Dynamics and Driving Factors of Soil Carbon Fractions in Corethrodendron scoparium (Fisch. & C. A. Mey.) Fisch. & Basiner. Sand-Fixing Plantations at the South Edge of Tengger Desert, Northwestern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Sampling and Indicator Measurement Methods
2.3.1. Sampling Methods
2.3.2. Index Determination and Methods
2.3.3. CPMI Assessment
2.3.4. Data Calculation and Analysis
3. Results
3.1. Vegetation Restoration Characteristics and Soil Physicochemical Properties in Artificial C. Scoparium Sand-Fixing Plantations
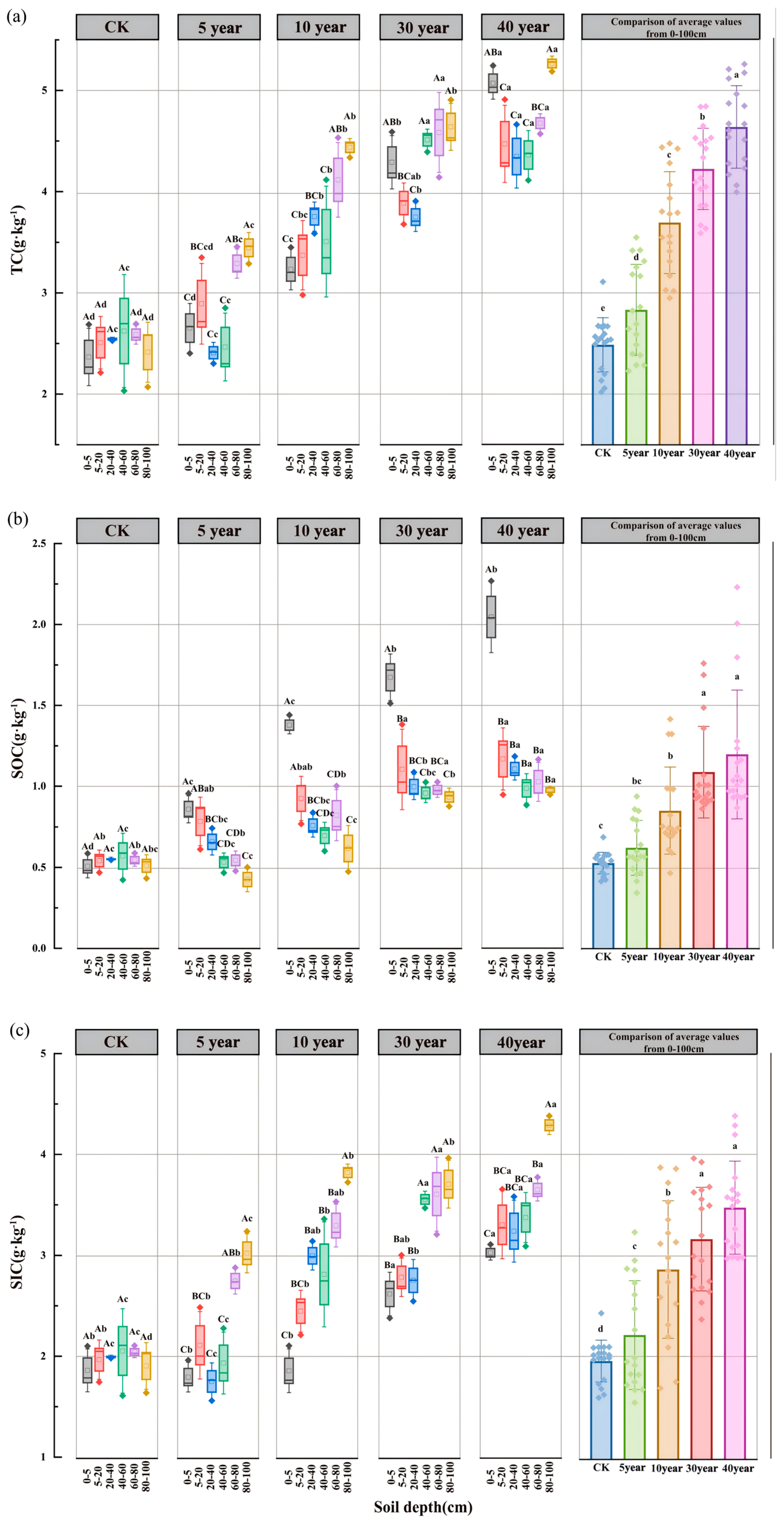
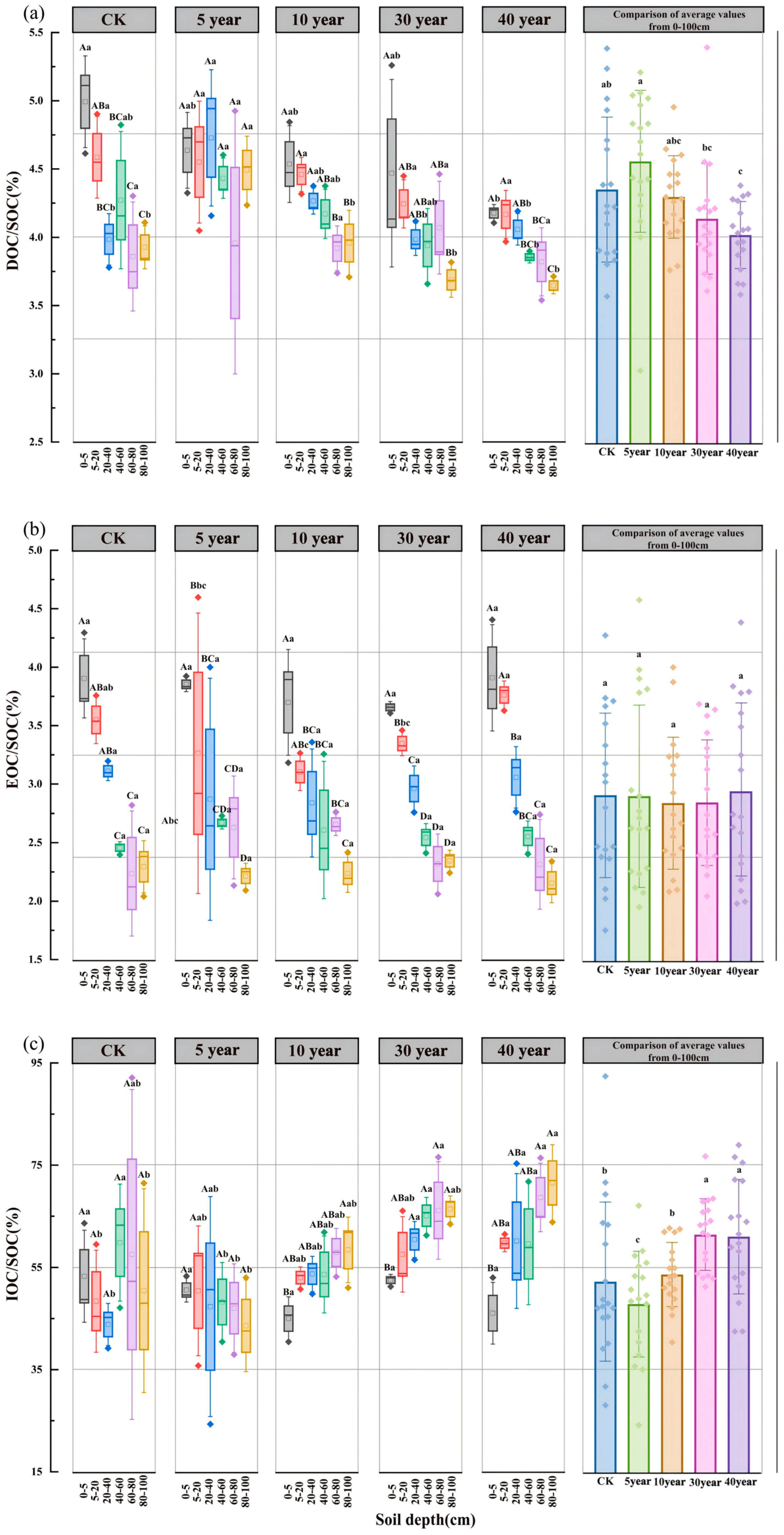
3.2. Spatiotemporal Dynamics of Soil Organic and Inorganic Carbon
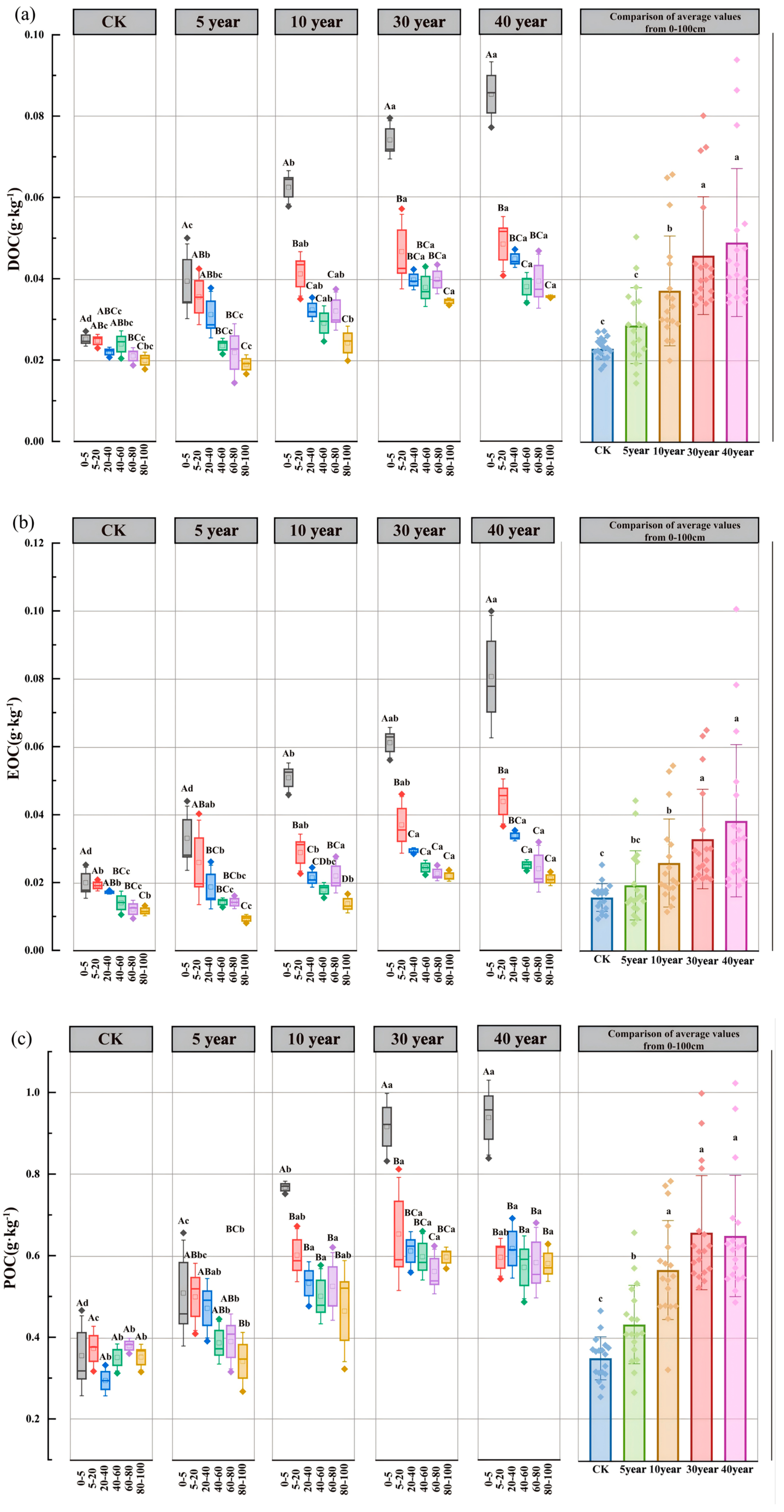
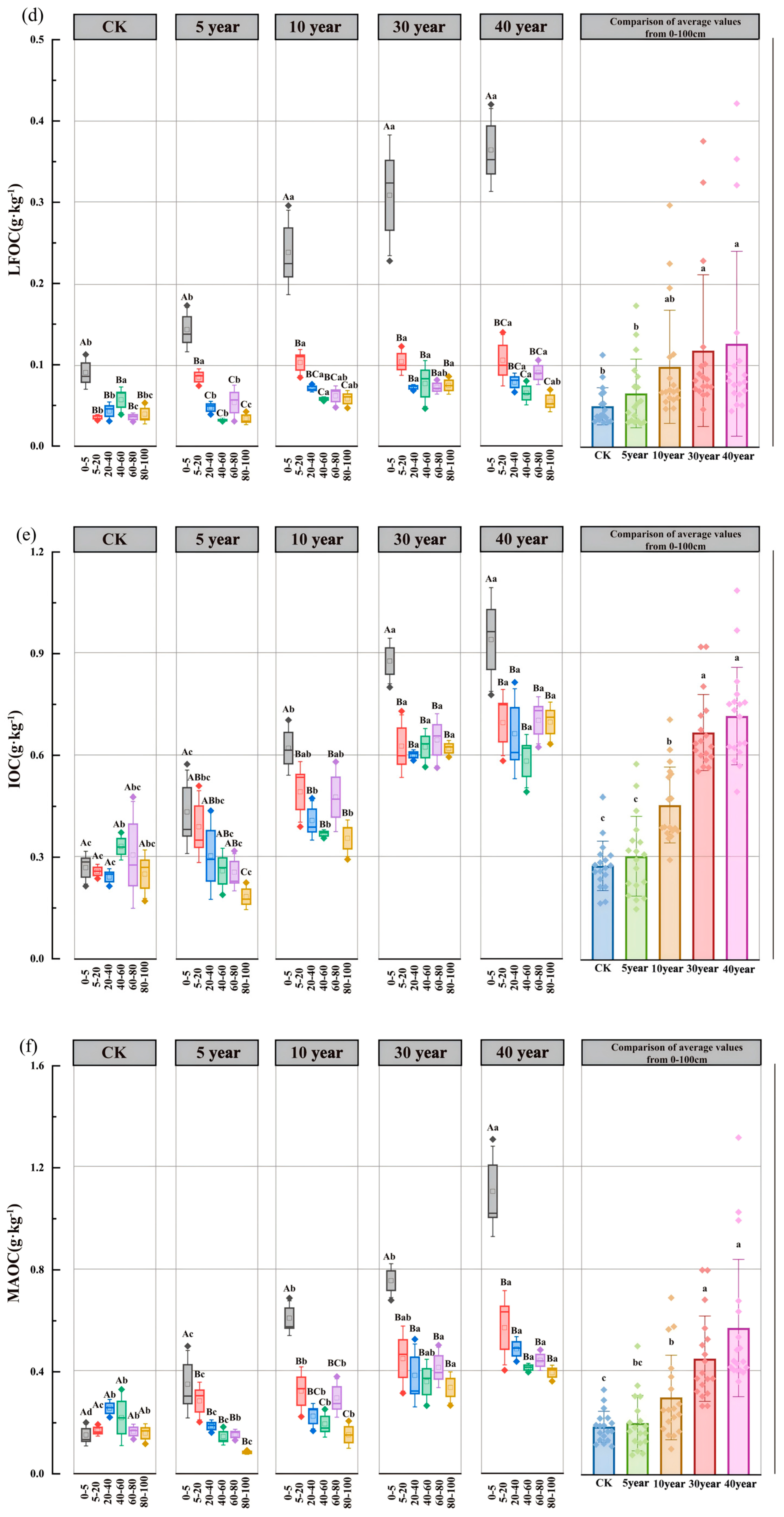
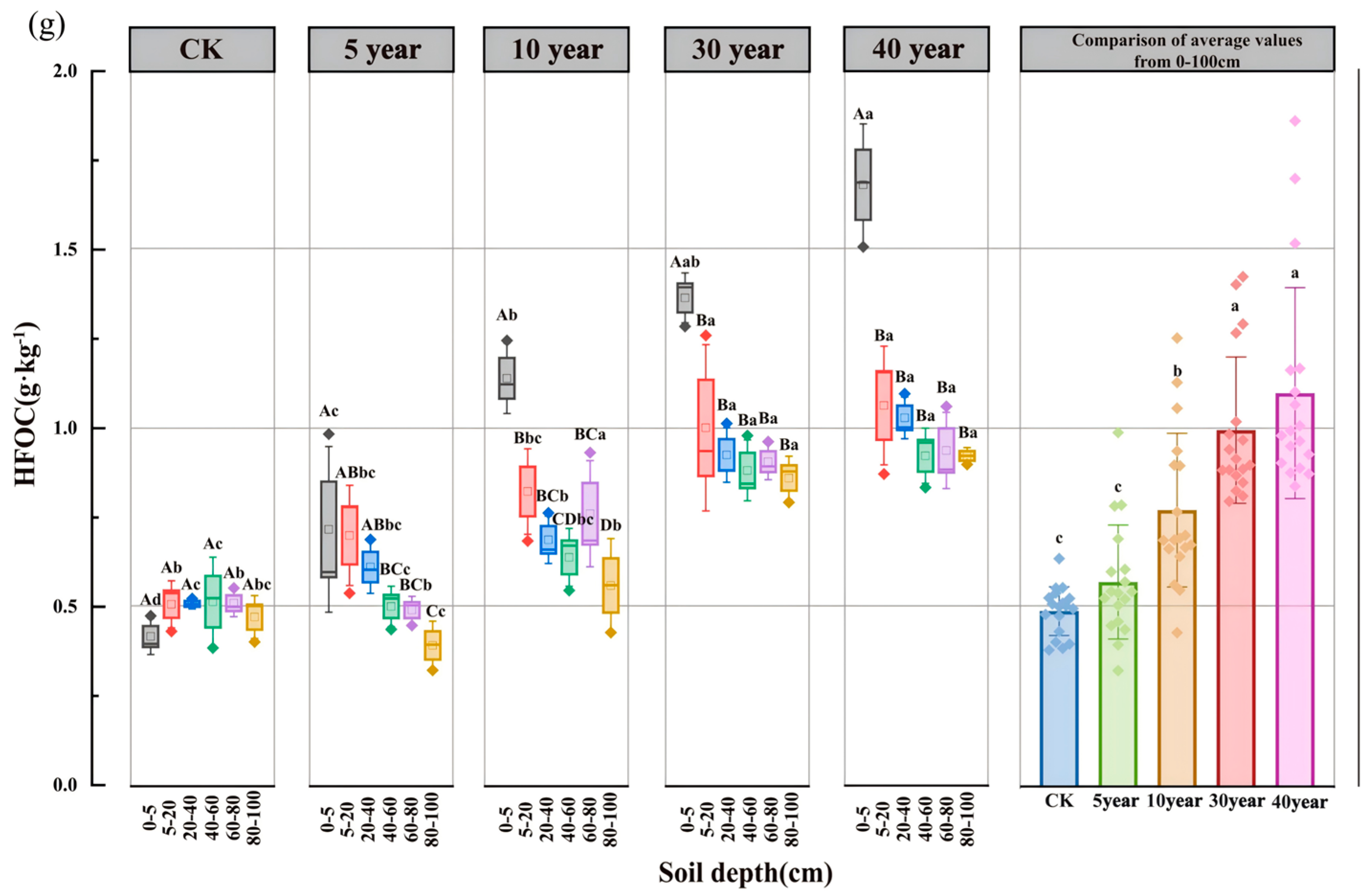
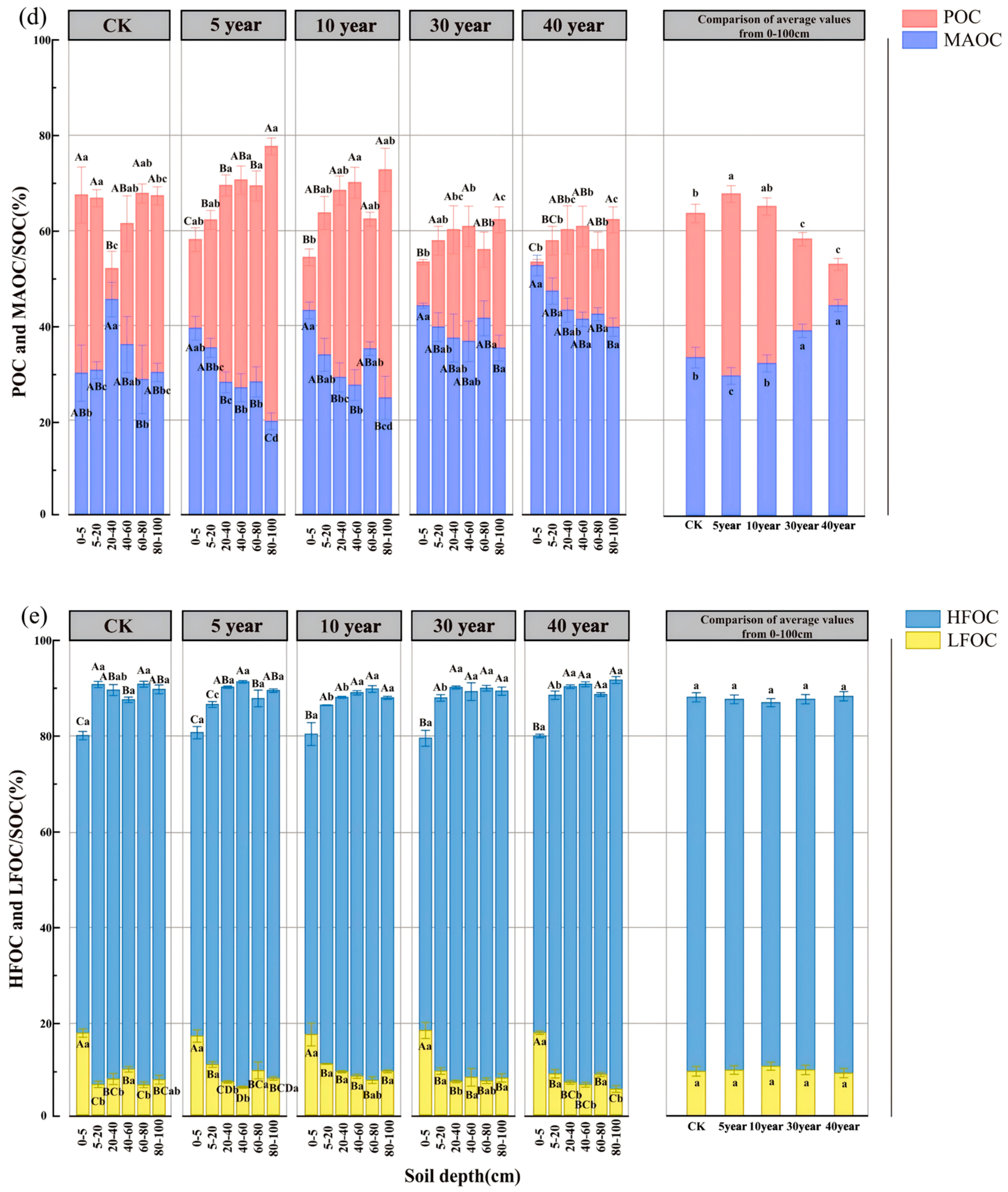
3.3. Spatiotemporal Dynamics of SOC Fractions and Their Proportional Distribution
3.4. Characteristics of Carbon Pool Management Indices in C. scoparium Sand-Fixing Plantations Across Restoration Chronosequences
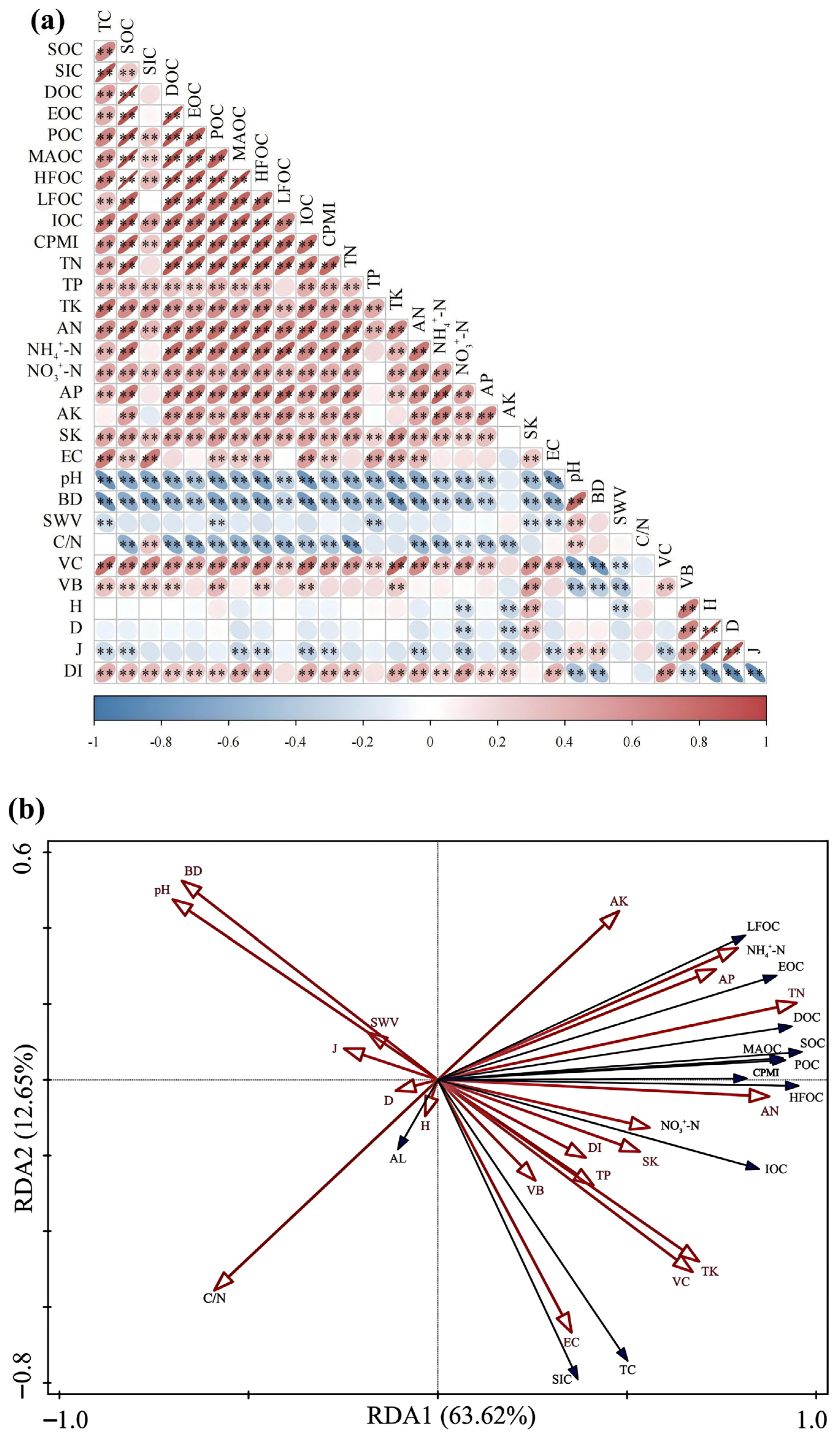
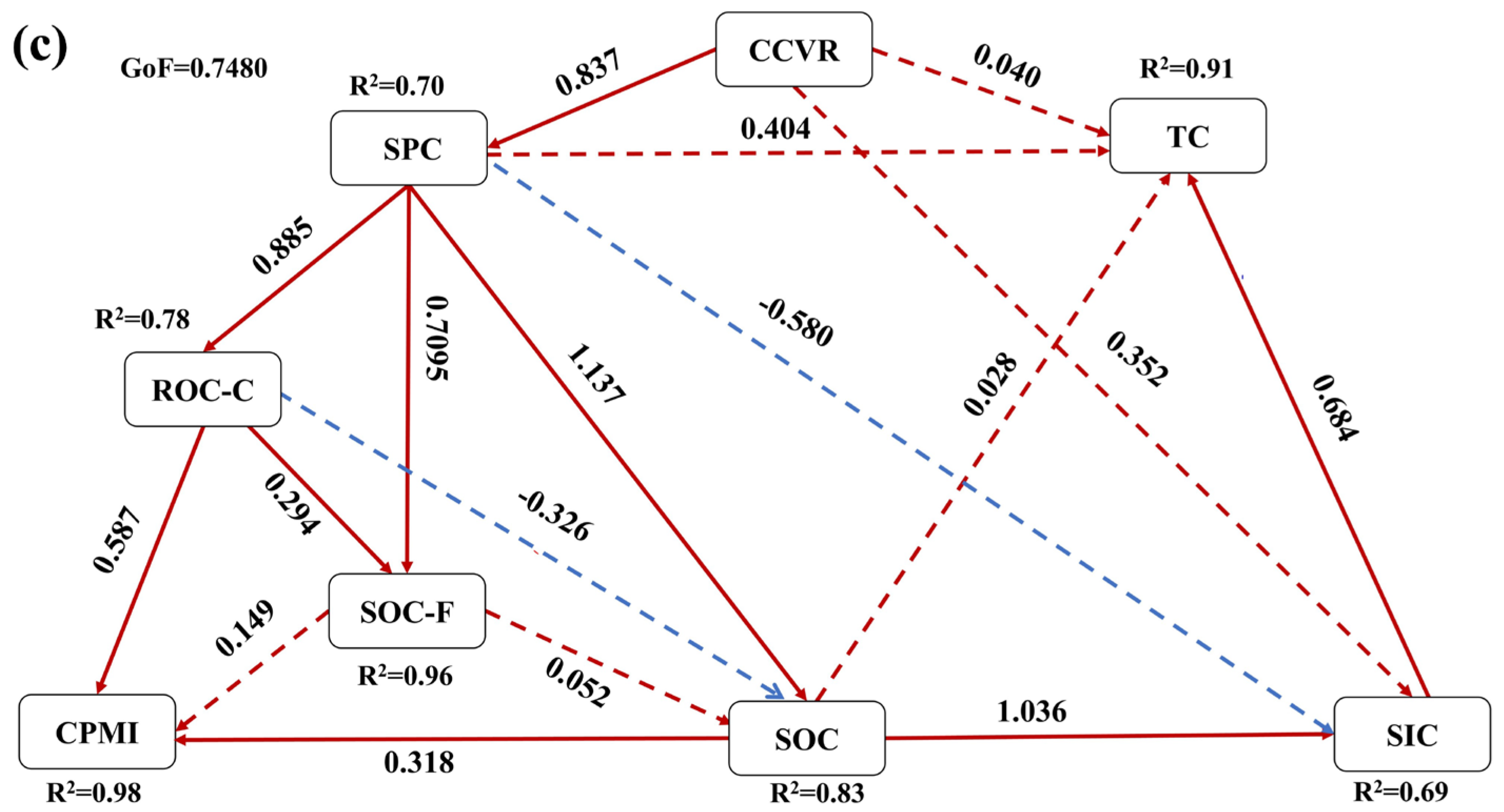
3.5. Effects of Soil Physicochemical Properties and Vegetation Restoration Characteristics on Soil Carbon Fractions
4. Discussion
4.1. Effects of Artificial C. scoparium Sand-Fixing Plantations on SOC, SIC, and CPMI
4.2. Effects of Artificial C. scoparium Sand-Fixing Plantations on SOC Fractions and Their Proportions
4.3. Drivers of Soil Carbon Fraction Dynamics and CPMI in C. scoparium Sand-Fixing Plantations
4.4. Research Limitations and Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.J.; Li, Y.F.; Xie, T.; Chang, Z.Q.; Li, X.R. Recovery of soil carbon and nitrogen stocks following afforestation with xerophytic shrubs in the Tengger Desert, North China. Catena 2022, 214, 106277. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Smith, D.M.S.; Lambin, E.F.; Turner, B.L., 2nd; Mortimore, M.; Batterbury, S.P.J.; Downing, T.E.; Dowlatabadi, H.; Fernandez, R.J.; Herrick, J.E.; et al. Global desertification: Building a science for dryland development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.P.; Yu, H.P.; Guan, X.D.; Wang, G.Y.; Guo, R.X. Accelerated dryland expansion under climate change. Nat. Clim. Chang. 2016, 6, 166–171. [Google Scholar] [CrossRef]
- Maestre, F.T.; Benito, B.M.; Berdugo, M.; Concostrina-Zubiri, L.; Delgado-Baquerizo, M.; Eldridge, D.J.; Guirado, E.; Gross, N.; Kéfi, S.; Le Bagousse-Pinguet, Y.; et al. Biogeography of global drylands. New Phytol. 2021, 231, 540–558. [Google Scholar] [CrossRef]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Shi, Y.; Baumann, F.; Ma, Y.; Song, C.; Kühn, P.; Scholten, T.; He, J.S. Organic and inorganic carbon in the topsoil of the Mongolian and Tibetan grasslands: Pattern, control and implications. Biogeosciences 2012, 9, 2287–2299. [Google Scholar] [CrossRef]
- Schlaepfer, D.R.; Bradford, J.B.; Lauenroth, W.K.; Munson, S.M.; Tietjen, B.; Hall, S.A.; Wilson, S.D.; Duniway, M.C.; Jia, G.; Pyke, D.A.; et al. Climate change reduces extent of temperate drylands and intensifies drought in deep soils. Nat. Commun. 2017, 8, 14196. [Google Scholar] [CrossRef]
- Lal, R. Carbon Cycling in Global Drylands. Curr. Clim. Chang. Rep. 2019, 5, 221–232. [Google Scholar] [CrossRef]
