Regulation of Water Use in the Southernmost European Fir (Abies pinsapo Boiss.): Drought Avoidance Matters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Field Sampling
2.2. Microclimatic Variables
2.3. Ecophysiological Variables
2.4. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Microclimatology
| Soil Profile | LowElevation (1200 m) | High Elevation (1550 m) | ||||||
|---|---|---|---|---|---|---|---|---|
| Soil Type 1 | TypicHaploxerept | TypicHaploxerept | ||||||
| Slope (%) | 36 | 42 | ||||||
| Elevation (m a.s.l.) | 1200 | 1550 | ||||||
| Aspect | NW | NW | ||||||
| Soil Horizon | Ah | Bw | Bw/R | Ah | Ah/C | C | ||
| Prof. (cm) | 0–12 | 12–25 | 25–50 | 0–12 | 12–25 | 25–50 | ||
| Texture (%) | Clay | 21.6 | 37.7 | 27.9 | 14.2 | 16.5 | 17.2 | |
| Silt | 49 | 46 | 52.8 | 47.8 | 39.4 | 28.6 | ||
| Sand | 29.4 | 16.3 | 19.3 | 38 | 44.1 | 54.2 | ||
| Gravel(%) | 13.1 | 8 | 19.8 | 26.3 | 37.7 | 42.2 | ||
| Bulk density 2 | 1.29 | 1.41 | 1.31 | 1.44 | 1.51 | 1.49 | ||
| Textural class 1 | Loam | Silty-clay-loam | Silty-clay-loam | Loam | Loam | Sandy-loam | ||
| pF 33 kPa (empirical) 3 | 44.3 | 46.5 | 46.3 | 42.4 | 37.8 | 35.2 | ||
| pF 1500 kPa (empirical) 3 | 24.7 | 31.0 | 30.3 | 21.4 | 20.3 | 19.3 | ||
| pF 33 kPa (estimated) 4 | 33.1 | 36.5 | 35.1 | 28.9 | 27.0 | 25.6 | ||
| pF 1500 kPa (estimated) 4 | 15.4 | 22.3 | 17.8 | 11.4 | 12.0 | 12.2 | ||
| Available water content (l·m−2·0.5m −1) | 82.1 | 81.7 | ||||||
| Roots (mg·g soil−1) | 4.41 | 3.81 | 4.07 | 0.29 | 1.57 | 1.35 | ||
| O. C. (%) | 3.25 | 1.93 | 3.06 | 1.58 | 0.9 | 1.09 | ||
| O. N. (%) | 0.17 | 0.12 | 0.19 | 0.1 | 0.04 | 0.05 | ||
| N-NO3− (ppm) | 6.1 | <1 | 1.98 | 5.3 | <1 | <1 | ||
| N-NH4+ (ppm) | 17.5 | 15.5 | 17.0 | 8.65 | 3.2 | 3.51 | ||
| P Olsen (ppm) | 2.9 | 2.4 | 2.9 | 4.4 | 8.1 | 13.7 | ||
| Ca2+ | 17.4 | 14.6 | 19.1 | 9.09 | 6.77 | 8.48 | ||
| Exchangeable bases | Mg2+ | 1.44 | 8.45 | 4.07 | 0.61 | 1.36 | 0.33 | |
| (cmolc·kg−1) | Na+ | 0.26 | 0.28 | 0.24 | 0.24 | 0.24 | 0.26 | |
| K+ | 0.59 | 0.3 | 0.27 | 0.5 | 0.19 | 0.18 | ||
| CEC (meq/100g) | 22.4 | 21.6 | 23.7 | 11.0 | 10.9 | 11.4 | ||
| % Base saturation | 88.2 | 100 | 100 | 95.3 | 78.8 | 81.2 | ||

