Assessment of Red-Edge Position Extraction Techniques: A Case Study for Norway Spruce Forests Using HyMap and Simulated Sentinel-2 Data
Abstract
:1. Introduction
2. Materials and Methods
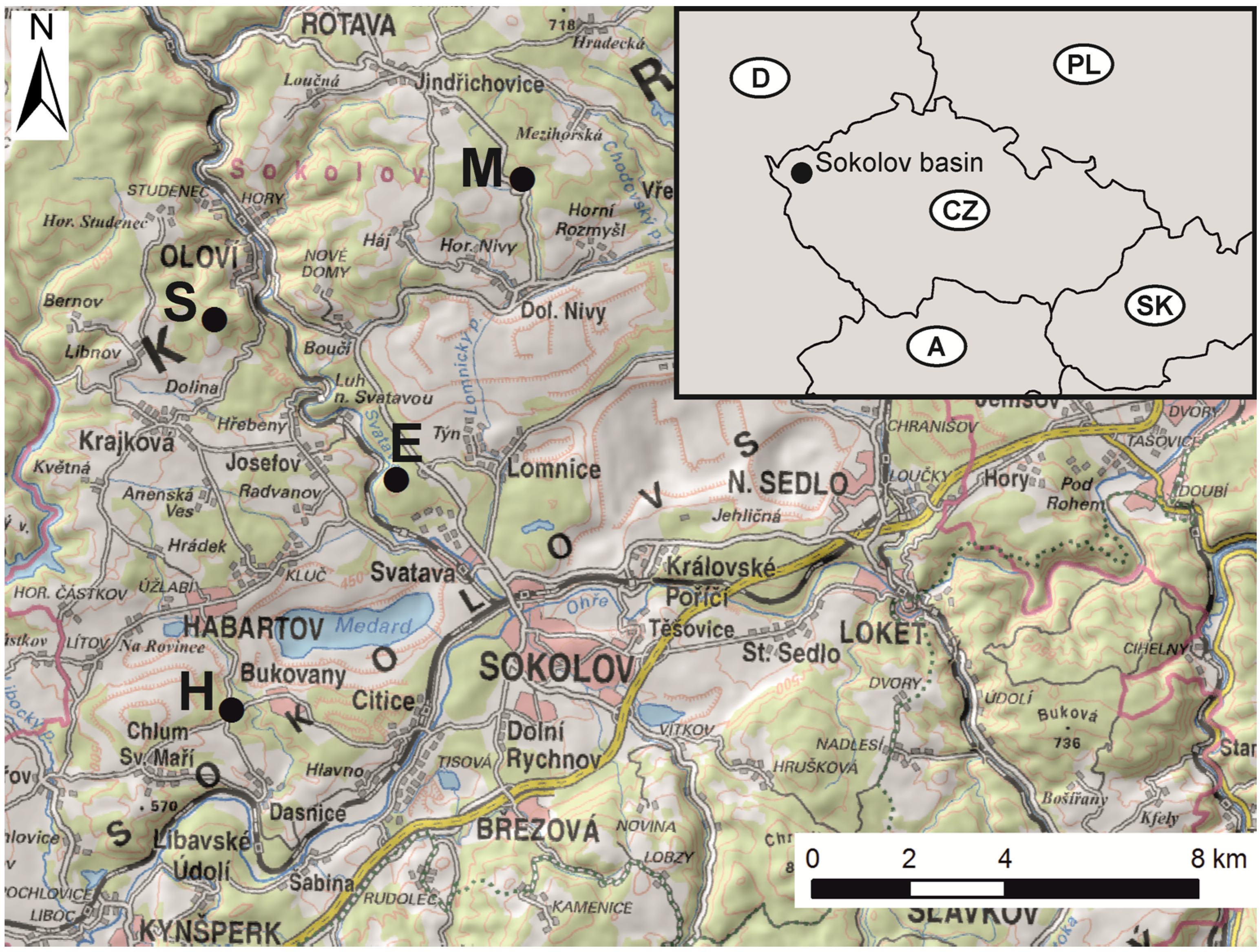
2.1. Study Area
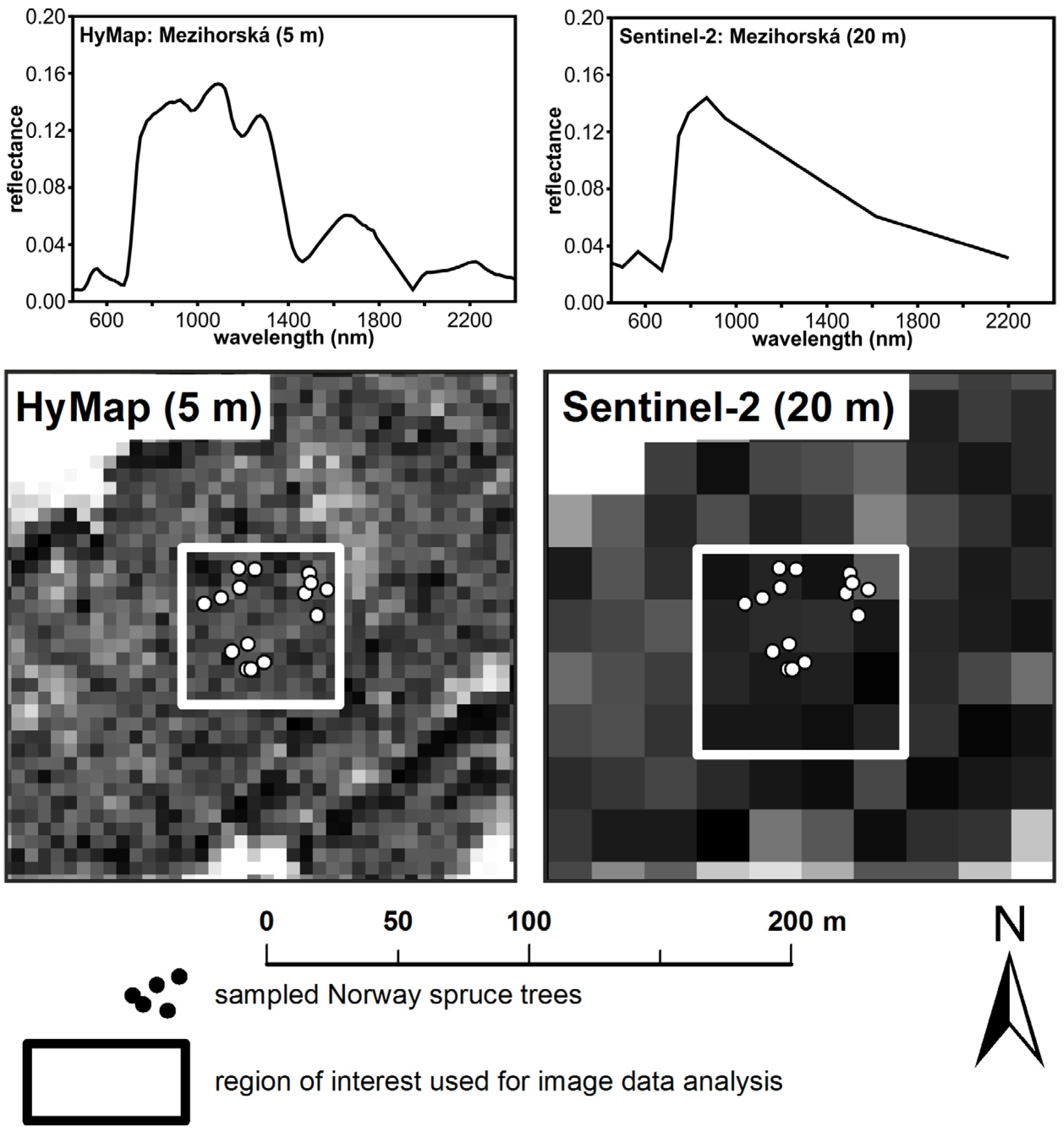
2.2. Airborne Hyperspectral Data Acquisition
2.3. Simulated Sentinel-2 Image Data
2.4. In-Situ and Laboratory Measurement of Vegetation Properties
2.5. Leaf and Canopy Radiative Transfer Modelling
2.6. Red-Edge Position Calculation
2.6.1. Polynomial Fitting (PF)
2.6.2. Linear Extrapolation (LE)
2.6.3. 4-Point Linear Interpolation (4PLI)
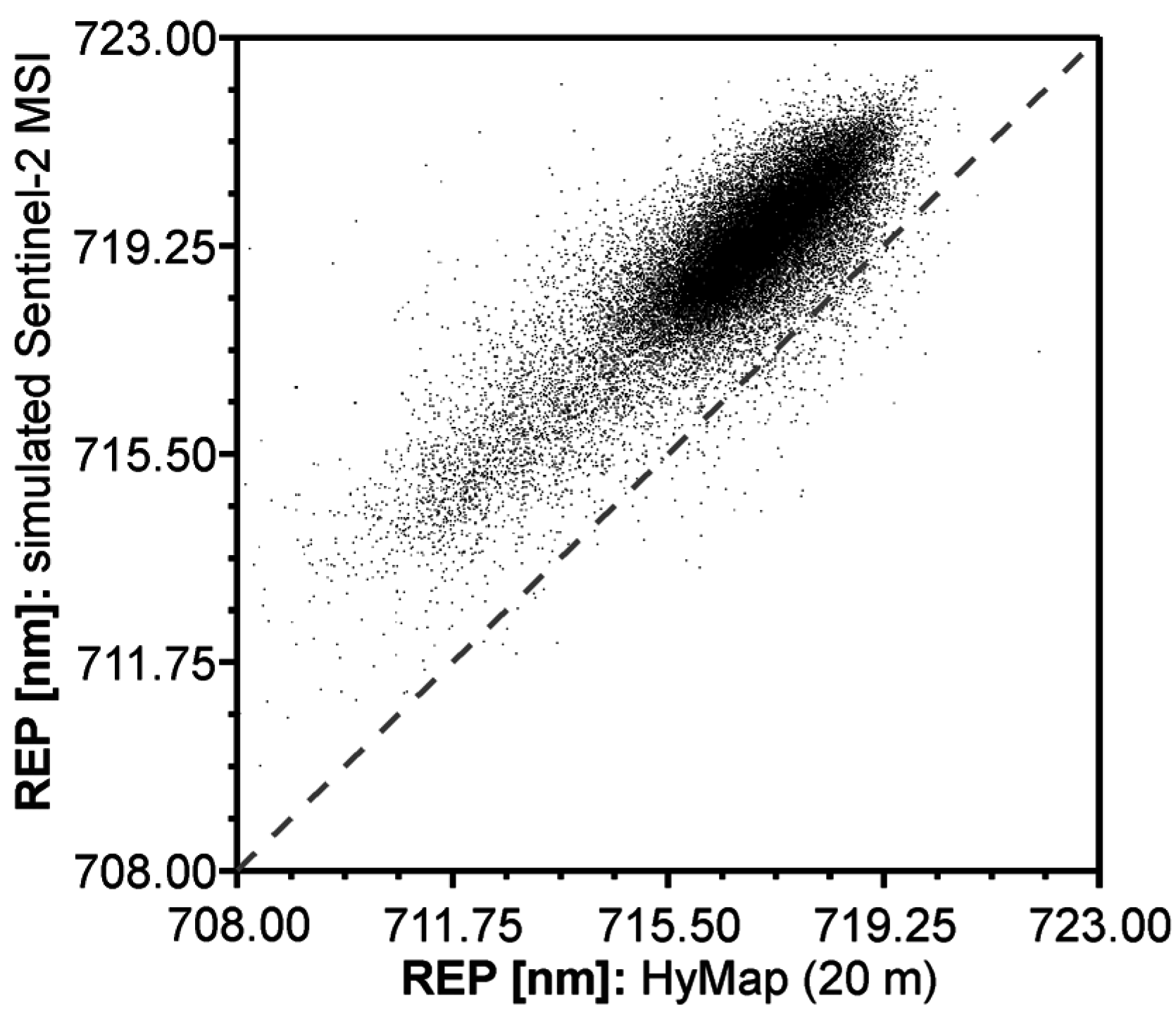
2.7. Comparison between REPIMG and REPSIM
2.8. Vegetation Properties and REP Correlation
3. Results
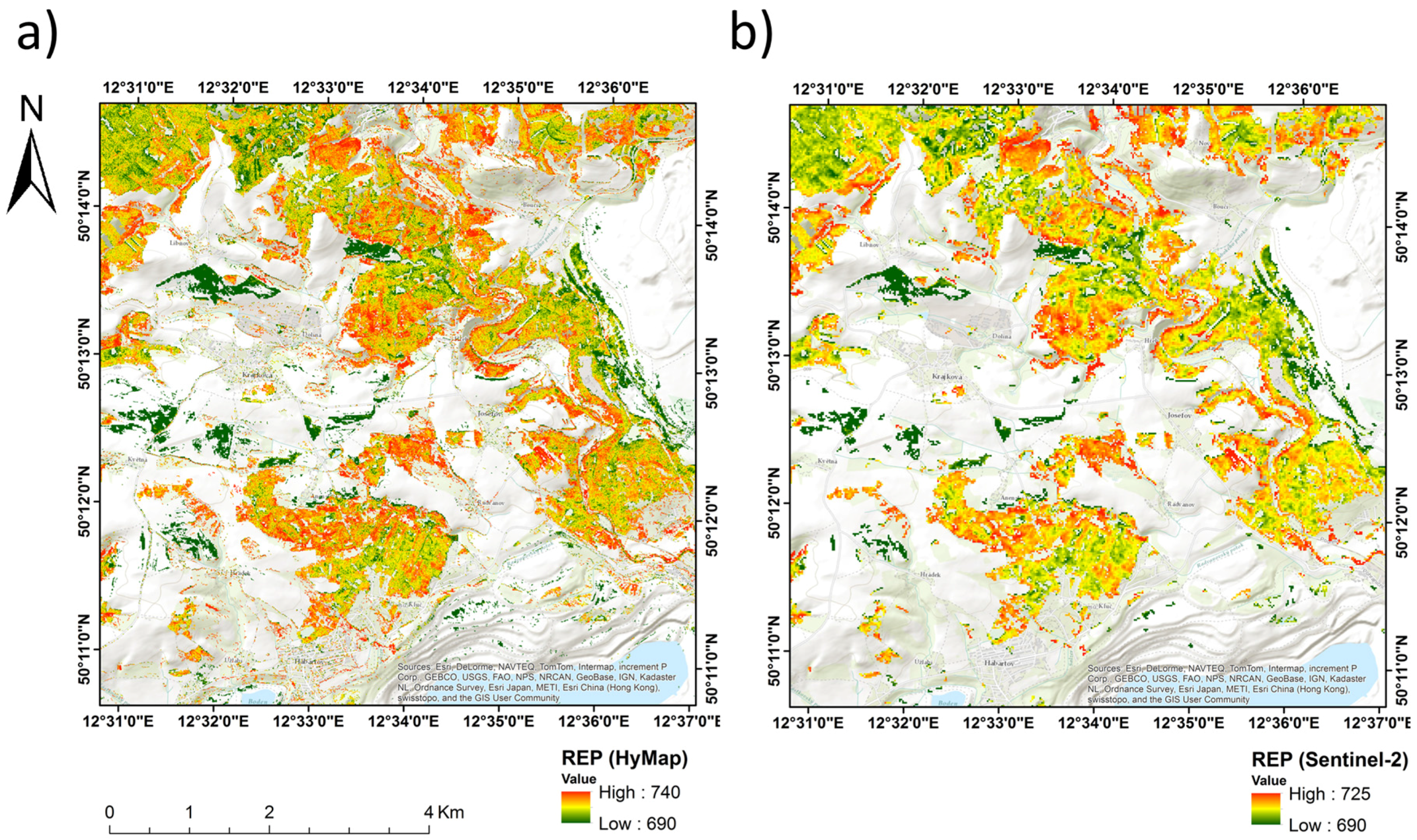
3.1. Comparison of REPSTD, REPIMG and REPSIM Values
3.2. Correlation of the REP Calculated by Different Methods with Chl × LAI Values
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Guzman, J.; Atkinson, P.M.; Dash, J.; Rioja-Nieto, R. Spatiotemporal variation in Mangrove chlorophyll concentration using Landsat 8. Remote Sens. 2015, 7, 14530–14558. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant. Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Saberioon, M.M.; Amin, M.S.M.; Gholizadeh, A.; Ezrin, M.H. A review of optical methods for assessing nitrogen contents during rice growth. Appl. Eng. Agric. 2014, 30, 657–669. [Google Scholar]