- Zhao, S.X.; Ta, N.; Li, Z.H.; Yang, Y.; Zhang, X.; Liu, D.; Zhang, A.; Wang, X.D. Varying pyrolysis temperature impacts application effects of biochar on soil labile organic carbon and humic fractions. Appl. Soil Ecol. 2018, 123, 484–493. [Google Scholar] [CrossRef]
- Prescott, C.E.; Vesterdal, L. Decomposition and transformations along the continuum from litter to soil organic matter in forest soils. For. Ecol. Manag. 2021, 498, 119522. [Google Scholar] [CrossRef]
- Kang, M.P.; Zhao, C.Z.; Ma, M.; Li, X.Y. Characteristics of soil organic carbon fractions in four vegetation communities of an inland salt marsh. Carbon Bal. Manag. 2024, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Han, X.; Liu, X.; Li, Z.W.; Li, J.; Yuan, Y.L.; Li, H.; Zhang, L.; Liu, S.N.; Wang, L.X.; You, C.M.; et al. Characteristics of Soil Organic Carbon Fractions and Stability Along a Chronosequence of Cryptomeria japonica var. sinensis Plantation in the Rainy Area of Western China. Forests 2022, 13, 1663. [Google Scholar] [CrossRef]
- Retallack, G.J. Carboniferous fossil plants and soils of an early tundra ecosystem. Palalso 1999, 14, 324–336. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, D.; Yang, X.; Zong, H.; Fu, X.; Zheng, J. Evolution of aeolian activities in the Tennger Desert during the Holocene: Comprehensive research based on geological records and simulated data. Quat. Sci. 2024, 44, 394–415. [Google Scholar]
- Azam, A.; Akhtar, M.S.; Rukh, S.; Mehmood, A.; Imran, M.; Khan, A.; Qayyum, A.; Ahmad, W.; Gurmani, A.R. Changes in Soil Organic Carbon Fractions Across a Loess Toposequence. J. Soil Sci. Plant Nutr. 2020, 20, 1193–1202. [Google Scholar] [CrossRef]
- Li, C.J.; Ran, M.; Song, L.Y.; Zhang, Y.Y.; Li, A.W.; Shi, W.J.; Li, W.D.; Cheng, J.L.; Zhao, B.; Luo, Y.L.; et al. Temperature effects on cropland soil particulate and mineral-associated organic carbon are governed by agricultural land-use types. Geoderma 2024, 448, 116942. [Google Scholar] [CrossRef]
- Blair, G.; Lefroy, R.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Ma, Q.L.; Wang, X.Y.; Chen, F.; Wei, L.Y.; Zhang, D.K.; Jin, H.J. Carbon Sequestration Characteristics of Typical Sand-Fixing Plantations in the Shiyang River Basin of Northwest China. Forests 2024, 15, 1548. [Google Scholar] [CrossRef]
- Lv, C.; Saba, T.; Wang, J.; Hui, W.; Kang, X.; Xie, Y.; Wang, K.; Wang, H.; Gong, W. Conversion effects of farmland to Zanthoxylum bungeanum plantations on soil organic carbon fractions in the arid valley of the upper reaches of the yangtze river, china. Catena 2022, 217, 106523. [Google Scholar] [CrossRef]
- Li, Z.; Guo, B.; Zhou, Y.; Li, T.; Shao, M.a. Forest management practices can increase the soil organic carbon sequestration potential of Robinia pseudoacacia plantations in the Loess Plateau. Soil Sci. Soc. Am. J. 2025, 89, e70082. [Google Scholar] [CrossRef]
- Li, H.X.; Man, X.L.; Cai, T.J. Long-term effects of thinning on soil organic carbon fractions and carbon pool management indices in secondary forests of heavily burned areas. J. Environ. Manag. 2024, 371, 123273. [Google Scholar] [CrossRef] [PubMed]
