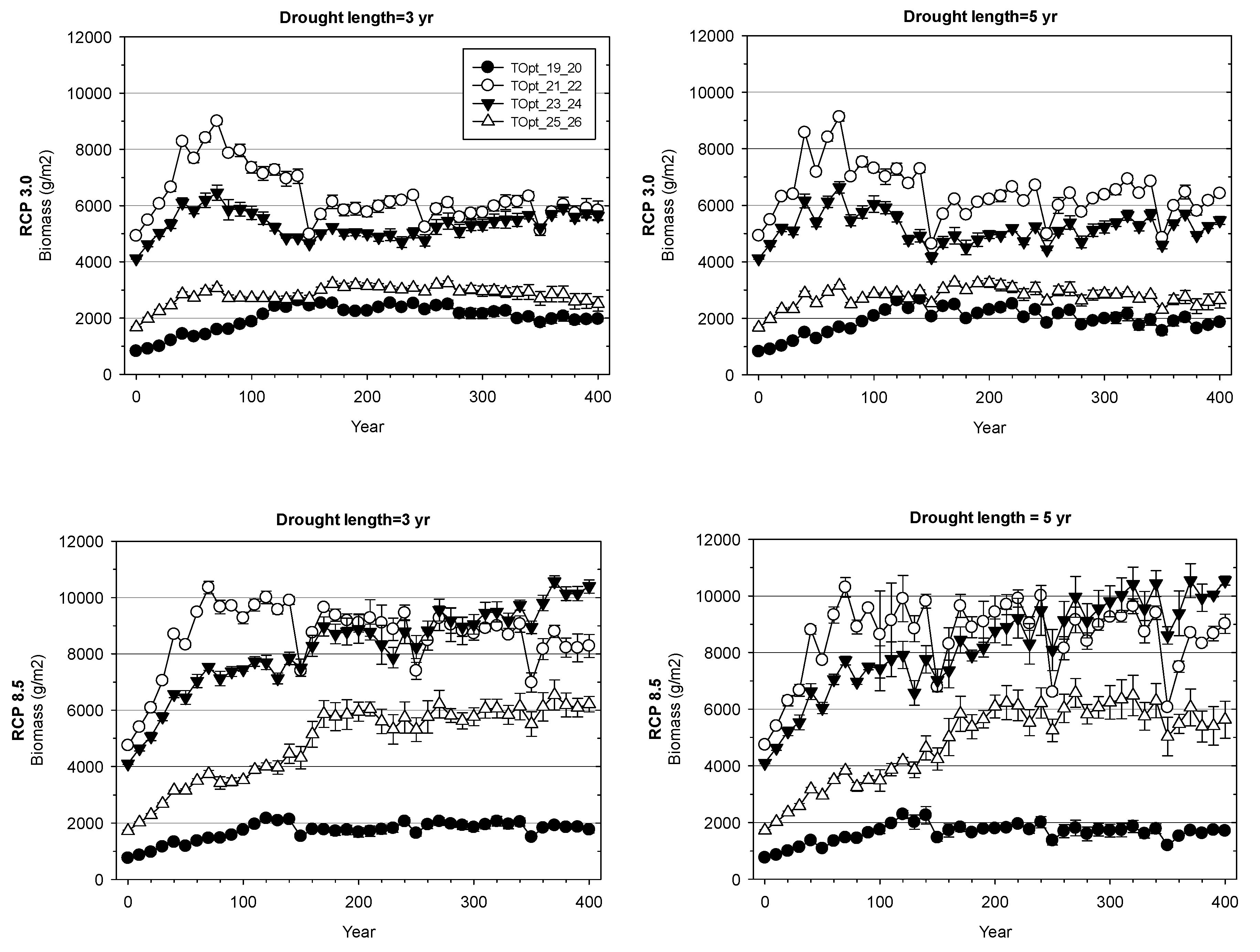
3. Results
3.1. Site-Scale Experiment
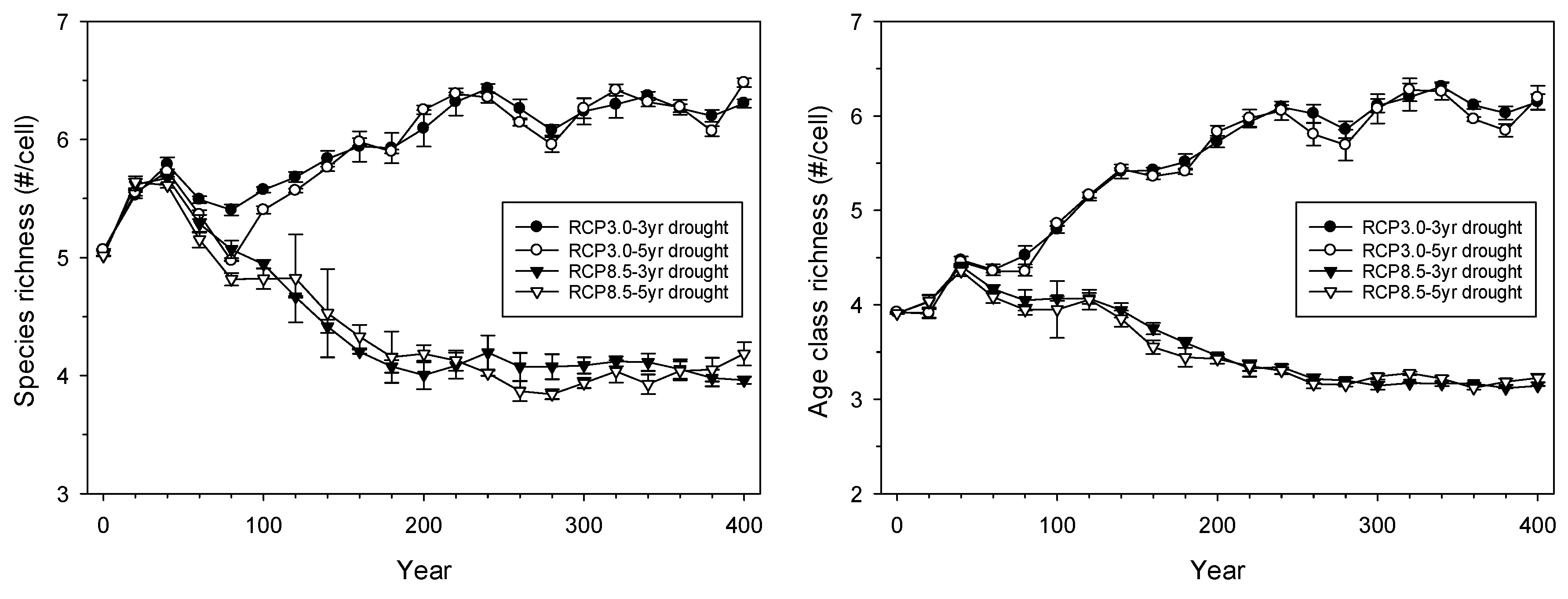
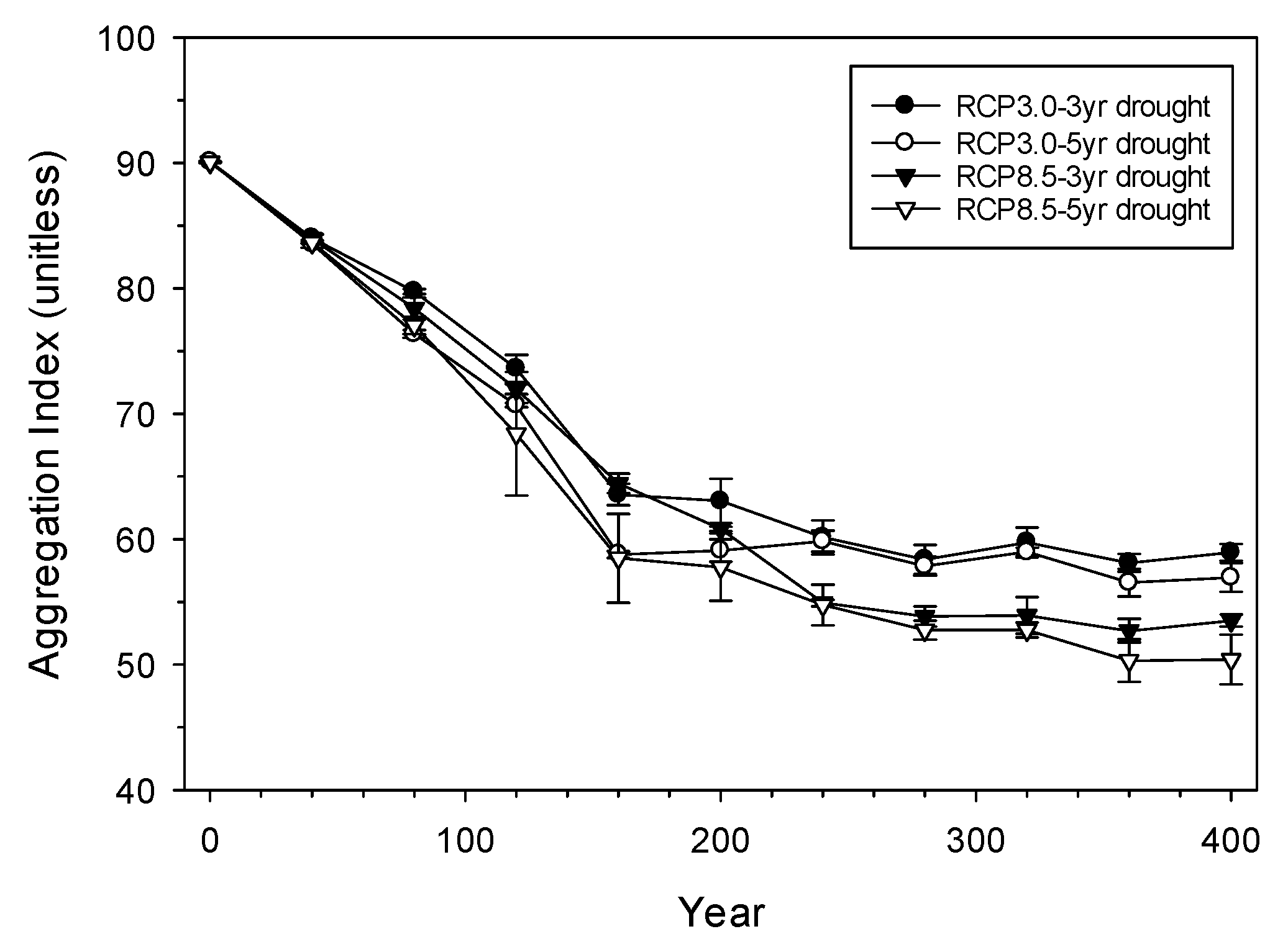
3.2. Landscape-Scale Experiment
4. Discussion
4.1. Site-Scale Experiment
4.2. Landscape-Scale Experiment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; ISBN 978-1-107-05799-1. [Google Scholar]
- Shao, H.-B.; Li-Ye, C.; Cheruth, A.J.; Chang-Xing, Z. Water-deficit stress-induced anatomical changes in higher plants. C. R. Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Fisher, R.A.; Xu, C.; Domec, J.C.; Hölttä, T.; Mackay, D.S.; Sperry, J.S.; Boutz, A.; Dickman, L.; Gehres, N.; et al. Evaluating theories of drought-induced vegetation mortality using a multimodel–experiment framework. New Phytol. 2013, 200, 304–321. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, E.J.; Miranda, B.R.; De Bruijn, A.M.G.; Sturtevant, B.R.; Kubiske, M.E. Do rising temperatures always increase forest productivity? Interacting effects of temperature, precipitation, cloudiness and soil texture on tree species growth and competition. Environ. Model. Softw. 2017, 97, 171–183. [Google Scholar] [CrossRef]
- Way, D.A.; Oren, R. Differential responses to changes in growth temperature between trees from different functional groups and biomes: A review and synthesis of data. Tree Physiol. 2010, 30, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Aber, J.D.; Ollinger, S.V.; Federer, C.A.; Reich, P.B.; Goulden, M.L.; Kicklighter, D.W.; Melillo, J.M.; Lathrop, R.G., Jr. Predicting the effects of climate change on water yield and forest production in the northeastern United States. Clim. Res. 1995, 5, 207–222. [Google Scholar] [CrossRef]