- Mišurec, J.; Kopačková, V.; Lhotáková, Z.; Hanus, J.; Weyermann, J.; Campbell, P.E.; Albrechtová, J. Utilization of hyperspectral image optical indices to assess the Norway spruce forest health status. J. Appl. Remote Sens. 2012, 6, 063545. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Atzberger, C.; Skidmore, A.K.; Abkar, A.A. Leaf area index derivation from hyperspectral vegetation indices and the red edge position. Int. J. Remote Sens. 2009, 30, 6199–6218. [Google Scholar] [CrossRef]
- Cho, M.A.; Skidmore, A.K. A new technique for extracting the red edge position from hyperspectral data: The linear extrapolation method. Remote Sens. Environ. 2006, 101, 181–193. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. PROSPECT: A model of leaf optical properties spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Cédric, B.; Zarco-Tejada, P.J.; Asner, G.P.; Francois, C.; Ustin, S.L. PROSPECT + SAIL models: A review of use for vegetation characteristics. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Ganapol, B.D.; Johnson, L.F.; Hammer, P.D.; Hlavka, C.A.; Peterson, D.L. A new within-leaf radiative transfer model. Remote Sens. Environ. 1998, 63, 182–193. [Google Scholar] [CrossRef]
- Verhoef, W. Light-scattering by leaf layers with application to canopy reflectance modelling—The SAIL model. Remote Sens. Environ. 1984, 16, 125–141. [Google Scholar] [CrossRef]