- Janzen, H.H.; Campbell, C.A.; Brandt, S.A.; Lafond, G.P.; Townley-Smith, L. Light-Fraction Organic Matter in Soils from Long-Term Crop Rotations. Soil Sci. Soc. Am. J. 1992, 56, 1799–1806. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, A.; Yang, Y.; Ma, Q.; Li, X.; Zhao, C. Long-Term Effects of Xerophytic Shrub Haloxylon ammodendron Plantations on Soil Properties and Vegetation Dynamics in Northwest China. PLoS ONE 2016, 11, e0168000. [Google Scholar] [CrossRef]
- Zhou, X.J.; An, X.L.; De Philippis, R.; Ye, C.R.; Ke, T.; Zhang, Y.R.; Chen, L.Z. The facilitative effects of shrub on induced biological soil crust development and soil properties. Appl. Soil Ecol. 2019, 137, 129–138. [Google Scholar] [CrossRef]
- Gao, X.; Liu, X.; Ma, L.; Wang, R. Root vertical distributions of two Artemisia species and their relationships with soil resources in the Hunshandake desert, China. Ecol. Evol. 2020, 10, 3112–3119. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, Y.; Song, M.; Yu, M.; Li, X.; Zhang, Y.; Huang, J.; Su, Z.; Sun, S.; Dai, H. Competition between shrubs and grasses in a shrub-encroached temperate grassland: Implications from nitrogen acquisition. Biol. Fertil. Soils 2025, 61, 1129–1144. [Google Scholar] [CrossRef]
- Cong, M.; Zhang, Z.; Zhao, G.; Dong, X.; Wang, W.; Mu, Z.; Tariq, A.; Graciano, C.; Sardans, J.; Peñuelas, J.; et al. Shrub Afforestation Increases Microbial-Derived Carbon in Arid Regions. Land Degrad. Dev. 2025, 36, 4691–4702. [Google Scholar] [CrossRef]
- Gao, Y.; Tian, J.; Pang, Y.; Liu, J. Soil Inorganic Carbon Sequestration Following Afforestation Is Probably Induced by Pedogenic Carbonate Formation in Northwest China. Front. Plant Sci. 2017, 8, 1282. [Google Scholar] [CrossRef]
- Lai, Z.; Jin, A.; Feng, W.; She, W.; Lang, T.; Liu, Z. Comparative Carbon Allocation and Soil Carbon Storage in Three Revegetated Shrublands in the Mu Us Desert. Forests 2025, 16, 586. [Google Scholar] [CrossRef]
- Jones, D.; Willett, V. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Leavitt, S.W.; Follett, R.F.; Paul, E.A. Estimation of the slow and fast cycling soil organic carbon fractions from 6 N HCl hydrolysis. Radiocarbon 1996, 38, 230–231. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, D.; Rong, J.; Ai, X.; Ai, S.; Su, X.; Sheng, M.; Yang, S.; Zhang, J.; Ai, Y. Landslide and aspect effects on artificial soil organic carbon fractions and the carbon pool management index on road-cut slopes in an alpine region. Catena 2021, 199, 105094. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef]
- Bayala, J.; Sanou, J.; Bazié, H.R.; Coe, R.; Kalinganire, A.; Sinclair, F.L. Regenerated trees in farmers’ fields increase soil carbon across the Sahel. Agroforest. Syst. 2020, 94, 401–415. [Google Scholar] [CrossRef]
- Petrie, M.D.; Collins, S.L.; Swann, A.M.; Ford, P.L.; Litvak, M.E. Grassland to shrubland state transitions enhance carbon sequestration in the northern Chihuahuan Desert. Glob. Change Biol. 2015, 21, 1226–1235. [Google Scholar] [CrossRef]
- Jia, X.X.; Wang, X.; Hou, L.C.; Wei, X.R.; Zhang, Y.; Shao, M.A.; Zhao, X.N. Variable response of inorganic carbon and consistent increase of organic carbon as a consequence of afforestation in areas with semiarid soils. Land Degrad. Dev. 2019, 30, 1345–1356. [Google Scholar] [CrossRef]