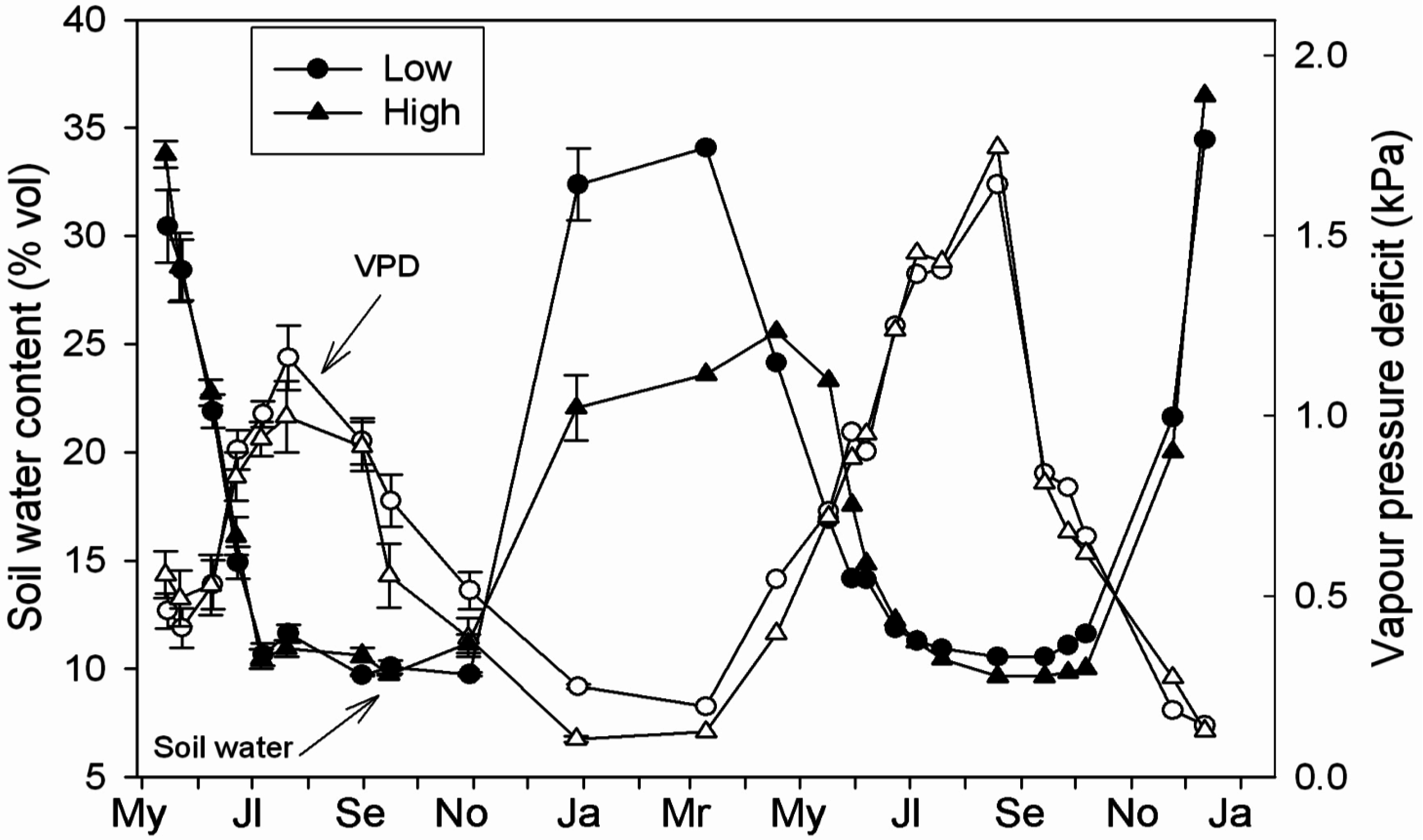
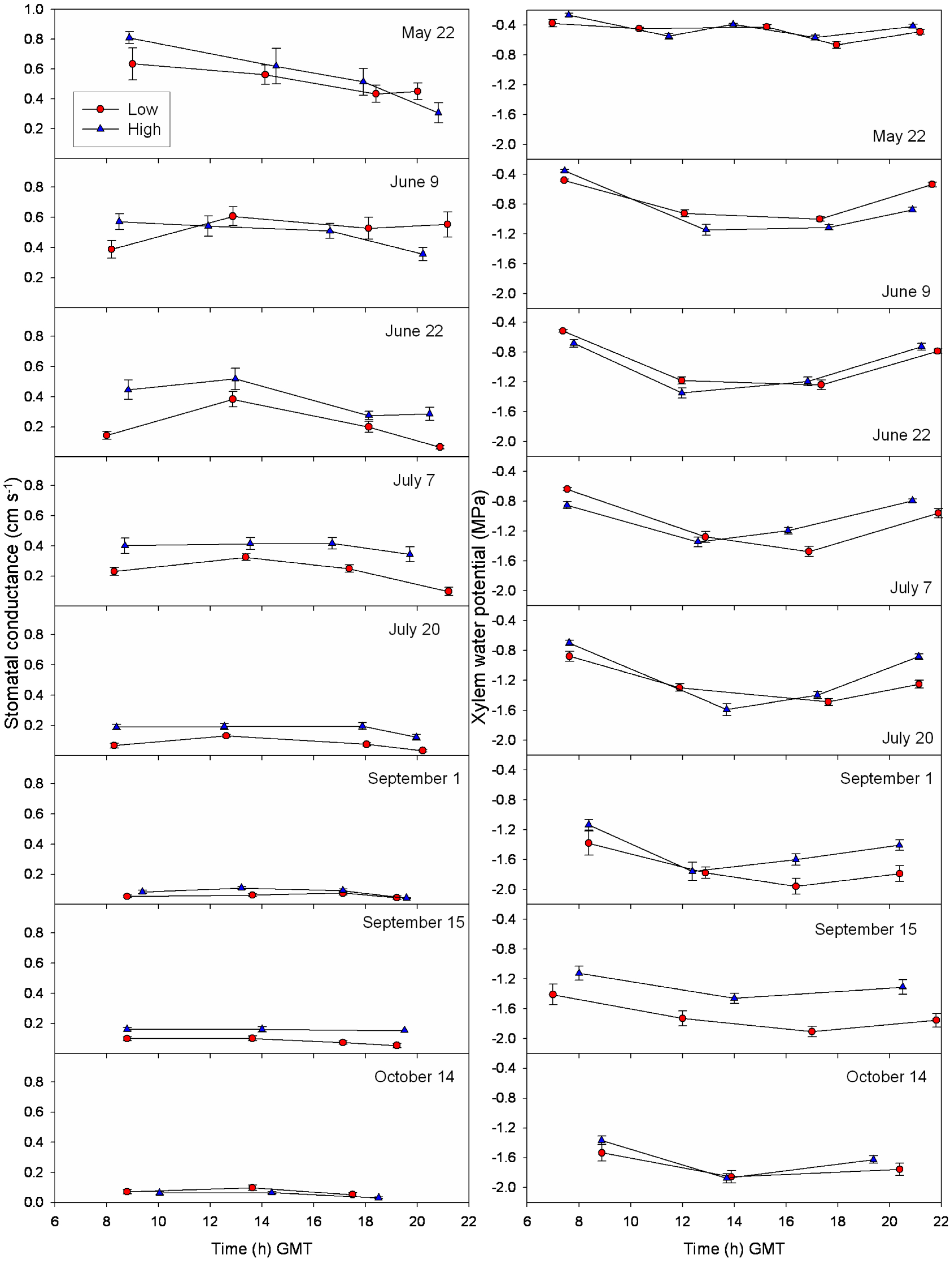
3.3. Daily Curves of Stomatal Conductance and Xylem Water Potential
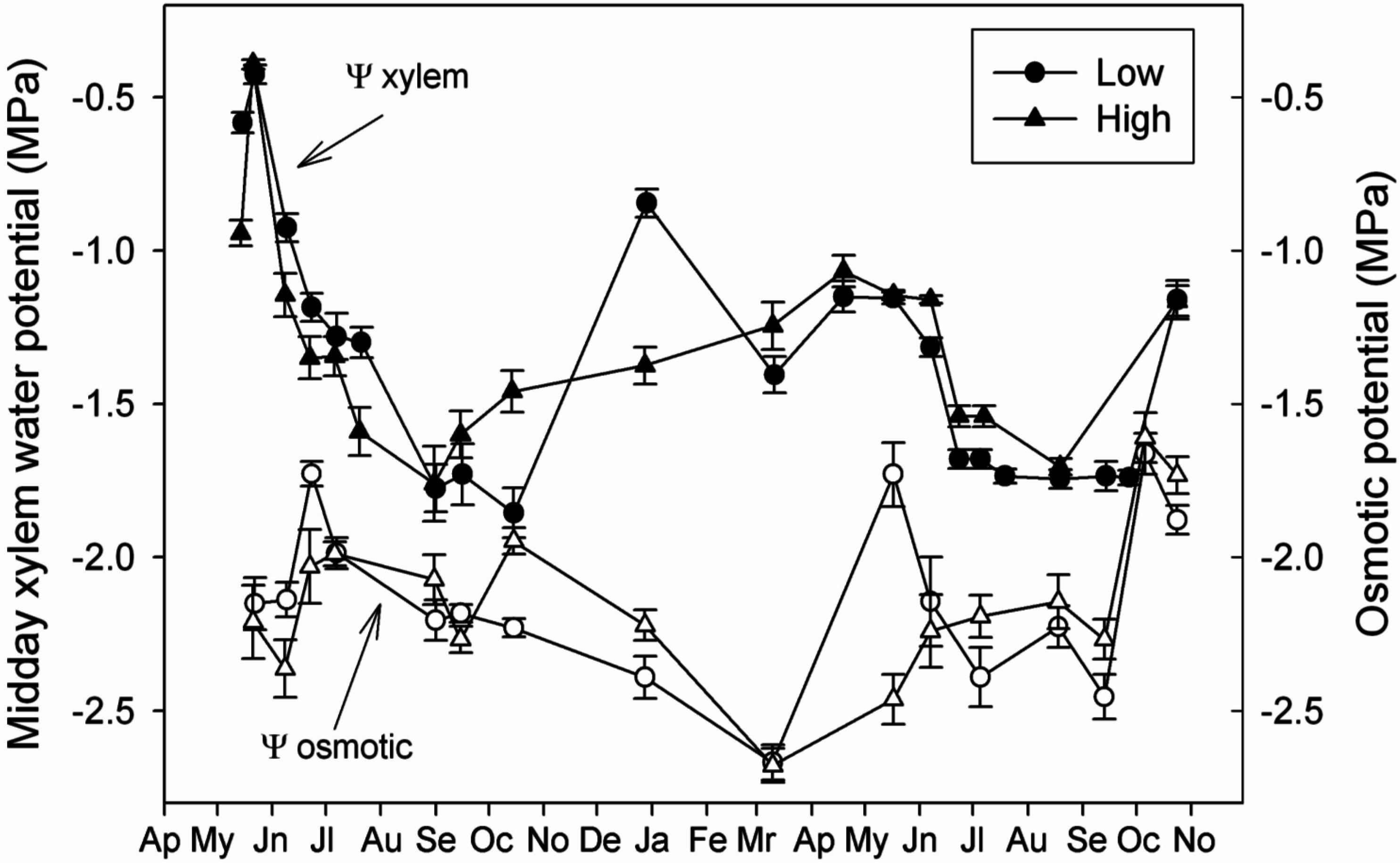
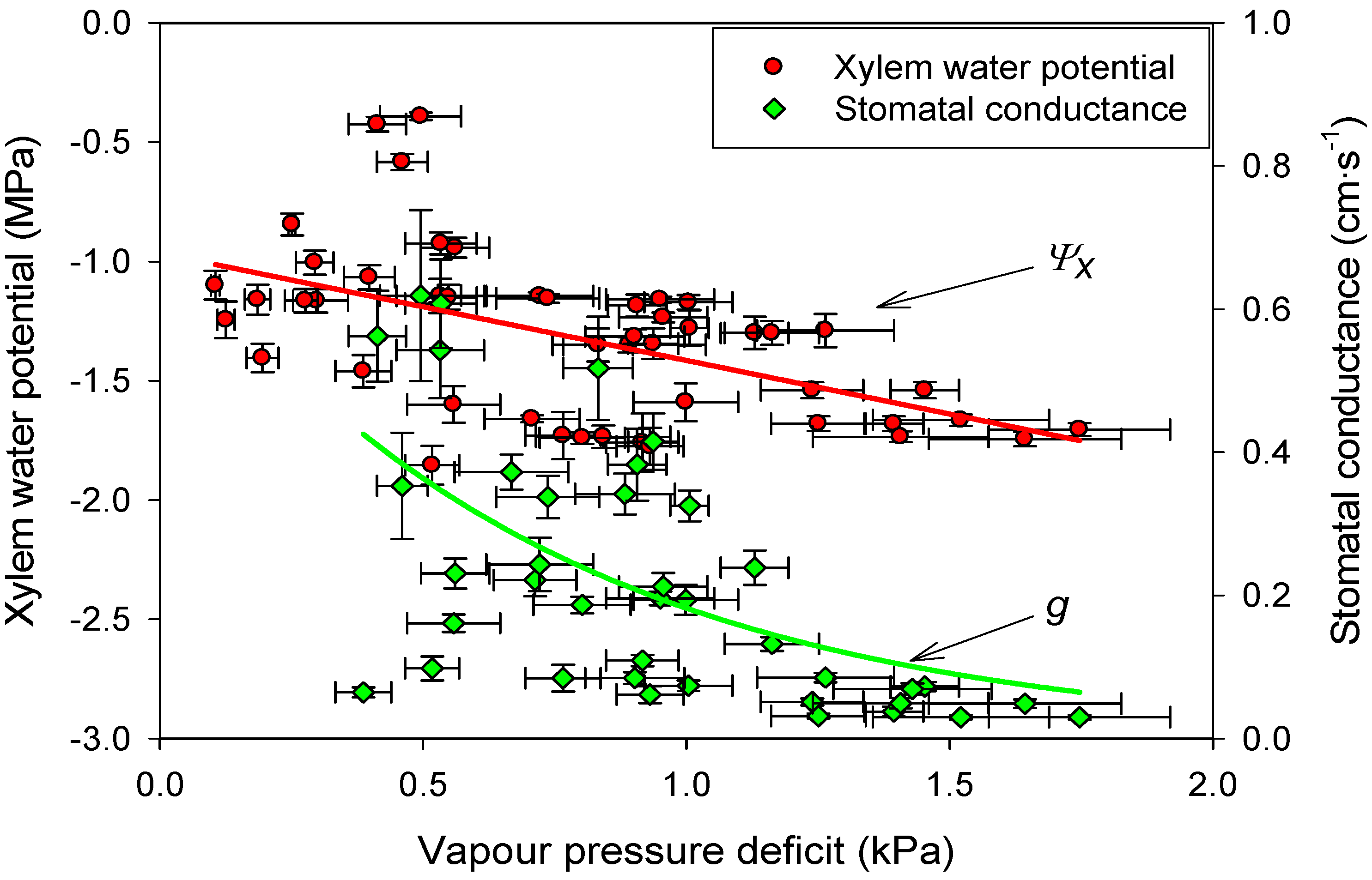
3.4. Linking Physiological and Environmental Variables Related to Water Balance
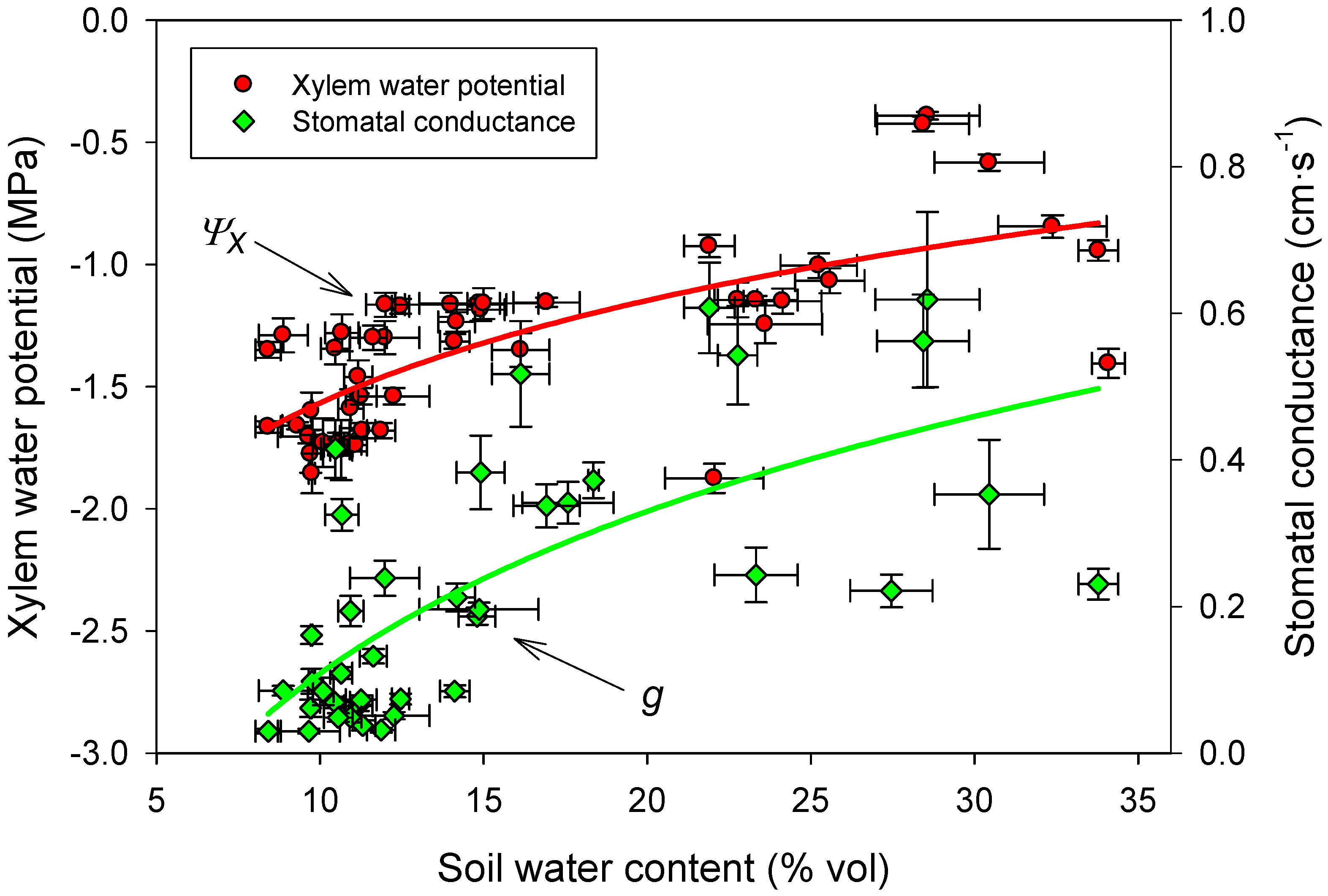
3.5. Foliar Nutrient Content
| Low elevation (1200 m) | High elevation (1550 m) | |||
|---|---|---|---|---|
| Time spam | Growing period (May–June) | Summer–Autumn (July–September) | Growing period (May–June) | Summer–Autumn (July–September) |
| Carbon (%) | 47.1 ± 0.16b | 47.8 ± 0.10c | 46.7 ± 0.13a | 47.2 ± 0.08b |
| Nitrogen (%) | 1.11 ± 0.06c | 0.82 ± 0.03a | 1.08 ± 0.08c | 0.94 ± 0.03b |
| C/N | 43.5 ± 2.00a | 59.7 ± 2.17c | 47.2 ± 3.19b | 50.9 ± 1.60b |
| P (mg/g) | 1.11 ± 0.12b | 0.74 ± 0.05a | 1.52 ± 0.10c | 1.14 ± 0.04b |
| N/P | 11 .0 ± 0.95b | 11.9 ± 0.88b | 7.47 ± 0.81a | 8.38 ± 0.31a |
| K (mg/g) | 9.49 ± 0.63b | 7.22 ± 0.63a | 8.68 ± 0.41ab | 7.54 ± 0.42ab |
| Ca (mg/g) | 3.42 ± 0.23a | 2.91 ± 0.38a | 4.33 ± 0.28b | 3.72 ± 0.31b |
| Mg (mg/g) | 2.01 ± 0.07 | 2.16 ± 0.13 | 1.93 ± 0.10 | 2.03 ± 0.10 |
| Fe (μg/g) | 190.1 ± 17.4c | 141.3 ± 4.56b | 184.2 ± 32.3c | 88.7 ± 7.41a |
| Mn (μg/g) | 71.1 ± 15.6b | 35.5 ± 3.95a | 141.8 ± 10.8c | 136.6 ± 13.7c |
| Zn (μg/g) | 22.1 ± 1.52b | 20.5 ± 1.50b | 19.2 ± 1.07ab | 18.2 ± 0.96a |
4. Discussion
4.1. Drought-Avoidance Matters in A. pinsapo
4.2. Capacity of Osmotic Adjustment
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Breshears, D.D.; Cobb, N.S.; Rich, P.M.; Price, K.P.; Allen, C.D.; Balice, R.G.; Romme, W.H.; Kastens, J.H.; Floyd, M.L.; Belnap, J.; et al. Regional vegetation die-off in response to global-change-type drought. Proc. Natl. Acad. Sci. USA 2005, 102, 15144–15148. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J. From pattern to process: Linking intrinsic water-use efficiency to drought-induced forest decline. Glob. Ch. Biol. 2012, 18, 1000–1015. [Google Scholar] [CrossRef]