- Van der Tol, C.; Verhoef, W.; Timmermans, J.; Verhoef, A.; Su, Z. An integrated model of soil-canopy spectral radiance observations, photosynthesis, fluorescence, temperature and energy balance. Biogeosci. Discuss. 2009, 6, 6025–6075. [Google Scholar] [CrossRef]
- Demarez, V.; Gastellu-Etchegorry, J.P. A modeling approach for studying forest chlorophyll content. Remote Sens. Environ. 2000, 71, 226–238. [Google Scholar] [CrossRef]
- Jacquemoud, S. Inversion of the PROSPECT + SAIL canopy reflectance model from AVIRIS equivalent spectra: Theoretical study. Remote Sens. Environ. 1993, 44, 281–292. [Google Scholar] [CrossRef]
- Chaurasia, S.; Dadhwal, V.K. Comparison of principal component inversion with VI-empirical approach for LAI estimation using simulated reflectance data. Int. J. Remote Sens. 2004, 25, 2881–2887. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; de Jong, S.M.; Epema, G.F.; van der Meer, F.; Bakker, W.; Skidmore, A.K. Derivation of the red edge index using MERIS standard band setting. Int. J. Remote Sens. 2002, 23, 3169–3184. [Google Scholar] [CrossRef]
- Curran, P.J.; Windham, W.R.; Gholz, H.L. Exploring the relationship between reflectance red edge and chlorophyll concentration in slash pine leaves. Tree Physiol. 1995, 15, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.P.; Curran, P.J. A new technique for interpolating the reflectance red edge position. Int. J. Remote Sens. 1998, 19, 2133–2139. [Google Scholar] [CrossRef]
