1. Introduction
Airborne particulate matter (PM) represents one of the major environmental pollutants worldwide. Long-term exposure to fine particulates (PM
2.5 and smaller) is well known to contribute to respiratory and cardiovascular diseases, but it has also been linked to less commonly recognized health outcomes, for example, low birth weight [
1,
2]. While outdoor air pollution is routinely monitored and regulated by legislation [
3], indoor environments still require more comprehensive investigation [
4,
5]. Indoor air may contain even higher concentrations of PM, particularly during the heating season, when polycyclic aromatic hydrocarbons (PAHs) and black carbon are released as by-products of organic fuel combustion [
4,
6,
7]. Cooking remains another major source for elevated fine PM concentrations indoors [
8]. In addition to indoor PM sources, polluted outdoor air can penetrate into buildings and substantially increase overall contamination [
5]. These factors, combined with reduced building ventilation, call for sustainable strategies to mitigate increasing indoor air pollution [
9].
Similarly to the application of large-scale green infrastructure in outdoor urban areas [
10,
11,
12], air phytoremediation using ornamental plants can also be implemented indoors [
9]. Although air phytoremediation efficiency in confined spaces has been questioned, especially when passive PM removal by potted plants is considered [
13], the advantages of the botanical air filters include their ecological character, aesthetic value, and relatively low-cost [
14,
15,
16,
17]. The two most common forms of indoor greenery include potted plants and green walls. So far, the species examined in the context of indoor PM removal include
Chlorophytum comosum (Thunb.) Jacq.,
Chlorophytum Orchidastrum Lindl.,
Epipremnum aureum (Linden & André) G.S.Bunting,
Ficus lyrate L.,
Nephrolepis exaltata (L.) Schott ‘bostoniensis’ and
Schefflera arboricola L. [
18,
19]. Among the factors influencing indoor PM removal, the air humidity, leaf area, and light intensity are known to enhance the process [
19]. The mechanisms involved in air purification include induced airflow through the green wall structure and substrate, as well as passive particulate deposition on foliage surfaces [
9,
20].
During the Christmas period, potted and/or fresh-cut plants such as Norway spruce (
Picea abies (L.) H. Karst). and Caucasian fir (
Abies nordmanniana (Steven) Spach) are widely introduced to houses as a part of long-standing cultural traditions [
21,
22]. In many cases, homes are adorned with cut Christmas trees, while a more environmentally conscious approach favors the use of potted specimens that can later be replanted outdoors, extending their ecological value beyond the festive season [
23]. The high effectiveness of air pollution removal by spruces and firs is well evidenced in outdoor conditions. Previous studies have demonstrated that the combination of morphological traits such as extensive foliage area, the presence of waxy layers, rough surfaces and brushy structure of conifers contributes to the capture of fine and coarse particles [
24,
25,
26]. It is because these features enhance the effective surface area for PM deposition, thereby increasing the overall capacity of plants to capture and retain airborne pollutants. However, this exceptional effectiveness comes at a cost—conifers planted in highly polluted areas can experience increased physiological stress and reduced performance.
Nevertheless, despite existing evidence of the high efficiency of conifers in outdoor PM removal, the potential of Christmas trees to alter indoor air quality remains unexplored to date. At the same time, in much of the world, the festive season coincides with winter and the heating period, when buildings are tightly sealed, cooking activities intensify, and people gather indoors—conditions that typically elevate PM levels. The mentioned species, due to their morphology and biological activity, can possibly act as temporary biofilters for indoor air, similarly to their conspecifics growing outdoors [
24]. On the other hand, commercially available trees that are typically kept and sold outdoors accumulate PM particles prior to purchase. Once brought inside, these pollutants may be released into the indoor environment, potentially contributing to further deterioration of already poor air quality.
This study aimed to determine the extent to which P. abies and A. nordmanniana affect air quality in household environments. We compared trees supplied in two commercial forms—potted (living) and cut—and assessed the potential of Christmas trees as (1) carriers of pre-accumulated outdoor PM into indoor spaces, and (2) temporary indoor biofilters that may capture indoor-generated PM. It was hypothesized that differences between species identity and commercial forms (potted vs. cut) Christmas trees are reflected in the initial PM deposition, PM accumulation and/or release, and in the amount of epicuticular waxes produced.