- Gao, Y.; Dang, P.; Zhao, Q.; Liu, J.; Liu, J. Effects of vegetation rehabilitation on soil organic and inorganic carbon stocks in the Mu Us Desert, northwest China. Land Degrad. Dev. 2017, 29, 1031–1040. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Gao, G.Y.; Ran, L.S.; Wang, Y.; Fu, B.J. Afforestation Reduces Deep Soil Carbon Sequestration in Semiarid Regions: Lessons from Variations of Soil Water and Carbon Along Afforestation Stages in China’s Loess Plateau. J. Geophys. Res. Biogeosci. 2024, 129, e2024JG008287. [Google Scholar] [CrossRef]
- Glaser, P.H.; Hansen, B.C.S.; Donovan, J.J.; Givnish, T.J.; Stricker, C.A.; Volin, J.C. Holocene dynamics of the Florida Everglades with respect to climate, dustfall, and tropical storms. Proc. Natl. Acad. Sci. USA 2013, 110, 17211–17216. [Google Scholar] [CrossRef]
- Huber, D.P.; Lohse, K.A.; Commendador, A.; Joy, S.; Aho, K.; Finney, B.; Germino, M.J. Vegetation and precipitation shifts interact to alter organic and inorganic carbon storage in cold desert soils. Ecosphere 2019, 10, e02655. [Google Scholar] [CrossRef]
- McAbee, K.; Reinhardt, K.; Germino, M.J.; Bosworth, A. Response of aboveground carbon balance to long-term, experimental enhancements in precipitation seasonality is contingent on plant community type in cold-desert rangelands. Oecologia 2017, 183, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Cable, J.M.; Ogle, K.; Barron-Gafford, G.A.; Bentley, L.P.; Cable, W.L.; Scott, R.L.; Williams, D.G.; Huxman, T.E. Antecedent Conditions Influence Soil Respiration Differences in Shrub and Grass Patches. Ecosystems 2013, 16, 1230–1247. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Raikes, J.A.; Hartley, A.E.; Cross, A.F. On the Spatial Pattern of Soil Nutrients in Desert Ecosystems. Ecology 1996, 77, 364–374. [Google Scholar] [CrossRef]
- Clark, R.B.; Zeto, S.K. Mineral acquisition by arbuscular mycorrhizal plants. J. Plant Nutr. 2000, 23, 867–902. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Z.; Tariq, A.; Sabit, R.; Sardans, J.; Graciano, C.; Li, X.; Zhu, Y.; Peñuelas, J.; Al-Bakre, D.A.; et al. Grazing exclusion significantly reduced soil organic carbon stocks but enhanced soil inorganic carbon stocks in desert steppe of northwest China. Ecol. Indic. 2025, 172, 113341. [Google Scholar] [CrossRef]
- Guan, Q.Y.; Sun, X.Z.; Yang, J.; Pan, B.T.; Zhao, S.L.; Wang, L. Dust Storms in Northern China: Long-Term Spatiotemporal Characteristics and Climate Controls. J. Clim. 2017, 30, 6683–6700. [Google Scholar] [CrossRef]
- Dong, L.; Ran, J.; Luo, J.; Bai, L.; Sun, Y.; Aqeel, M.; Zhang, Y.; Wang, X.; Du, Q.; Xiong, J. Inorganic Carbon Pools and Their Drivers in Grassland and Desert Soils. Glob. Change Biol. 2024, 30, e17536. [Google Scholar] [CrossRef]
- Li, X.R.; Ma, F.Y.; Xiao, H.L.; Wang, X.P.; Kim, K.C. Long-term effects of revegetation on soil water content of sand dunes in arid region of Northern China. J. Arid Environ. 2004, 57, 1–16. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Liu, B.; Lyu, H.; Duan, Y.; Yang, Y.; Wang, S.; Li, Z. Rhizosphere enzyme activities and microorganisms drive the transformation of organic and inorganic carbon in saline–alkali soil region. Sci. Rep. 2022, 12, 1314. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Zhang, Y.; Feng, W.; Lai, Z.; Qin, S. Soil Microbes Transform Inorganic Carbon Into Organic Carbon by Dark Fixation Pathways in Desert Soil. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG006047. [Google Scholar] [CrossRef]