- Buschmann, C.; Nagel, E. In vivo spectroscopy and internal optics of leaves as basis for remote sensing of vegetation. Int. J. Remote Sens. 1993, 14, 711–722. [Google Scholar] [CrossRef]
- Boochs, F.; Kupfer, G.; Dockter, K.; Kuhbauch, W. Shape of the red-edge as vitality indicator for plants. Int. J. Remote Sens. 1990, 11, 1741–1753. [Google Scholar] [CrossRef]
- Gates, D.M.; Keegan, H.J.; Schleter, J.C.; Weidner, V.R. Spectral properties of plants. Appl. Opt. 1965, 4, 11–20. [Google Scholar] [CrossRef]
- Horler, D.N.H.; Dockray, M.; Barber, J. The red edge of plant leaf reflectance. Int. J. Remote Sens. 1983, 4, 273–288. [Google Scholar] [CrossRef]
- Ramoelo, A.; Skidmore, A.K.; Cho, M.A.; Schlerf, M.; Mathieu, R.; Heitkönig, I.M.A. Regional estimation of savanna grass nitrogen using the red-edge band of the spaceborne rapideye sensor. Int. J. Appl. Earth Obs. Geoinform. 2012, 19, 151–162. [Google Scholar] [CrossRef]
- Verrelst, J.; Camps-Valls, G.; Muñoz-Marí, J.; Rivera, J.P.; Veroustraete, F.; Clevers, J.G.P.W.; Moreno, J.J. Optical remote sensing and the retrieval of terrestrial vegetation bio-geophysical properties—A review. ISPRS J. Photogramm. Remote Sens. 2015, 108, 273–290. [Google Scholar] [CrossRef]
- Description of Derivative-Based High Spectral-Resolution (AVIRIS) Green Vegetation Index. Available online: http://proceedings.spiedigitallibrary.org/proceeding.aspx?articleid=1011206 (accessed on 8 October 2016).