- Carnicer, J.; Barbeta, A.; Sperlich, D.; Coll, M.; Peñuelas, J. Contrasting trait syndromes in angiosperms and conifers are associated with different responses of tree growth to temperature on a large scale. Front. Plant Sci. 2013, 4, 409. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modeling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- Hsiao, T.C. Plant responses to water stress. Annu. Rev. Plant Physiol. Plant. Mol. Biol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Ditmarová, L.; Kurjak, D.; Palmroth, S.; Kmet, J.; Strelcová, K. Physiological responses of Norway spruce (Picea abies) seedlings to drought stress. Tree Physiol. 2010, 30, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, T.C.; Acevedo, E.; Fereres, E.; Henderson, D.W. Stress metabolism—Water stress, growth, and osmotic adjustment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1976, 273, 479–500. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.C.; Covelo, F.; Carreira, J.A.; Merino, J. Phenological and water-use patterns underlying maximum growing season length at the highest elevations: Implications under climate change. Tree Physiol. 2012, 32, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.C.; Tíscar, P.A.; Camarero, J.J.; Taïqui, L.; Viñegla, B.; Seco, J.I.; Merino, J.; Carreira, J.A. Tree growth decline on relict western-Mediterranean mountain forests: Causes and impacts. In Forest Decline: Causes and Impacts; Jenkins, J.A., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 91–110. [Google Scholar]
- Trigo, R.M.; Palutikof, J.P. Precipitation scenarios over Iberia: A comparison between direct GCM output and different downscaling techniques. J. Clim. 2001, 14, 4422–4446. [Google Scholar] [CrossRef]
- Räisänen, J.; Hansson, U.; Ullerstig, A.; Döscher, R.; Graham, L.P.; Jones, C.; Meier, H.E.M.; Samuelsson, P.; Willén, U. European climate in the late twenty-first century: Regional simulations with two driving global models and two forcing scenarios. Clim. Dyn. 2004, 22, 13–31. [Google Scholar] [CrossRef]
- IPCC. IPCC Fifth Assessment Report (AR5). In Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Breshears, D.D.; Adams, H.D.; Eamus, D.; McDowell, N.G.; Law, D.J.; Will, R.E.; Williams, A.P.; Zou, C.B. The critical amplifying role of increasing atmospheric moisture demand on tree mortality and associated regional die-off. Front. Plant Sci. 2013, 4, 266. [Google Scholar] [CrossRef] [PubMed]