- Gustafson, E.J.; Sturtevant, B.R. Modeling forest mortality caused by drought stress: Implications for climate change. Ecosystems 2013, 16, 60–74. [Google Scholar] [CrossRef]
- Gustafson, E.J.; De Bruijn, A.M.G.; Miranda, B.R.; Sturtevant, B.R. Implications of mechanistic modeling of drought effects on growth and competition in forest landscape models. Ecosphere 2016, 7, e01253. [Google Scholar] [CrossRef]
- Franks, P.J.; Adams, M.A.; Amthor, J.S.; Barbour, M.M.; Berry, J.A.; Ellsworth, D.S.; Farquhar, G.D.; Ghannoum, O.; Lloyd, J.; McDowell, N.; et al. Sensitivity of plants to changing atmospheric CO2 concentration: From the geological past to the next century. New Phytol. 2013, 197, 1077–1094. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, W.I.; Vicca, S.; Dijkstra, F.A.; Hagedorn, F.; Hovenden, M.J.; Larsen, K.S.; Morgan, J.A.; Volder, A.; Beier, C.; Dukes, J.S.; et al. Simple additive effects are rare: A quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Glob. Chang. Biol. 2012, 18, 2681–2693. [Google Scholar] [CrossRef] [PubMed]
- van der Kooi, C.J.; Reich, M.; Löw, M.; De Kok, L.J.; Tausz, M. Growth and yield stimulation under elevated CO2 and drought: A meta-analysis on crops. Environ. Exp. Bot. 2016, 122, 150–157. [Google Scholar] [CrossRef]