2. Materials and Methods
2.1. Plant Material
The plant material consisted of two conifer species commonly used as Christmas trees: Norway spruce, Picea abies (L.) H. Karst, and Caucasian fir Abies nordmanniana (Steven) Spach (Pinales: Pinaceae). Trees of both species were obtained from the same commercial Christmas tree nursery located in Glinojeck (Mazovia region, Poland). The experimental material included trees with intact root systems (potted) as well as cut trees without roots. All trees were of comparable size and age and had been cultivated under similar field conditions before being transported to indoor environments.
For the experiment, unwashed trees with Christmas decorations were placed in detached single-family houses located in the Mazovia region (Poland), representing typical residential indoor conditions during the Christmas period with regular human activities (
Figure 1).
A total of six houses located in urban areas were selected (three houses with both cut and potted P. abies each; three houses with both cut and potted A. nordmaniana each), serving as a separate experimental unit. Houses and rooms were selected randomly, while ensuring broadly comparable characteristics, including similar living room size, room function, and typical household ventilation systems. Trees were placed separately in living rooms, while kitchens were excluded, to prevent the influence of microclimatic factors (e.g., increased humidity and temperature related to cooking).
Throughout the experimental period, both P. abies and A. nordmanniana trees were maintained under as-similar-as-possible indoor environmental conditions characteristic of inhabited households, including stable room temperature, natural lighting, and typical indoor humidity. No watering, fertilization, or treatments were applied beyond standard practices typical for indoor display of Christmas trees. Although basic environmental similarity was ensured, households differed in occupancy, ventilation habits, and cleaning practices; these factors were not experimentally controlled and were treated as inherent sources of variability representative of real-life residential conditions.
2.2. Needle Sampling
The sampling period covered December and January of the 2020/2021 winter season. Needles of P. abies and A. nordmanniana were collected according to a uniform procedure. Each tree constituted one biological replicate from which approximately 50–60 needles were collected. To minimize the influence of micro-environmental variability, needles were consistently collected from the outer parts of the crown at approximately human breathing height (about 1.3–1.6 m above the floor). Sampling was preferentially conducted on the side of the tree facing the main room space and areas of human activity, and opposite to walls, windows, or other vertical surfaces, in order to better reflect conditions associated with airborne particle deposition and resuspension in occupied indoor environments.
Needle collections were performed at four time intervals: immediately after the trees were brought indoors (day 0), and subsequently on the 10th, 20th, and 30th day of the experiment. Needles of both species and forms were sampled from branches located in exposed parts of the crown to obtain representative material.
Immediately after collection, the needles were put in labeled paper envelopes and stored in a dry, controlled environment with stable temperature and humidity until further analyses. All collected material was later used for laboratory analyses.
2.3. Determination of Surface (SPM) and Wax-Retained (WPM) Particulate Matter and Wax Content
A protocol established by Dzierżanowski et al. [
27] was followed. To determine the amount of water-insoluble PM deposited on the surface of needles, three types of filters with a diameter of 47 mm were used: paper filters type 91 (pore size 10 μm), type 42 (pore size 2.5 μm), and PTFE membrane filters (pore size 0.2 μm) (Whatman, Maidstone, UK). Before use, the filters were dried for 30 min at 60 °C in a laboratory dryer (KCW-100, PREMED, Łódź, Poland) and then stabilized under room conditions for another 30 min. After equilibration, the filters were weighed (XS105DU, Mettler-Toledo, Greifensee, Switzerland) and neutralized using a deionization gate (HAUG, St. Gallen, Switzerland) to eliminate electrostatic charge.