- Joffre Infante, J.M.; Mauchamp, A.; Fernández-Alés, R.; Joffre, R.; Rambal, S. Within-tree variation in transpiration in isolated evergreen oak trees: Evidence in support of the pipe model theory. Tree Physiol. 2001, 21, 409–414. [Google Scholar] [CrossRef]
- Rambal, S.; Ourcival, J.M.; Joffre, R.; Mouillot, F.; Nouvellon, Y.; Reichstein, M.; Rocheteau, A. Drought controls over conductance and assimilation of a Mediterranean evergreen ecosystem: Scaling from leaf to canopy. Glob. Chang. Biol. 2003, 9, 1813–1824. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J.; Carreira, J.A. Plastic responses of Abies pinsapo xylogenesis to drought and competition. Tree Physiol. 2009, 29, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Soil Survey Staff. Keys to Soil Taxonomy, 8th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 1999. [Google Scholar]
- Rawls, W.J.; Pachepsky, Y.A.; Ritchie, J.C.; Sobecki, T.M.; Bloodworth, H. Effect of soil organic carbon on soil water retention. Geoderma 2003, 116, 61–76. [Google Scholar] [CrossRef]
- De Vos, B.; van Meirvenne, M.; Quataert, P.; Deckers, J.; Muys, B. Predictive Quality of Pedotransfer Functions for Estimating Bulk Density of Forest Soils. Soil Sci. Soc. Am. J. 2005, 69, 500–510. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 1998. [Google Scholar]
- Schulze, E.-D.; Beck, E.; Müller-Hohenstein, K. Plant Ecology; Springer-Verlag: Heidelberg, Germany, 2002. [Google Scholar]
- Castellvi, F.; Perez, P.J.; Stockle, C.O.; Ibanez, M. Methods for estimating vapour pressure deficit at a regional scale depending on data availability. Agric. For. Meteorol. 1997, 87, 243–252. [Google Scholar] [CrossRef]
- Maier-Maercker, U. Dynamics of change in stomatal response and water status of Picea abies during a persistent drought period: A contribution to the traditional view of plant water relations. Tree Physiol. 1998, 18, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Sommers, L.E.; Nelson, D.W. Determination of total phosphorus in soils: A rapid perchloric acid digestion procedure. Soil Sci. Soc. Am. J. 1972, 36, 902–904. [Google Scholar] [CrossRef]