- De Bruijn, A.; Gustafson, E.J.; Sturtevant, B.R.; Foster, J.R.; Miranda, B.R.; Lichti, N.I.; Jacobs, D.F. Toward more robust projections of forest landscape dynamics under novel environmental conditions: Embedding PnET within LANDIS-II. Ecol. Model. 2014, 287, 44–57. [Google Scholar] [CrossRef]
- Scheller, R.M.; Domingo, J.B.; Sturtevant, B.R.; Williams, J.S.; Rudy, A.; Gustafson, E.J.; Mladenoff, D.J. Design, development, and application of LANDIS-II, a spatial landscape simulation model with flexible temporal and spatial resolution. Ecol. Model. 2007, 201, 409–419. [Google Scholar] [CrossRef]
- Gustafson, E.J.; Kubiske, M.E.; Sturtevant, B.R.; Miranda, B.R.; Hoshika, Y.; Paoletti, E. Extrapolating plot-scale CO2 and ozone enrichment experimental results to novel conditions and scales using mechanistic modeling. Ecol. Process. 2018, 7, 31. [Google Scholar] [CrossRef]
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Wythers, K.R.; Reich, P.B.; Bradford, J.B. Incorporating temperature-sensitive Q10 and foliar respiration acclimation algorithms modifies modeled ecosystem responses to global change. J. Geophys. Res. BioGeosci. 2013, 118, 1–14. [Google Scholar] [CrossRef]
- Gustafson, E.J.; De Bruijn, A.M.; Pangle, R.E.; Limousin, J.M.; McDowell, N.G.; Pockman, W.T.; Sturtevant, B.R.; Muss, J.D.; Kubiske, M.E. Integrating ecophysiology and forest landscape models to better project drought effects under climate change. Glob. Chang. Biol. 2015, 21, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Hajima, T.; Sudo, K.; Nagashima, T.; Takemura, T.; Okajima, H.; Nozawa, T.; Kawase, H.; Abe, M.; Yokohata, T.; et al. MIROC-ESM 2010: Model description and basic results of CMIP5-20c3m experiments. Geosci. Model Dev. 2011, 4, 845–872. [Google Scholar] [CrossRef]
- Saxton, K.E.; Rawls, W.J. Soil Water Characteristic Equations. 2004. Available online: https://www.ars.usda.gov/research/software/download/?softwareid=492&modecode=80-42-05-10ml (accessed on 2 April 2018).
- Searle, S.R.; Speed, F.M.; Milliken, G.A. Population Marginal Means in the Linear Model: An Alternative to Least Squares Means. Am. Stat. 1980, 34, 216–221. [Google Scholar]
- White, J.W.; Rassweiler, A.; Samhouri, J.F.; Stier, A.C.; White, C. Ecologists should not use statistical significance tests to interpret simulation model results. Oikos 2014, 123, 385–388. [Google Scholar] [CrossRef]
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Soil Survey Geographic (SSURGO) Database. 2013. Available online: http://sdmdataaccess.nrcs.usda.gov/ (accessed on 1 May 2018).
- Janowiak, M.K.; Iverson, L.R.; Mladenoff, D.J.; Peters, E.; Wythers, K.R.; Xi, W.; Brandt, L.A.; Butler, P.R.; Handler, S.D.; Shannon, P.D.; et al. Forest Ecosystem Vulnerability Assessment and Synthesis for Northern Wisconsin and Western Upper Michigan: A Report from the Northwoods Climate Change Response Framework Project; Gen. Tech. Rep. NRS-136; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2014; p. 247.