For the determination of surface-deposited PM (SPM), each needle sample was placed in a glass crystallizer and rinsed with 250 mL of distilled water for 60 s to wash off particles loosely attached to the needle surface. The obtained suspension was passed through a 100 μm metal sieve (Haver & Boecker, Oelde, Germany) to remove coarse debris and then filtered under vacuum using a filtration set (PALL Corp., Port Washington, NY, USA) connected to a vacuum pump (KNF Neuberger, Inc., Trenton, NJ, USA). Filtration was carried out sequentially through filters with pore sizes of 10 μm, 2.5 μm, and 0.2 μm. To reduce surface tension, several drops of isopropyl alcohol were applied to PTFE filters prior to filtration. Particles collected on the filters represented large (10–100 μm), coarse (2.5–10 μm), and fine (0.2–2.5 μm) fractions. After filtration, filters were dried and reweighed under the same conditions. The difference in mass before and after filtration was used to calculate the amount of PM deposited on the needle surface.
To assess the amount of PM retained within epicuticular waxes (WPM), needle samples were rinsed with 150 mL of chloroform and mixed for 40 s to dissolve the wax layer along with embedded particles. The suspension was filtered in the same manner as the water washings, except that isopropyl alcohol was not used before filtration through 0.2 μm PTFE filters.
The total wax content on the needle surface was determined from the chloroform filtrate. The filtrate containing dissolved waxes was transferred into pre-weighed glass beakers, which were left under a fume hood until complete solvent evaporation. The remaining wax layer was then weighed, and the difference in beaker mass before and after evaporation was used to determine the wax amount covering the needles.
The surface area of needles was measured using an Image Analysis System (Skye Instruments Ltd., Llandrindod Wells, UK), and the data were processed with the SkyeLeaf v2 software. The results were expressed as micrograms of PM or wax per square centimeter of needle surface (µg·cm−2).
2.4. Statistical Analysis
All data obtained from laboratory measurements and indoor air monitoring were statistically analyzed using Statgraphics Plus 4.1 (Statgraphics Technologies, Inc., The Plains, VA, USA) and OriginPro 2021 (OriginLab Corp., Northampton, MA, USA) software. A one-way analysis of variance (ANOVA) was applied to determine the effect of the tree species and form on PM deposition/release, and wax content. When statistically significant differences were detected, comparisons between mean values were performed using Student’s t-test based on the Least Significant Difference (LSD) procedure at a significance level of α = 0.05. The results are presented in figures and tables as mean values with standard error (±SE), reflecting the variability within each treatment.
3. Results
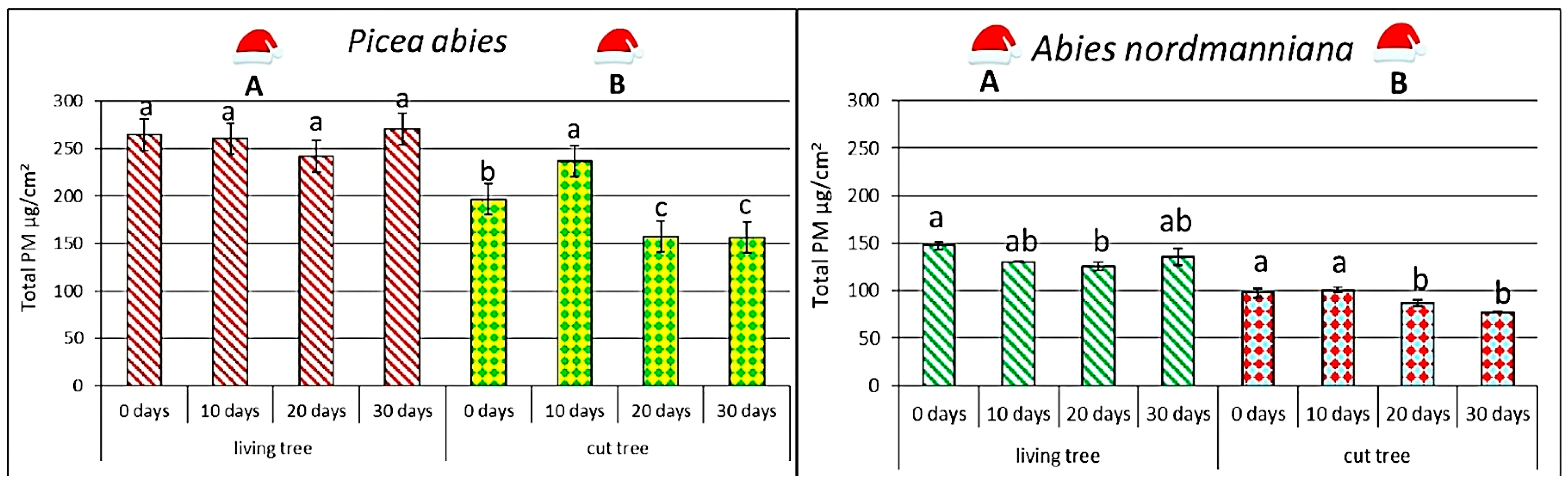
(
Picea abies (L.) H. Karst.) and
Abies nordmanniana (Steven) Spach differed markedly in the initial total PM deposition on needles (
Figure 2).