- Von Ende, C.N. Repeated-Measures Analysis: Growth and Other Time-Dependent Measures. Design and Analysis of Ecological Experiments; Scheiner, S.M., Gurevitch, J., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 134–157. [Google Scholar]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Cochard, H.; Barredo, G.; Villarroya, D.; Gil-Pelegrín, E. Hydraulic traits are associated with the distribution range of two closely related Mediterranean firs, Abies alba Mill. And Abies pinsapo Boiss. Tree Physiol. 2001, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mooney, H.A. Habitat, plant form, and plant water relations in Mediterranean-climate regions. Ecol. Medit. 1982, 8, 481–488. [Google Scholar]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Shpringer, I.; Fikler, B.; Elbaz, G.; Cohen, S.; Yakir, D. Relationships between stomatal regulation, water-use, and water-use efficiency of two coexisting key Mediterranean tree species. For. Ecol. Manag. 2013, 302, 34–42. [Google Scholar] [CrossRef]
- Baquedano, F.J.; Castillo, F.J. Drought tolerance in the Mediterranean species Quercus coccifera, Quercus ilex, Pinus halepensis, and Juniperus phoenicea. Photosynthetica 2007, 45, 229–238. [Google Scholar] [CrossRef]
- Martinez-Vilalta, J.; Mangirón, M.; Ogaya, R.; Sauret, M.; Serrano, L.; Peñuelas, J.; Piñol, J. Sap flow of three co-occurring Mediterranean woody species under varying atmospheric and soil water conditions. Tree Physiol. 2003, 23, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Knapik, D.; Peguero-Pina, J.J.; Flexas, J.; Herbette, S.; Cochard, H.; Niinemets, U.; Gil-Pelegrín, E. Coping with low light under high atmospheric dryness: Shade acclimation in a Mediterranean conifer (Abies pinsapo Boiss.). Tree Phys. 2015. [Google Scholar] [CrossRef] [PubMed]
- Abrams, M.D.; Kubiske, M.E. Photosynthesis and water relations during drought in Acer rubrum L. genotypes from contrasting sites in Central Pennsylvania. Funct. Ecol. 1990, 4, 727–733. [Google Scholar] [CrossRef]