- Wilson, B.T.; Lister, A.J.; Riemann, R.I. A nearest-neighbor imputation approach to mapping tree species over large areas using forest inventory plots and moderate resolution raster data. For. Ecol. Manag. 2012, 271, 182–198. [Google Scholar] [CrossRef]
- Kittel, T.G.F.; Rosenbloom, N.A.; Kaufman, C.; Royle, J.A.; Daly, C.; Fisher, H.H.; Gibson, W.P.; Aulenbach, S.; Yates, D.N.; McKeown, R.; et al. VEMAP 2: U.S. Monthly Climate Change Scenarios, Version 2; ORNL DAAC: Oak Ridge, TN, USA, 2000; Available online: https://doi.org/10.3334/ORNLDAAC/567 (accessed on 2 April 2018).
- Scheller, R.M.; Domingo, J.B. LANDIS-II Base Fire v3.1: Extension User Guide; LANDIS-II Foundation: Madison, WI, USA, 2017; Available online: http://www.landis-ii.org/extensions/base-fire (accessed on 2 April 2018).
- Sturtevant, B.; Miranda, B.; Yang, J.; He, H.; Gustafson, E.; Scheller, R. Studying fire mitigation strategies in multi-ownership landscapes: Balancing the management of fire-dependent ecosystems and fire risk. Ecosystems 2009, 12, 445–461. [Google Scholar] [CrossRef]
- Scheller, R.M.; Domingo, J.B. LANDIS-II Base Wind v2.0: Extension User Guide; LANDIS-II Foundation: Madison, WI, USA, 2011; Available online: http://www.landis-ii.org/extensions/base-wind (accessed on 2 April 2018).
- Rich, R.L.; Frelich, L.E.; Reich, P.B. Wind-throw mortality in the southern boreal forest: Effects of species, diameter and stand age. J. Ecol. 2007, 95, 1261–1273. [Google Scholar] [CrossRef]
- Gustafson, E.J.; Miranda, B.R.; Sturtevant, B.R. Linear Wind Disturbance v1.0 Extension User Guide; LANDIS-II Foundation: Madison, WI, USA, 2016; Available online: http://www.landis-ii.org/extensions/linear-wind (accessed on 2 April 2018).
- Johnson, E.A.; Miyanishi, K. (Eds.) Plant Disturbance Ecology; Academic Press: Burlington, MA, USA, 2007; pp. 59–101. ISBN 978012088778. [Google Scholar]
- About Tornadoes. Available online: http://www.wunderground.com/resources/education/tornadoFAQ.asp?MR=1 (accessed on 23 October 2018).
- Gustafson, E.J.; Shifley, S.R.; Mladenoff, D.J.; He, H.S.; Nimerfro, K.K. Spatial simulation of forest succession and timber harvesting using LANDIS. Can. J. For. Res. 2000, 30, 32–43. [Google Scholar] [CrossRef]
- He, H.S.; DeZonia, B.E.; Mladenoff, D.J. An aggregation index (AI) to quantify spatial patterns of landscapes. Landsc. Ecol. 2000, 15, 591–601. [Google Scholar] [CrossRef]
- Charney, N.D.; Babst, F.; Poulter, B.; Record, S.; Trouet, V.M.; Frank, D.; Enquist, B.J.; Evans, M.E.; Calcagno, V. Observed forest sensitivity to climate implies large changes in 21st century North American forest growth. Ecol. Lett. 2016, 19, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.E.; Lewin, K.F.; Isebrands, J.G.; Coleman, M.D.; Heilman, W.E.; Riemenschneider, D.E.; Sober, J.; Host, G.E.; Zak, D.R.; Hendrey, G.R.; et al. Forest Atmosphere Carbon Transfer and Storage (FACTS-II) the Aspen Free-Air CO2 and O3 Enrichment (FACE) Project: An Overview; USDA Forest Service Gen. Tech. Rep. NC-214, North Central Research Station: St. Paul, MN, USA, 2000. [Google Scholar]
- Girardin, M.P.; Bouriaud, O.; Hogg, E.H.; Kurz, W.; Zimmermann, N.E.; Metsaranta, J.M.; de Jong, R.; Frank, D.C.; Esper, J.; Büntgen, U.; et al. No growth stimulation of Canada’s boreal forest under half-century of combined warming and CO2 fertilization. Proc. Nat. Acad. Sci. USA 2016, 113, E8406–E8414. [Google Scholar] [CrossRef] [PubMed]