At the beginning of the experiment (0 days), clear quantitative differences in total PM loads were observed between tree species as well as between potted and cut individuals. Potted P. abies carried by far the highest PM loads, exceeding those on potted A. nordmanniana by approximately 80%. Cut P. abies also contained substantially more PM than cut A. nordmanniana at the start, with spruce exceeding fir by around 100%. Within each species, potted individuals exhibited markedly higher initial PM loads than cut ones: in P. abies by about 34%, and in A. nordmanniana by roughly 50%.
In P. abies, differences between potted and cut trees persisted throughout the 30-day period, but their temporal dynamics were distinct. In potted spruce, total PM loads remained relatively stable, with fluctuations generally within ±10% of the initial values, indicating sustained retention capacity under indoor conditions. In contrast, cut P. abies showed a clear two-phase pattern: PM loads increased by about 20% after 10 days, followed by a systematic decline, reaching levels 15%–25% below the initial value by day 30. When compared to the peak at day 10, this represented a total drop of nearly 35%, confirming that cut spruce needles lost PM more readily over time than potted ones.
In A. nordmanniana, initial PM loads were consistently lower than those of P. abies across both potted and cut trees, with fir carrying 40%–60% less PM on average at the start. Potted fir trees exhibited a gradual PM decline of 10%–15% during the first 20 days, followed by a 5%–10% increase by day 30, likely reflecting indoor re-deposition processes. Cut A. nordmanniana displayed a different pattern: PM loads decreased continuously, reaching values 20%–25% lower than the initial measurement by day 30, with no sign of redeposition.
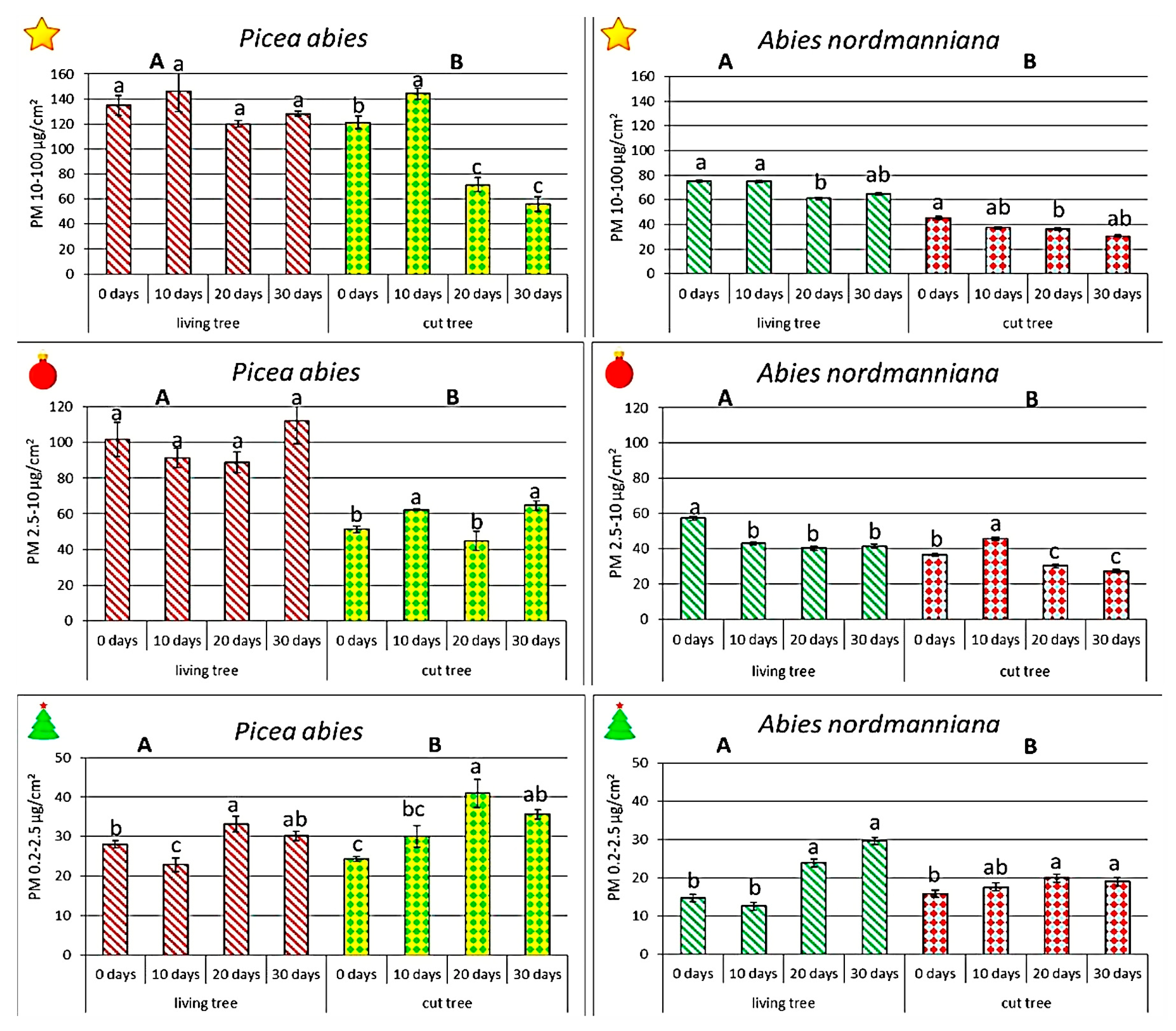