- Zhu, X.L.; Si, J.H.; He, X.H.; Jia, B.; Zhou, D.M.; Wang, C.L.; Qin, J.; Liu, Z.J.; Ndayambaza, B.; Bai, X.; et al. The distribution and driving mechanism of soil inorganic carbon in semi-arid and arid areas: A case study of Alxa region in China. Catena 2024, 247, 108475. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.J.; Guo, T.W.; Zhang, P.L.; Wang, J.P. Soil organic and inorganic carbon in the loess profiles of Lanzhou area: Implications of deep soils. Catena 2015, 126, 68–74. [Google Scholar] [CrossRef]
- Tamir, G.; Shenker, M.; Heller, H.; Bloom, P.R.; Fine, P.; Bar-Tal, A. Dissolution and Re-crystallization Processes of Active Calcium Carbonate in Soil Developed on Tufa. Soil Sci. Soc. Am. J. 2012, 76, 1606–1613. [Google Scholar] [CrossRef]
- Bughio, M.A.; Wang, P.L.; Meng, F.Q.; Qing, C.; Kuzyakov, Y.; Wang, X.J.; Junejo, S.A. Neoformation of pedogenic carbonates by irrigation and fertilization and their contribution to carbon sequestration in soil. Geoderma 2016, 262, 12–19. [Google Scholar] [CrossRef]
- Han, J.; Pan, C.; Sun, Y.; Chen, Z.; Xiong, Y.; Huang, G. Impact of Land Use Conversion on Soil Structure and Hydropedological Functions in an Arid Region. Land Degrad. Dev. 2025, 36, 643–654. [Google Scholar] [CrossRef]
- Chadwick, O.A.; Sowers, J.M.; Amundson, R.G. Morphology of Calcite Crystals in Clast Coatings From Four Soils in the Mojave Desert Region. Soil Sci. Soc. Am. J. 1989, 53, 211. [Google Scholar] [CrossRef]
- Zamanian, K.; Pustovoytov, K.; Kuzyakov, Y. Pedogenic carbonates: Forms and formation processes. Earth-Sci. Rev. 2016, 157, 1–17. [Google Scholar] [CrossRef]
- Singh, H.; Mishra, D.; Nahar, N.M. Energy use pattern in production agriculture of a typical village in arid zone, India––Part I. Energy Convers. Manag. 2002, 43, 2275–2286. [Google Scholar] [CrossRef]
- Raheb, A.; Heidari, A.; Mahmoodi, S. Organic and inorganic carbon storage in soils along an arid to dry sub-humid climosequence in northwest of Iran. Catena 2017, 153, 66–74. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, Z.Q.; Tian, F.P.; Wang, D.; Wu, G.L. Soil Organic Carbon and Inorganic Carbon Accumulation Along a 30-year Grassland Restoration Chronosequence in Semi-arid Regions (China). Land Degrad. Dev. 2016, 28, 189–198. [Google Scholar] [CrossRef]
- Ferdush, J.; Paul, V. A review on the possible factors influencing soil inorganic carbon under elevated CO2. Catena 2021, 204, 105434. [Google Scholar] [CrossRef]
- Liu, S.S.; Zhou, L.H.; Li, H.; Zhao, X.; Yang, Y.H.; Zhu, Y.K.; Hu, H.F.; Chen, L.Y.; Zhang, P.J.; Shen, H.H.; et al. Shrub encroachment decreases soil inorganic carbon stocks in Mongolian grasslands. J. Ecol. 2020, 108, 678–686. [Google Scholar] [CrossRef]
- He, X.X.; Sheng, M.Y.; Wang, L.J.; Zhang, S.L.; Luo, N.N. Effects on soil organic carbon accumulation and mineralization of long-term vegetation restoration in Southwest China karst. Ecol. Indic. 2022, 145, 109622. [Google Scholar] [CrossRef]
- Kleber, M.; Bourg, I.C.; Coward, E.K.; Hansel, C.M.; Myneni, S.C.B.; Nunan, N. Dynamic interactions at the mineral-organic matter interface. Nat. Rev. Earth Environ. 2021, 2, 402–421. [Google Scholar] [CrossRef]
- An, S.S.; Mentler, A.; Mayer, H.; Blum, W.E.H. Soil aggregation, aggregate stability, organic carbon and nitrogen in different soil aggregate fractions under forest and shrub vegetation on the Loess Plateau, China. Catena 2010, 81, 226–233. [Google Scholar] [CrossRef]