- Wythers, K.R.; Reich, P.B.; Tjoelker, M.G.; Bolstad, P.B. Foliar respiration acclimation to temperature and temperature variable Q10 alter ecosystem carbon balance. Glob. Chang. Biol. 2005, 11, 435–449. [Google Scholar] [CrossRef]
- McCann, K.S. The diversity–stability debate. Nature 2000, 405, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, E.J. When relationships estimated in the past cannot be used to predict the future: Using mechanistic models to predict landscape ecological dynamics in a changing world. Landsc. Ecol. 2013, 28, 1429–1437. [Google Scholar] [CrossRef]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C.; et al. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Duveneck, M.; Thompson, J.R.; De Bruijn, A.; Gustafson, E. Recovery dynamics and climate change effects to future New England forests. Landsc. Ecol. 2016, 32, 1385–1397. [Google Scholar] [CrossRef]
- Wang, W.J.; He, H.S.; Thompson, F.R.; Fraser, J.S.; Dijak, W.D. Changes in forest biomass and tree species distribution under climate change in the Northeastern United States. Landsc. Ecol. 2016, 32, 1399–1413. [Google Scholar] [CrossRef]
- Giardin, M.P.; Hogg, E.H.; Bernier, P.Y.; Kurz, W.A.; Guo, X.J.; Cyr, G. Negative impacts of high temperatures on growth of black spruce forests intensify with the anticipated climate warming. Glob. Chang. Biol. 2016, 22, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Hermle, S.; Lavigne, M.B.; Bernier, P.Y.; Bergeron, O.; Paré, D. Component respiration, ecosystem respiration and net primary production of a mature black spruce forest in northern Quebec. Tree Phys. 2010, 30, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salguero, R.; Camarero, J.J.; Gutiérrez, E.; González Rouco, F.; Gazol, A.; Sangüesa-Barreda, G.; Andreu-Hayles, L.; Linares, J.C.; Seftigen, K. Assessing forest vulnerability to climate warming using a process-based model of tree growth: Bad prospects for rear-edges. Glob. Chang. Biol. 2017, 23, 2705–2719. [Google Scholar] [CrossRef] [PubMed]
- Nemani, R.R.; Keeling, C.D.; Hashimoto, H.; Jolly, W.M.; Piper, S.C.; Tucker, C.J.; Myneni, R.B.; Running, S.W. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 2003, 300, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Oren, R.; Ellsworth, D.S.; Johnsen, K.H.; Phillips, N.; Ewers, B.E.; Maier, C.; Schäfer, K.V.R.; McCarthy, H.; Hendrey, G.; McNulty, S.G.; et al. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 2001, 411, 411–469. [Google Scholar] [CrossRef] [PubMed]
- De Graaff, M.; Van Groenigen, K.; Six, J.; Hungate, B.; Van Kessel, C. Interactions between plant growth and soil nutrient cycling under elevated CO2: A meta-analysis. Glob. Chang. Biol. 2006, 12, 2077–2091. [Google Scholar] [CrossRef]

| Life History Trait | Parameter | Low | Mid | High |
|---|---|---|---|---|
| Productivity | FolN (% wt.) 1 | 2.2 | 2.5 | 2.8 |
| Shade intolerance | HalfSat (μmol/m2/s) 2 | 275 | 437.5 | 600 |
| Drought tolerance | H3/H4 (MPa) 3 | −0.98/−1.37 | −1.07/−1.47 | −1.16/−1.57 |
| Optimal temperature | PsnTOpt (°C) 4 | 19 | 23 | 27 |
| Treatment | Parameter | Low | Mid | High |
|---|---|---|---|---|
| Temperature | Monthly min. and max. temperature (°C) 1 | +0 | +3 | +6 |