Considering the different particle size fractions (PM
10–100, PM
2.5–10, and PM
0.2–2.5), both species exhibited clear, size-dependent differences in PM deposition under indoor conditions. At the beginning of the experiment, on potted
P. abies, there was substantially more PM deposited across all particle sizes than on potted
A. nordmanniana. For large particles (PM
10–100), spruce exceeded fir by approximately 79%. The coarse fraction (PM
2.5–10) showed a similarly big difference of 77%, while the fine fraction (PM
0.2–2.5) was nearly 90% higher in spruce (
Figure 3).
Cut trees showed a similar pattern. Cut P. abies was characterized by accumulated c. 170% more large PM deposited than cut A. nordmanniana, by 40% more coarse PM, and c. 55% more fine PM. Thus, in line with the total PM patterns, spruce—especially potted trees—introduced markedly more PM of every size fraction into indoor spaces on day 0.
In P. abies, large particles (PM10–100) on potted trees remained relatively stable across the 30-day indoor exposure, fluctuating within ±10% of initial values. In turn, cut spruce exhibited a two-phase pattern: a short-lived increase of roughly 20% by day 10, followed by a pronounced decline of about 50% by day 30. This indicates that coarse PM is released far more readily from cut than from potted spruces. Potted A. nordmanniana showed a decline of ~15%, whereas cut individuals lost ~35% of their initial PM10–100 load by day 30.
In the case of coarse particles (PM2.5–10), potted P. abies maintained high and stable retention, while cut spruce retained nearly 50% less of this fraction and showed fluctuations over time. In A. nordmanniana, coarse PM loads remained much lower. Potted firs decreased by ~25%, while cut ones declined by almost 40%, reinforcing the weaker retention capacity of fir in this particle range.