- Demetriades-Shah, T.H.; Steven, M.D.; Clark, J.A. High resolution derivative spectra in remote sensing. Remote Sens. Environ. 1990, 33, 55–64. [Google Scholar] [CrossRef]
- Railyan, V.Y.; Korobov, R.M. Red edge structure of canopy refectance spectra of triticale. Remote Sens. Environ. 1993, 46, 173–182. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Pu, R.; Gong, P.; Biging, G.S.; Larrieu, M.R. Extraction of red edge optical parameters from Hyperion data for estimation of forest leaf area index. IEEE Trans. Geosci. Remote Sens. 2003, 41, 916–921. [Google Scholar]
- Hare, E.W.; Miller, J.R.; Hollinger, A.B.; Sturgeon, D.R.; O’Neil, N.T.; Ward, T.V. Measurements of the vegetation reflectance red edge with an airborne programmable imaging spectrometer. In Proceedings of the Fifth Thematic Conference on Remote Sensing For Exploration Geology, Reno, NV, USA, 29 October 1986; Environmental Institute of Michigan: Ann Arbor, MI, USA; p. 657.
- Frampton, W.J.; Dash, J.; Watmough, G.; Milton, E.J. Evaluating the capabilities of Sentinel-2 for quantitative estimation of biophysical variables in vegetation. ISPRS J. Photogramm. Remote Sens. 2013, 82, 83–92. [Google Scholar] [CrossRef]
- Dotzler, S.; Hill, J.; Buddenbaum, H.; Stoffels, J. The potential of EnMAP and Sentinel-2 data for detecting drought stress phenomena in deciduous forest communities. Remote Sens. 2015, 7, 14227–14258. [Google Scholar] [CrossRef]
- Laurin, G.V.; Puletti, N.; Hawthorne, W.; Liesenberg, V.; Corona, P.; Papale, D.; Chen, Q.; Valentini, R. Discrimination of tropical forest types, dominant species, and mapping of functional guilds by hyperspectral and simulated multispectral Sentinel-2 data. Remote Sens. Environ. 2016, 176, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Verrelst, J.; Rivera, J.P.; Veroustraete, F.; Muñoz-Marí, J.; Clevers, J.G.P.W.; Camps-Valls, G.; Moreno, J.J. Experimental Sentinel-2 LAI estimation using parametric, non-parametric and physical retrieval methods—A comparison. ISPRS J. Photogramm. Remote Sens. 2015, 108, 260–272. [Google Scholar] [CrossRef]
- Delegido, J.; Verrelst, J.; Alonso, L.; Moreno, J. Evaluation of Sentinel-2 red-edge bands for empirical estimation of green LAI and chlorophyll content. Sensors 2011, 11, 7063–7081. [Google Scholar] [CrossRef] [PubMed]
- Atzberger, C.; Richter, K. Spatially constrained inversion of radiative transfer models for improved LAI mapping from future Sentinel-2 imagery. Remote Sens. Environ. 2012, 120, 208–218. [Google Scholar] [CrossRef]
- Richter, K.; Atzberger, C.; Vuolo, F.; Weihs, P.; D’Urso, G. Experimental assessment of the Sentinel-2 band setting for RTM-based LAI retrieval of sugar beet and maize. Can. J. Remote Sens. 2009, 35, 230–247. [Google Scholar] [CrossRef]
- Richter, K.; Hank, T.B.; Vuolo, F.; Mauser, W.; D’Urso, G. Optimal exploitation of the Sentinel-2 spectral capabilities for crop leaf area index mapping. Remote Sens. 2012, 4, 561–582. [Google Scholar] [CrossRef] [Green Version]
- Clevers, J.G.P.W.; Gitelson, A.A. Remote estimation of crop and grass chlorophyll and nitrogen content using red-edge bands on Sentinel-2 and -3. Int. J. Appl. Earth Obs. Geoinform. 2013, 23, 344–351. [Google Scholar] [CrossRef]
- Verrelst, J.; Munoz, J.; Alonso, L.; Delegido, J.; Rivera, J.P.; Camps-Valls, G.; Moreno, J. Machine learning regression algorithms for biophysical parameter retrieval: Opportunities for Sentinel-2 and -3. Remote Sens. Environ. 2012, 118, 127–139. [Google Scholar] [CrossRef]
- Delegido, J.; Verrelst, J.; Meza, C.M.; Rivera, J.P.; Alonso, L.; Moreno, J. A red-edge spectral index for remote sensing estimation of green LAI over agroecosystems. Eur. J. Agron. 2013, 46, 42–52. [Google Scholar] [CrossRef]