- Benbi, D.K.; Brar, K.; Toor, A.S.; Sharma, S. Sensitivity of Labile Soil Organic Carbon Pools to Long-Term Fertilizer, Straw and Manure Management in Rice-Wheat System. Pedosphere 2015, 25, 534–545. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Ren, C.J.; Wang, C.K.; Delgado-Baquerizo, M.; Luo, Y.Q.; Luo, Z.K.; Du, Z.G.; Zhu, B.; Yang, Y.H.; Jiao, S.; et al. Global turnover of soil mineral-associated and particulate organic carbon. Nat. Commun. 2024, 15, 5329. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Chen, J.; Wang, X.J.; Zhang, R.Z.; Cai, L.Q.; Jiao, Y.P.; Li, Z.Q.; Han, G.J. Changes in soil particulate and mineral-associated organic carbon concentrations under nitrogen addition in China-a meta-analysis. Plant Soil 2023, 489, 439–452. [Google Scholar] [CrossRef]
- Chen, J.G.; Ji, C.J.; Fang, J.Y.; He, H.B.; Zhu, B. Dynamics of microbial residues control the responses of mineral-associated soil organic carbon to N addition in two temperate forests. Sci. Total Environ. 2020, 748, 141318. [Google Scholar] [CrossRef]
- Wang, B.R.; An, S.S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Batool, M.; Cihacek, L.J.; Alghamdi, R.S. Soil Inorganic Carbon Formation and the Sequestration of Secondary Carbonates in Global Carbon Pools: A Review. Soil Syst. 2024, 8, 15. [Google Scholar] [CrossRef]
- Granse, D.; Wanner, A.; Stock, M.; Jensen, K.; Mueller, P. Plant-sediment interactions decouple inorganic from organic carbon stock development in salt marsh soils. Limnol. Oceanogr. Lett. 2024, 9, 469–477. [Google Scholar] [CrossRef]
- Zang, H.; Mehmood, I.; Kuzyakov, Y.; Jia, R.; Gui, H.; Blagodatskaya, E.; Xu, X.; Smith, P.; Chen, H.; Zeng, Z.; et al. Not all soil carbon is created equal: Labile and stable pools under nitrogen input. Glob. Change Biol. 2024, 30, e17405. [Google Scholar] [CrossRef]
- Mori, T.; Lu, X.K.; Aoyagi, R.; Mo, J.M. Reconsidering the phosphorus limitation of soil microbial activity in tropical forests. Funct. Ecol. 2018, 32, 1145–1154. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Zhang, X.Y.; Wang, H.M.; Meng, S.W.; Yang, F.T.; Chen, F.S.; Wang, S.Q.; Dong, Q.X.; Wang, J. Warming causes variability in SOM decomposition in N- and P-fertiliser-treated soil in a subtropical coniferous forest. Eur. J. Soil Sci. 2022, 73, e13320. [Google Scholar] [CrossRef]
- Wu, B.; Bai, T.S.; Yu, W.J.; Zhu, T.B.; Li, D.M.; Ye, C.L.; Liu, M.Q.; Hu, S.J. Soil pH and precipitation controls on organic carbon retention from organic amendments across soil orders: A meta-analysis. Soil Biol. Biochem. 2025, 207, 109819. [Google Scholar] [CrossRef]
- Peng, F.; Xue, X.; You, Q.G.; Huang, C.H.; Dong, S.Y.; Liao, J.; Duan, H.C.; Tsunekawa, A.; Wang, T. Changes of soil properties regulate the soil organic carbon loss with grassland degradation on the Qinghai-Tibet Plateau. Ecol. Indic. 2018, 93, 572–580. [Google Scholar] [CrossRef]
- Hao, Y.; Mao, J.; Bachmann, C.M.; Hoffman, F.M.; Koren, G.; Chen, H.; Tian, H.; Liu, J.; Tao, J.; Tang, J.; et al. Soil moisture controls over carbon sequestration and greenhouse gas emissions: A review. NPJ Clim. Atmos. Sci. 2025, 8, 16. [Google Scholar] [CrossRef]