The finest and most health-relevant particles (PM0.2–2.5) behaved differently. Potted P. abies increased its fine PM loads by about 20%, while cut spruce increased them by 30%–40% over the 30-day period. Although A. nordmanniana initially retained 40%–50% less fine PM than spruce, its dynamics diverged: potted firs showed a consistent upward trend, whereas cut firs remained nearly unchanged. Importantly, the increase in fine particle retention was most evident in cut P. abies and potted A. nordmanniana.
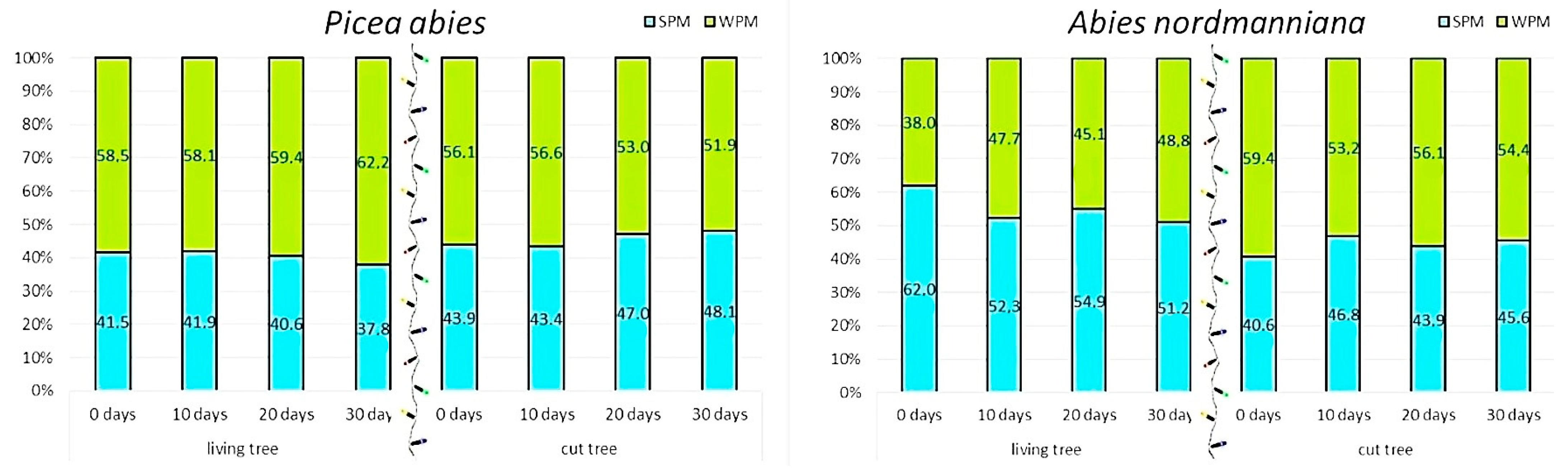
The shares of PM retained on the needles (
SPM) and embedded within the waxes (
WPM) differed between vitality classes and showed relatively consistent patterns over the 30-day exposure period (
Figure 4).
In potted trees, the share of wax-embedded PM remained stable with a slight increase on day 30. In P. abies, WPM stayed near 58%–59% throughout the 20-day period, with 62.2% on day 30. In A. nordmanniana, WPM increased noticeably—from 38% on day 0 to 48% on day 30. In cut trees, the pattern shifted toward increasing surface deposition. In P. abies, SPM rose from 43.9% to 48.1% by day 30. In A. nordmanniana, overall SPM increased from 40.6% to 45.6% on day 30, with some fluctuations in between.