- Abrams, M.D. Adaptations and responses to drought in Quercus species of North America. Tree Physiol. 1990, 7, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Abrams, M.D.; Schultz, J.C.; Kleiner, K.W. Ecophysiological responses in mesic versus xeric hardwood species to an early-season drought in central Pennsylvania. For. Sci. 1990, 36, 970–981. [Google Scholar]
- Maherali, H.; DeLucia, E.H. Xylem conductivity and vulnerability to cavitation of ponderosa pine growing in contrasting climates. Tree Physiol. 2000, 20, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Cochard, H.; Mencuccini, M.; Sterck, F. Hydraulic adjustment of Scots pine across Europe. New Phytol. 2009, 184, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Hinckley, T.M.; Meinzer, F.C.; Sprugel, D.G. Boundary layer conductance, leaf temperature and transpiration of Abies amabilis branches. Tree Physiol. 1999, 19, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Schoonmaker, A.L.; Hacke, U.G.; Landhäusser, S.M.; Lieffers, V.J.; Tyree, M.T. Hydraulic acclimation to shading in boreal conifers of varying shade tolerance. Plant. Cell. Environ. 2010, 33, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Peltoniemi, M.; Duursma, R.; Medlyn, B. Co-optimal distribution of leaf nitrogen and hydraulic conductance in plant canopies. Tree Physiol. 2012, 32, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; McAdam, S.A.M.; Jordan, G.J.; Martins, S.C.V. Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proc. Natl. Acad. Sci. USA 2014, 111, 14489–14493. [Google Scholar] [CrossRef] [PubMed]
- Kollist, H.; Nuhkat, M.; Roelfsema, M.R.G. Closing gaps: Linking elements that control stomatal movement. New Phytol. 2014, 203, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; McAdam, S.A.M. Abscisic acid mediates a divergence in the drought response of two conifers. Plant Physiol. 2013, 162, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Babita, M.; Maheswari, M.; Rao, L.M.; Shanker, A.K.; Rao, D.G. Osmotic adjustment, drought tolerance and yield in castor (Ricinus communis) L.) hybrids. Environ. Exp. Bot. 2010, 69, 243–249. [Google Scholar] [CrossRef]