- Setia, R.; Gottschalk, P.; Smith, P.; Marschner, P.; Baldock, J.; Setia, D.; Smith, J. Soil salinity decreases global soil organic carbon stocks. Sci. Total Environ. 2013, 465, 267–272. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.J.; Jiang, L.M.; Li, E.; Yang, X.D.; Yang, J.J. Salinity affects microbial function genes related to nutrient cycling in arid regions. Front. Microbiol. 2024, 15, 1407760. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, X.J.; Li, X.L.; Wang, J.P.; Xu, M.G.; Li, D.W. Dynamics of soil organic and inorganic carbon in the cropland of upper Yellow River Delta, China. Sci. Rep. 2016, 6, 36105. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Z.; Guan, Q.; Zhang, E.; Sun, Y.; Yan, Y.; Du, Q. Coupling mechanism between vegetation and multi-depth soil moisture in arid–semiarid area: Shift of dominant role from vegetation to soil moisture. For. Ecol. Manag. 2023, 546, 121323. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Xie, J.B.; Wang, Y.G.; Li, Y. Biotic and Abiotic Contribution to Diurnal Soil CO2 Fluxes from Saline/Alkaline Soils. Sci. Rep. 2020, 10, 5396. [Google Scholar] [CrossRef]

| Type | CK | 5 Year | 10 Year | 30 Year | 40 Year |
|---|---|---|---|---|---|
| Number of species | 3 | 6 | 14 | 11 | 11 |
| Dominant species | C. squarrosum | C. scoparium A. ordosica | C. scoparium A. ordosica | A. ordosica | A. ordosica C. alaschanica |
| Community companion plants | P. villosa | C. squarrosum P. villosa etc. | S. glareosa C. alaschanica E. gmelinii etc. | A. scoparia E. gmelinii Suaeda glauca Bunge. etc. | S. glareosa A. melilotoides A. hispidus etc. |
| Indicators | CK | Years | |||
|---|---|---|---|---|---|
| 5 Year | 10 Year | 30 Year | 40 Year | ||
| TN (g·kg−1) | 0.08 ± 0.01 c | 0.09 ± 0.01 c | 0.13 ± 0.01 b | 0.17 ± 0.02 ab | 0.19 ± 0.02 a |
| TP (g·kg−1) | 0.24 ± 0.01 c | 0.24 ± 0.01 c | 0.25 ± 0.01 c | 0.50 ± 0.05 a | 0.34 ± 0.02 b |
| TK (g·kg−1) | 15.48 ± 0.09 b | 14.77 ± 0.07 c | 15.73 ± 0.11 b | 15.71 ± 0.15 b | 16.64 ± 0.12 a |
| NH4+-N (mg·kg−1) | 0.63 ± 0.09 c | 0.75 ± 0.05 c | 0.97 ± 0.1 bc | 1.37 ± 0.27 ab | 1.61 ± 0.34 a |
| NO3−-N (mg·kg−1) | 1.66 ± 0.24 c | 1.71 ± 0.06 bc | 2.18 ± 0.15 bc | 2.4 ± 0.34 b | 3.73 ± 0.3 a |
| AN (mg·kg−1) | 7.89 ± 0.44 d | 7.79 ± 0.37 d | 10 ± 0.52 c | 12.35 ± 0.86 b | 14.59 ± 0.68 a |
| AP (mg·kg−1) | 0.75 ± 0.13 b | 0.68 ± 0.07 b | 1.22 ± 0.16 ab | 1.3 ± 0.28 ab | 1.67 ± 0.31 a |
| AK (mg·kg−1) | 77.17 ± 3.85 ab | 72.94 ± 1.37 b | 72.44 ± 2.9 b | 72.06 ± 4.61 b | 85.44 ± 3.63 a |
| SK (mg·kg−1) | 142.46 ± 3.36 b | 209.28 ± 8.22 a | 212.97 ± 5.71 a | 215.86 ± 5.65 a | 218.86 ± 9.25 a |
| SWV (%) | 3.01 ± 0.28 a | 1.10 ± 0.06 c | 2.55 ± 0.31 ab | 0.81 ± 0.10 c | 2.12 ± 0.23 b |
| pH | 8.89 ± 0.01 a | 8.72 ± 0.03 b | 8.67 ± 0.01 c | 8.36 ± 0.01 e | 8.42 ± 0.02 d |
| BD (g·cm−3) | 1.62 ± 0.02 a | 1.55 ± 0.01 b | 1.49 ± 0.01 c | 1.4 ± 0.01 d | 1.38 ± 0.01 d |
| EC (μS/cm) | 61.07 ± 1.70 b | 64.88 ± 0.72 b | 75.59 ± 1.39 b | 166.22 ± 20.5 a | 148.99 ± 11.61 a |
| C/N | 32.57 ± 1.45 a | 35.53 ± 3.76 a | 33.36 ± 3.49 a | 28.89 ± 2.40 a | 28.55 ± 2.17 a |
| Shrub cover (%) | 0.00 ± 0.00 c | 24.09 ± 0.88 a | 19.74 ± 1.49 b | 17.72 ± 1.15 b | 11.19 ± 0.65 c |
| Herb cover (%) | 2.44 ± 0.69 d | 3.03 ± 0.44 d | 9.65 ± 1.08 c | 12.92 ± 1.59 b | 45.61 ± 0.44 a |
| VC (%) | 2.44 ± 0.69 c | 27.13 ± 0.80 b | 29.39 ± 2.03 b | 30.64 ± 0.84 b | 56.8 ± 0.47 a |
| H | 0.29 ± 0.15 c | 1.09 ± 0.04 b | 1.59 ± 0.13 a | 1.04 ± 0.26 b | 0.24 ± 0.06 c |