- Schlerf, M.; Atzberger, C.; Hill, J. Remote sensing of forest biophysical variables using HyMap imaging spectrometer data. Remote Sens. Environ. 2005, 95, 177–194. [Google Scholar] [CrossRef]
- Fernández-Manso, A.; Fernández-Manso, O.; Quintano, C. SENTINEL-2A red-edge spectral indices suitability for discriminating burn severity. Int. J. Appl. Earth Obs. Geoinform. 2016, 50, 170–175. [Google Scholar] [CrossRef]
- Kopačková, V.; Mišurec, J.; Lhotáková, Z.; Oulehle, F.; Albrechtová, J. Using multi-date high spectral resolution data to assess the physiological status of macroscopically undamaged foliage on a regional scale. Int. J. Appl. Earth Obs. Geoinform. 2014, 27, 169–186. [Google Scholar] [CrossRef]
- Hu, B.; Lucht, W.; Li, X.; Strahler, A.H. Validation of kernel-driven models for the BRDF of land surfaces. Remote Sens. Environ. 1997, 62, 201–214. [Google Scholar] [CrossRef]
- Schaaf, C.; Gao, F.; Strahler, A.; Lucht, W.; Li, X.; Tsang, T.; Strugnell, N.; Zhang, X.; Jin, Y.; Muller, J.P.; et al. First operational BRDF, Albedo and nadir reflectance products from MODIS. Remote Sens. Environ. 2002, 83, 135–148. [Google Scholar] [CrossRef]
- Segl, K.; Guanter, L.; Gascon, F.; Kuester, T.; Rogass, C.; Mielke, C. S2eteS: An end-to-end modeling tool for simulation of S2image products. IEEE Trans. Geosci. Remote Sens. 2015, 53, 5560–5571. [Google Scholar] [CrossRef]
- Mielke, C.; Boesche, N.K.; Rogass, C.; Segl, K.; Gauert, C.; Kaufmann, H. Potential applications of the Sentinel-2 multispectral sensor and the EnMap hyperspectral sensor in mineral exploration. EARSeL eProc. 2014, 13, 93–102. [Google Scholar]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectrometry. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Homolová, L.; Malenovský, Z.; Clevers, J.G.P.W.; García-Santos, G.; Schaepman, M.E. Review of optical-based remote sensing for plant trait mapping. Ecol. Complex. 2013, 15, 1–16. [Google Scholar] [CrossRef]
- Lhotáková, Z.; Brodský, L.; Kupková, L.; Kopačková, V.; Potůčková, M.; Mišurec, J.; Klement, A.; Kovářová, M.; Albrechtová, J. Detection of multiple stresses in Scots pine growing at post-mining sites using visible to near-infrared spectroscopy. J. Environ. Monit. 2013, 15, 2004–2015. [Google Scholar]
- Féret, J.B.; François, C.; Asner, G.P.; Gitelson, A.A.; Martin, R.E.; Bidel, L.P.R.; Ustin, S.L.; le Maire, G.; Jacquemoud, S. PROSPECT-4 and 5: Advances in the leaf optical properties model separating photosynthetic pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R. Land cover mapping at BOREAS using red edge spectral parameters from CASI imagery. J. Geophys. Res. 1999, 104, 27921–27933. [Google Scholar] [CrossRef]
- Rautiainen, M.; Mõttus, M.; Yáñez-Rausell, L.; Homolová, L.; Malenovský, Z.; Schaepman, M.E. A note on upscaling coniferous needle spectra to shoot spectral albedo. Remote Sens. Environ. 2012, 117, 469–474. [Google Scholar] [CrossRef]
- Knyazikhin, Y.; Schull, M.A.; Xu, L.; Myneni, R.B.; Samanta, A. Canopy spectral invariants. Part 1: A new concept in remote sensing of vegetation. J. Quant. Spectrosc. Radiat. Transf. 2011, 112, 727–735. [Google Scholar] [CrossRef]
- Huang, D.; Knyazikhin, Y.; Dickinson, R.E.; Rautiainen, M.; Stenberg, P.; Disney, M.; Lewis, P.; Cescatti, A.; Tian, Y.; Verhoef, W.; et al. Canopy spectral invariants for remote sensing and model applications. Remote Sens. Environ. 2007, 106, 106–122. [Google Scholar] [CrossRef]
- Kuusk, A.; Nilson, T. A directional multispectral forest reflectance model. Remote Sens. Environ. 2000, 72, 244–252. [Google Scholar] [CrossRef]
- Allometric Relationships of Selected European Tree Species. Available online: http://publications.jrc.ec.europa.eu/repository/bitstream/JRC26286/EUR%2020855%20EN.pdf (accessed on 28 September 2016).