- Khosravifar, S.; Yarnia, M.; Benam, M.B.K.; Moghbeli, A.H.H. Effect of potassium on drought tolerance in potato cv. Agria. J. Food Agric. Environ. 2008, 6, 236–241. [Google Scholar]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Breshears, D.D.; Myers, O.B.; Meyer, C.W.; Barnes, F.J.; Zou, C.B.; Allen, C.D.; McDowell, N.G.; Pockman, W.T. Tree die-off in response to global-change-type drought: Mortality insights from a decade of plant water potential measurements. Front. Ecol. Environ. 2009, 7, 185–189. [Google Scholar] [CrossRef]
- Guehl, J.M.; Aussenac, G.; Boucachrine, J.; Zimmermann, R.; Pennees, A.; Ferhi, A.; Grieu, P. Sensitivity of leaf gas exchange to atmospheric drought, soil drought, and water-use efficiency in some Mediterranean Abies species. Can. J. For. Res. 1991, 21, 1507–1515. [Google Scholar] [CrossRef]
- Aussenac, G. Ecology and ecophysiology of circum-Mediterranean firs in the context of climate change. Ann. For. Sci. 2002, 59, 823–832. [Google Scholar] [CrossRef]
- Picon, C.; Ferhi, A.; Guehl, J.M. Concentration and d13C of leaf carbohydrates in relation to gas exchange in Quercus robur under elevated CO2 and drought. J. Exp. Bot. 1997, 48, 1547–1556. [Google Scholar]
- Martínez-Ferri, E.; Balaguer, L.; Valladares, F.; Chico, J.M.; Manrique, E. Energy dissipation in drought-avoiding and drought-tolerant tree species at midday during the Mediterranean summer. Tree Physiol. 2000, 20, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.H.; Schlesinger, W.H. Forest Ecosystems. Concepts and Management; Academic Press, INC: San Diego, CA, USA, 1985. [Google Scholar]
- Emmingham, W.H.; Waring, R.H. An index of photosynthesis for comparing forest sites in western Oregon. Can. J. For. Res. 1977, 7, 165–174. [Google Scholar] [CrossRef]
- Peguero-Pina, J.; Camarero, J.J.; Abadía, A.; Martín, E.R.; González-Cascón, A.; Morales, F.; Gil-Pelegrín, E. Physiological performance of silver-fir (Abies alba Mill.) populations under contrasting climates near the south-western distribution limit of the species. Flora 2007, 202, 226–236. [Google Scholar] [CrossRef]