| D | 0.21 ± 0.12 b | 0.54 ± 0.03 a | 0.75 ± 0.03 a | 0.5 ± 0.13 a | 0.09 ± 0.03 b |
| J | 0.40 ± 0.20 b | 0.61 ± 0.02 ab | 0.78 ± 0.03 a | 0.55 ± 0.14 ab | 0.14 ± 0.04 c |
| DI | 0.48 ± 0.02 b | 0.46 ± 0.03 b | 0.25 ± 0.03 c | 0.5 ± 0.13 b | 0.91 ± 0.03 a |
| herbaceous biomass/(g·m−2) | 10.95 ± 1.31 d | 166.03 ± 22.69 c | 214.99 ± 19.37 bc | 271.04 ± 11.42 ab | 304.25 ± 37.77 a |
| shrub biomass/(g·m−2) | 0.00 ± 0.00 c | 460.21 ± 4.25 a | 548.6 ± 41.74 a | 571.43 ± 102.92 a | 228.97 ± 53.89 b |
| VB/(g/·m−2) | 10.95 ± 1.31 c | 626.23 ± 18.57 b | 763.58 ± 60.21 a | 842.47 ± 42.93 a | 533.22 ± 59.92 b |
| Indicators | CK | Years | |||
|---|---|---|---|---|---|
| 5 Year | 10 Year | 30 Year | 40 Year | ||
| SC | 0.52 ± 0.02 c | 0.61 ± 0.04 c | 0.84 ± 0.06 b | 1.08 ± 0.06 a | 1.18 ± 0.09 a |
| A | 0.03 ± 0 a | 0.03 ± 0 a | 0.03 ± 0 a | 0.03 ± 0 a | 0.03 ± 0 a |
| AL | 1 ± 0 a | 1.03 ± 0.07 a | 1.01 ± 0.05 a | 1 ± 0.04 a | 1.02 ± 0.04 a |
| CPI | 1 ± 0 d | 1.18 ± 0.08 cd | 1.64 ± 0.14 bc | 2.11 ± 0.16 ab | 2.33 ± 0.22 a |
| CPMI | 100 ± 0 d | 119.44 ± 9.59 cd | 165.01 ± 16.67 bc | 207.89 ± 14.98 ab | 239.86 ± 26.43 a |
| Impact Factors | Explains (%) | Contribution (%) | Pseudo-F | p |
|---|---|---|---|---|
| TN | 57.7 | 70.40 | 120.00 | 0.01 |
| BD | 9.60 | 11.70 | 25.60 | 0.01 |
| VC | 1.90 | 2.30 | 5.20 | 0.01 |
| EC | 2.30 | 2.80 | 6.80 | 0.01 |
| C/N | 2.30 | 2.80 | 7.30 | 0.01 |
| AP | 1.70 | 2.10 | 5.80 | 0.01 |
| AN | 0.80 | 1.00 | 2.70 | 0.03 |
| NH4+-N | 0.60 | 0.80 | 2.2 | 0.07 |
| pH | 0.40 | 0.50 | 1.40 | 0.18 |
| TP | 0.40 | 0.50 | 1.40 | 0.22 |
| TK | 0.40 | 0.50 | 1.60 | 0.14 |
| SWV | 0.30 | 0.40 | 1.20 | 0.26 |
| VB | 0.40 | 0.40 | 1.30 | 0.21 |
| SK | 0.50 | 0.60 | 1.90 | 0.13 |
| AK | 0.40 | 0.50 | 1.60 | 0.16 |
| NO3−-N | 0.30 | 0.30 | 1.00 | 0.35 |
| H | 0.30 | 0.40 | 1.20 | 0.28 |
| D | 0.80 | 0.90 | 2.80 | 0.04 |
| DI | 0.30 | 0.40 | 1.30 | 0.21 |
| J | 0.40 | 0.50 | 1.70 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Ma, Q.; Ma, R.; Wei, L.; Cheng, F.; Wu, G.; Wang, R.; Wei, Q. Dynamics and Driving Factors of Soil Carbon Fractions in Corethrodendron scoparium (Fisch. & C. A. Mey.) Fisch. & Basiner. Sand-Fixing Plantations at the South Edge of Tengger Desert, Northwestern China. Forests 2025, 16, 1499. https://doi.org/10.3390/f16091499
Shi L, Ma Q, Ma R, Wei L, Cheng F, Wu G, Wang R, Wei Q. Dynamics and Driving Factors of Soil Carbon Fractions in Corethrodendron scoparium (Fisch. & C. A. Mey.) Fisch. & Basiner. Sand-Fixing Plantations at the South Edge of Tengger Desert, Northwestern China. Forests. 2025; 16(9):1499. https://doi.org/10.3390/f16091499
Chicago/Turabian StyleShi, Linqi, Quanlin Ma, Rui Ma, Linyuan Wei, Fang Cheng, Guohong Wu, Runjuan Wang, and Qian Wei. 2025. "Dynamics and Driving Factors of Soil Carbon Fractions in Corethrodendron scoparium (Fisch. & C. A. Mey.) Fisch. & Basiner. Sand-Fixing Plantations at the South Edge of Tengger Desert, Northwestern China" Forests 16, no. 9: 1499. https://doi.org/10.3390/f16091499
APA StyleShi, L., Ma, Q., Ma, R., Wei, L., Cheng, F., Wu, G., Wang, R., & Wei, Q. (2025). Dynamics and Driving Factors of Soil Carbon Fractions in Corethrodendron scoparium (Fisch. & C. A. Mey.) Fisch. & Basiner. Sand-Fixing Plantations at the South Edge of Tengger Desert, Northwestern China. Forests, 16(9), 1499. https://doi.org/10.3390/f16091499