- Bachmann, M.; Richter, R.; Holzwarth, S.; Weide, S.; Fischer, C.; Ehrler, C. Quality Report on HyMap 2009 Sokolov Data; Internal Report of the EO-MINERS Project, DLR; DLR’s Research Center Oberpfaffenhofen: Bavaria, Germany, 2011; pp. 1–25. [Google Scholar]
- Glenn, E.P.; Huete, A.R.; Nagler, P.L.; Nelson, S.G. Relationship between remotely-sensed vegetation indices, canopy attributes and plant physiological processes: What vegetation indices can and cannot tell us about the landscape. Sensors 2008, 8, 2136–2160. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Skidmore, A.; Schlerf, M.; Atzberger, C.; Corsi, F.; Cho, M. LAI and chlorophyll estimation for a heterogeneous grassland using hyperspectral measurements. ISPRS J. Photogramm. Remote Sens. 2008, 63, 409–426. [Google Scholar] [CrossRef]
- Verrelst, J.; Alonso, L.; Camps-Valls, G.; Delegido, J.; Moreno, J. Retrieval of canopy parameters using gaussian processes techniques. IEEE Trans. Geosci. Remote Sens. 2011. [Google Scholar] [CrossRef]
- Smola, J.; Schölkopf, B. A tutorial on support vector regression. Stat. Comput. 2004, 14, 199–222. [Google Scholar] [CrossRef]
- Camps-Valls, G.; Bruzzone, L.; Rojo-Alvarez, J.L.; Melgani, F. Robust support vector regression for biophysical variable estimation from remotely sensed images. IEEE Geosci. Remote Sens. Lett. 2006, 3, 339–343. [Google Scholar] [CrossRef]
- Baret, F.; Hagolle, O.; Geiger, B.; Bicheron, P.; Miras, B.; Huc, M.; Berthelot, B.; Niño, F.; Weiss, M.; Samain, O.; et al. LAI, fAPAR and fCover CYCLOPES global products derived from VEGETATION: Part 1: Principles of the algorithm. Remote Sens. Environ. 2007, 110, 275–286. [Google Scholar] [CrossRef] [Green Version]
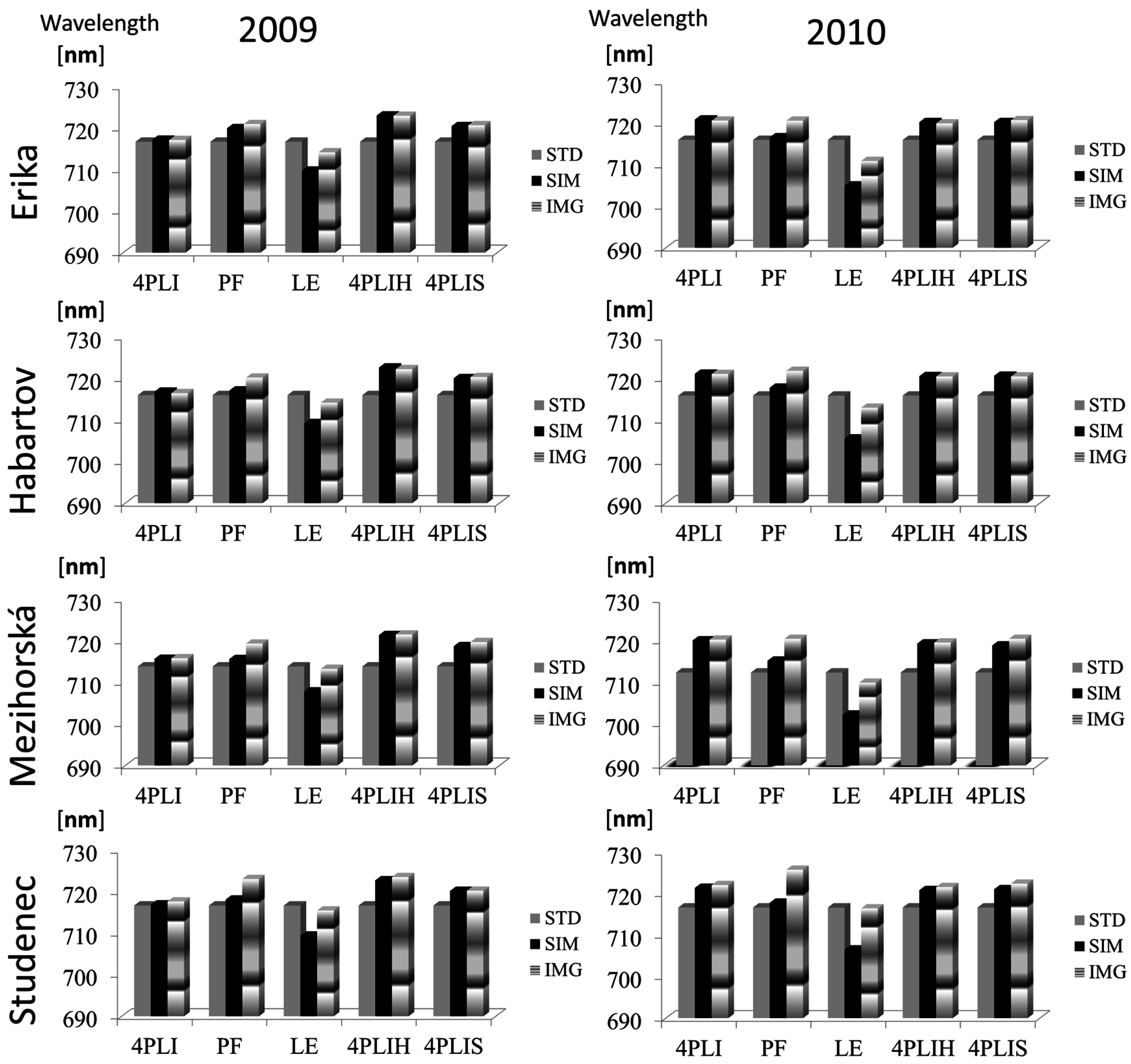
| Method | Year | RMSE (nm) | R2 |
|---|---|---|---|
| 4PLI | 2009 | 0.45 | 0.66 |
| 2010 | 0.43 | 0.74 | |
| PF | 2009 | 3.51 | 0.28 |
| 2010 | 5.57 | 0.48 | |
| LE | 2009 | 5.25 | 0.49 |
| 2010 | 7.88 | 0.71 | |
| 4PLIH | 2009 | 0.42 | 0.66 |
| 2010 | 0.38 | 0.74 | |
| 4PLIS | 2009 | 0.75 | 0.67 |
| 2010 | 1.11 | 0.69 |
| Method | Year | Data | RMSE (nm) | R2 |
|---|---|---|---|---|
| 4PLI | 2009 | REPSIM/STD | 5.14 | 0.96 |
| REPIMG/STD | 5.31 | 0.81 | ||
| 2010 | REPSIM/STD | 5.76 | 0.96 | |
| REPIMG/STD | 5.92 | 0.56 | ||
| PF | 2009 | REPSIM/STD | 6.04 | 0.75 |
| REPIMG/STD | 6.15 | 0.56 | ||
| 2010 | REPSIM/STD | 7.81 | 0.83 | |