- Nguyen, A.; Lamant, A. Variation in growth and osmotic regulation of roots of water-stressed maritime pine (Pinus pinaster Ait.) provenances. Tree Physiol. 1989, 5, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Dobberfuhl, D.R.; MacKay, N.A.; Schampel, J.H. Organism size, life history, and N: P stoichiometry. Bioscience 1996, 46, 674–684. [Google Scholar] [CrossRef]
- Abrams, M.D. Sources of variation in osmotic potentials with special reference to North American tree species. For. Sci. 1988, 34, 1030–1046. [Google Scholar]
- Rivas-Ubach, A.; Sardans, J.; Pérez-trujillo, M.; Estiarte, M.; Peñuelas, J. Strong relationship between elemental stoichiometry and metabolome in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 4181–4186. [Google Scholar] [CrossRef] [PubMed]
- Green, T.G.A.; Jane, G.T. Changes in osmotic potential during bud break and leaf development of Nothofagu smenziesii, Weinmannia racemosa,Quintinia acutifolia, and Ixerba brexioides. NZ. J. Bot. 1983, 21, 391–395. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Salguero, R.; Ortíz, C.; Covelo, F.; Ochoa, V.; García-Ruíz, R.; Seco, J.I.; Carreira, J.A.; Merino, J.Á.; Linares, J.C. Regulation of Water Use in the Southernmost European Fir (Abies pinsapo Boiss.): Drought Avoidance Matters. Forests 2015, 6, 2241-2260. https://doi.org/10.3390/f6062241
Sánchez-Salguero R, Ortíz C, Covelo F, Ochoa V, García-Ruíz R, Seco JI, Carreira JA, Merino JÁ, Linares JC. Regulation of Water Use in the Southernmost European Fir (Abies pinsapo Boiss.): Drought Avoidance Matters. Forests. 2015; 6(6):2241-2260. https://doi.org/10.3390/f6062241
Chicago/Turabian StyleSánchez-Salguero, Raúl, Cristina Ortíz, Felisa Covelo, Victoria Ochoa, Roberto García-Ruíz, José Ignacio Seco, José Antonio Carreira, José Ángel Merino, and Juan Carlos Linares. 2015. "Regulation of Water Use in the Southernmost European Fir (Abies pinsapo Boiss.): Drought Avoidance Matters" Forests 6, no. 6: 2241-2260. https://doi.org/10.3390/f6062241
APA StyleSánchez-Salguero, R., Ortíz, C., Covelo, F., Ochoa, V., García-Ruíz, R., Seco, J. I., Carreira, J. A., Merino, J. Á., & Linares, J. C. (2015). Regulation of Water Use in the Southernmost European Fir (Abies pinsapo Boiss.): Drought Avoidance Matters. Forests, 6(6), 2241-2260. https://doi.org/10.3390/f6062241