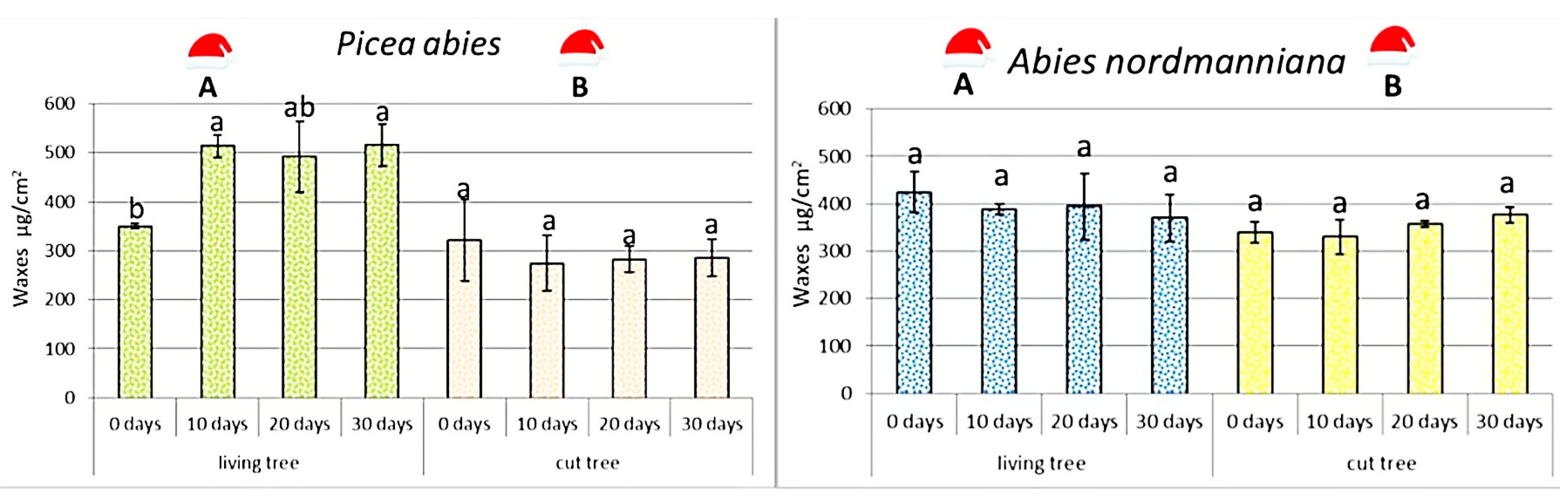
The amount of epicuticular waxes differed clearly between species and was influenced by tree form (
Figure 5).
At the beginning of the experiment, potted A. nordmanniana possessed about 21% more waxes than potted P. abies. However, in potted P. abies the wax content increased sharply during the first 10 days indoors, representing a 46.6% rise. Although values fluctuated afterwards, wax amounts remained elevated, with a 40%–47% increase compared with day 0 across the entire 30-day period.
Potted A. nordmanniana exhibited a gradual but non-significant decline in wax content: by 8.3% after 10 days, 7.1% after 20 days, and 12.8% after 30 days. Despite occasional increases in individual samples, the overall trend suggests modest wax loss rather than accumulation.
Cut trees displayed lower and more variable wax levels. In cut P. abies, wax content decreased during the first 10 days by 14.4%, followed by partial stabilization (−12% at day 20 and −10% at day 30). In cut A. nordmanniana, wax content initially dropped by 18% but subsequently increased above the starting value, reaching 5.3% more on day 20 and 11% on day 30.
5. Conclusions
The results obtained in this study are somewhat counter-intuitive and do not fully support our initial hypothesis that potted Christmas trees are invariably the best choice for indoor air quality improvement. Based on total PM loads alone, potted specimens—regardless of species—initially appeared the most favorable option, particularly for environmentally conscious consumers. However, a deeper analysis of fraction-specific PM accumulation and release revealed a more complex picture. An unexpected finding was that cut trees, although less sustainable from an ecological perspective, introduced substantially less PM into indoor environments than potted ones. Furthermore, cut P. abies revealed the highest accumulation capacity of fine PM, the fraction of greatest concern for human health. Thus, even the less “green” option shows its own environmental advantage.
Therefore, while consumer preferences often favor firs for their dense crowns and ornamental appeal, our findings indicate that from an indoor air-quality viewpoint, P. abies trees (both forms) offer the most reliable phytoremediation performance.
Taken together, these results demonstrate that neither species identity nor commercial form can be overlooked when evaluating the impact of Christmas trees on indoor air quality. Their environmental function is shaped by a nuanced interplay of morphology, vitality, transport history and particulate behavior—elements that do not always align with consumer expectations.