| REPIMG/STD | 7.15 | 0.37 | ||
| LE | 2009 | REPSIM/STD | 6.78 | 0.92 |
| REPIMG/STD | 6.71 | 0.62 | ||
| 2010 | REPSIM/STD | 10.42 | 0.86 | |
| REPIMG/STD | 7.18 | 0. 53 | ||
| 4PLIH | 2009 | REPSIM/STD | 5.02 | 0.96 |
| REPIMG/STD | 5.13 | 0.81 | ||
| 2010 | REPSIM/STD | 6.64 | 0.96 | |
| REPIMG/STD | 6.76 | 0.56 | ||
| 4PLIS | 2009 | REPSIM/STD | 4.06 | 0.98 |
| REPIMG/STD | 4.73 | 0.81 | ||
| 2010 | REPSIM/STD | 5.05 | 0.96 | |
| REPIMG/STD | 6.11 | 0.52 |
| Method | Year | REPSIM | REPIMG |
|---|---|---|---|
| Chl × LAI | Chl × LAI | ||
| HyMap Level | |||
| 4PLI | 2009 | 0.72 (RMSE: 35.99 mg/m2) | 0.58 (RMSE: 44.05 mg/m2) |
| 2010 | 0.49 (RMSE 40.12 mg/m2) | 0.67 (RMSE: 32.04 mg/m2) | |
| PF | 2009 | 0.91 (RMSE: 5.99 mg/m2) | 0.30 (RMSE: 57.26 mg/m2) |
| 2010 | 0.28 (RMSE: 47.61 mg/m2) | 0.61 (RMSE: 34.46 mg/m2) | |
| LE | 2009 | 0.74 (RMSE: 35.13 mg/m2) | 0.22 (RMSE: 60.27 mg/m2) |
| 2010 | 0.51 (RMSE: 39.21 mg/m2) | 0.61 (RMSE: 34.98 mg/m2) | |
| 4PLIH | 2009 | 0.72 (RMSE: 35.99 mg/m2) | 0.58 (RMSE: 44.05 mg/m2) |
| 2010 | 0.49 (RMSE: 40.12 mg/m2) | 0.67 (RMSE: 32.04 mg/m2) | |
| Sentinel-2 Level | |||
| 4PLIS | 2009 | 0.71 (RMSE: 36.61 mg/m2) | 0.48 (RMSE: 49.28 mg/m2) |
| 2010 | 0.48 (RMSE: 40.60 mg/m2) | 0.67 (RMSE: 32.11 mg/m2) | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gholizadeh, A.; Mišurec, J.; Kopačková, V.; Mielke, C.; Rogass, C. Assessment of Red-Edge Position Extraction Techniques: A Case Study for Norway Spruce Forests Using HyMap and Simulated Sentinel-2 Data. Forests 2016, 7, 226. https://doi.org/10.3390/f7100226
Gholizadeh A, Mišurec J, Kopačková V, Mielke C, Rogass C. Assessment of Red-Edge Position Extraction Techniques: A Case Study for Norway Spruce Forests Using HyMap and Simulated Sentinel-2 Data. Forests. 2016; 7(10):226. https://doi.org/10.3390/f7100226
Chicago/Turabian StyleGholizadeh, Asa, Jan Mišurec, Veronika Kopačková, Christian Mielke, and Christian Rogass. 2016. "Assessment of Red-Edge Position Extraction Techniques: A Case Study for Norway Spruce Forests Using HyMap and Simulated Sentinel-2 Data" Forests 7, no. 10: 226. https://doi.org/10.3390/f7100226
APA StyleGholizadeh, A., Mišurec, J., Kopačková, V., Mielke, C., & Rogass, C. (2016). Assessment of Red-Edge Position Extraction Techniques: A Case Study for Norway Spruce Forests Using HyMap and Simulated Sentinel-2 Data. Forests, 7(10), 226. https://doi.org/10.3390/f7100226