| CO2 | Mean monthly CO2 concentration (ppm) | 400 | 575 | 750 |
| Drought length | Drought length (yr) | 1 | 3 | 5 |
| Soil texture | AWC (mm) 2 | 60.5 | 107.5 | 150.8 |
| Species | Lon-Gevity (Years) | FolN (%) | Drought Tolerance Class 1 | Shade Tolerance Class 1 | PsnTOpt (°C) | Forest Type |
|---|---|---|---|---|---|---|
| Abies balsamea | 150 | 0.9 | S-intol | S-tol | 19 | SpruceFir |
| Acer rubrum | 200 | 2.2 | S-tol | S-tol | 26 | RedMaple |
| A. saccharum | 300 | 2.1 | S-intol | Tol | 23 | SmapBchBassYbirch |
| Betula alleghaniensis | 300 | 2.2 | S-intol | S-tol | 21 | SmapBchBassYbirch |
| B. papyrifera | 130 | 2.4 | Intol | S-intol | 21 | Aspen-birch |
| Carya cordiformis | 200 | 2.5 | S-intol | S-intol | 25 | Rare |
| Fagus grandifolia | 250 | 2.0 | S-tol | Tol | 23 | SmapBchBassYbirch |
| Fraxinus americana | 200 | 2.5 | S-tol | Interm. | 25 | CherryAsh |
| F. nigra | 150 | 2.6 | Intol | S-intol | 23 | Rare |
| F. pennsylvanica | 200 | 2.5 | S-tol | Interm. | 25 | CherryAsh |
| Picea glauca | 200 | 1.1 | S-tol | Interm. | 21 | SpruceFir |
| P. mariana | 200 | 1.0 | S-tol | Interm. | 20 | MxdSwampConif |
| Pinus banksiana | 100 | 1.3 | Tol | Intol | 20 | JackPine |
| P. resinosa | 250 | 1.5 | Tol | S-intol | 21 | PineOakHemlock |
| P. strobus | 300 | 1.8 | S-tol | Interm. | 21 | PineOakHemlock |
| Populus balsamifera | 150 | 2.4 | Intol | Intol | 19 | Aspen-birch |
| P. grandidentata | 90 | 2.5 | Intol | Intol | 22 | Aspen-birch |
| P. tremuloides | 90 | 2.5 | Intol | Intol | 21 | Aspen-birch |
| Prunus serotina | 150 | 2.5 | S-tol | S-intol | 25 | CherryAsh |
| Quercus alba | 300 | 2.7 | Tol | S-intol | 26 | MixedOak |
| Q. ellipsoidalis | 200 | 2.6 | Tol | S-intol | 21 | MixedOak |
| Q. macrocarpa | 300 | 2.7 | Tol | Interm. | 23 | MixedOak |
| Q. rubra | 210 | 2.6 | S-tol | Interm. | 24 | PineOakHemlock |
| Q. velutina | 200 | 2.3 | S-tol | Interm. | 24 | MixedOak |
| Thuja occidentalis | 400 | 1.0 | S-intol | Interm. | 20 | MxdSwampConif |
| Tilia americana | 200 | 2.5 | S-tol | S-tol | 23 | SmapBchBassYbirch |
| Tsuga canadensis | 450 | 1.4 | S-intol | Tol | 21 | PineOakHemlock |
| Treatment Factor | Low | High |
|---|---|---|
| Emission scenario | RCP3.0 | RCP8.5 |
| CO2 (ppm) * | 381–443 | 381–1962 |
| Approx. temperature rise (°C) * | +1.5 | +8 |
| Length of droughts (yrs) | 3 | 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gustafson, E.J.; Miranda, B.R.; Sturtevant, B.R. Can Future CO2 Concentrations Mitigate the Negative Effects of High Temperature and Longer Droughts on Forest Growth? Forests 2018, 9, 664. https://doi.org/10.3390/f9110664
Gustafson EJ, Miranda BR, Sturtevant BR. Can Future CO2 Concentrations Mitigate the Negative Effects of High Temperature and Longer Droughts on Forest Growth? Forests. 2018; 9(11):664. https://doi.org/10.3390/f9110664
Chicago/Turabian StyleGustafson, Eric J., Brian R. Miranda, and Brian R. Sturtevant. 2018. "Can Future CO2 Concentrations Mitigate the Negative Effects of High Temperature and Longer Droughts on Forest Growth?" Forests 9, no. 11: 664. https://doi.org/10.3390/f9110664
APA StyleGustafson, E. J., Miranda, B. R., & Sturtevant, B. R. (2018). Can Future CO2 Concentrations Mitigate the Negative Effects of High Temperature and Longer Droughts on Forest Growth? Forests, 9(11), 664. https://doi.org/10.3390/f9110664





