The Impact of Climate Change on Anatomical Characteristics of Silver Fir and European Beech Wood from Three Sites in the Carpathians, Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Climate Data
2.2. Sample Collection and Preparation
2.3. Laboratory Methods and Quantitative Analysis of Wood Anatomy
2.4. Statistical Analysis
3. Results
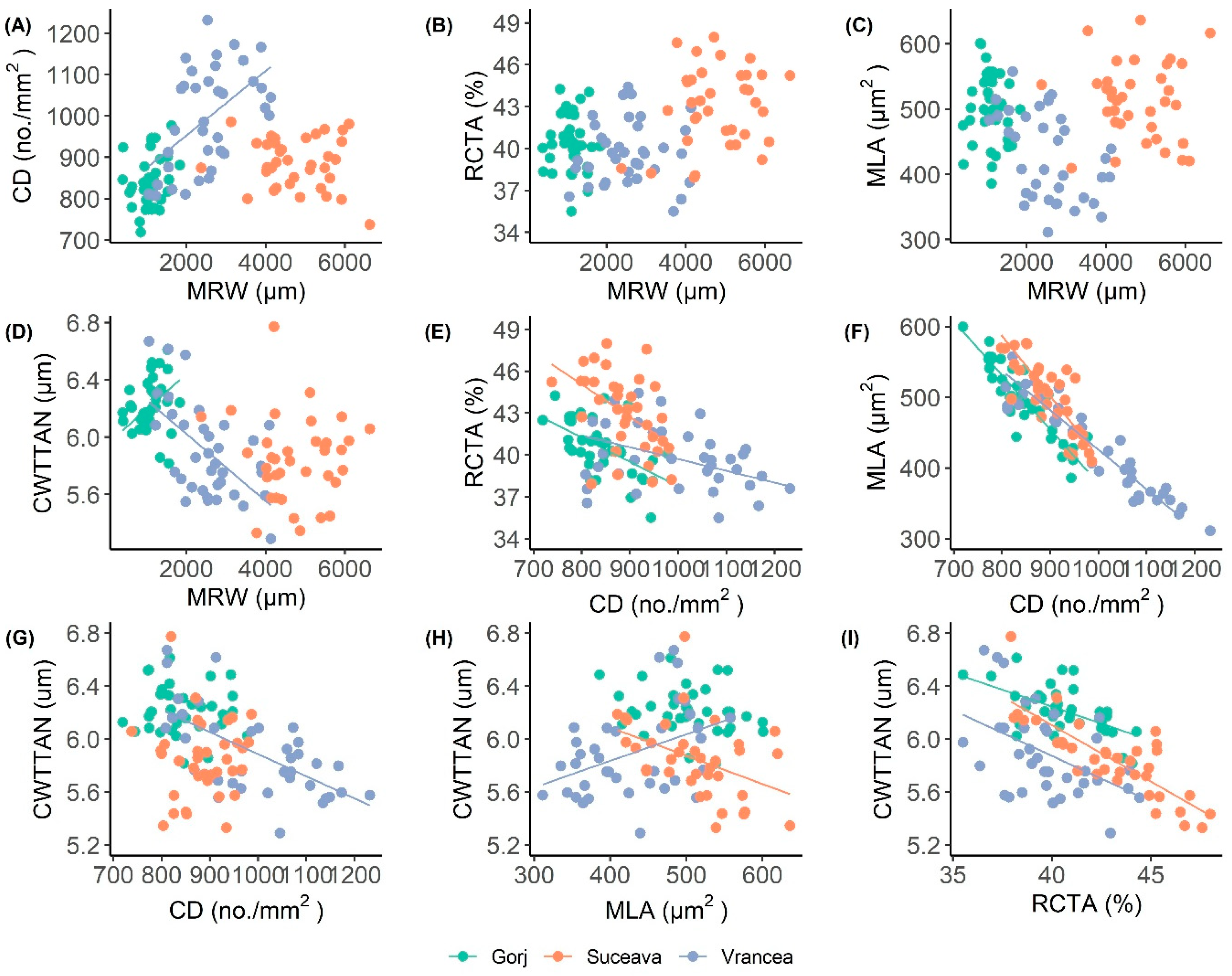
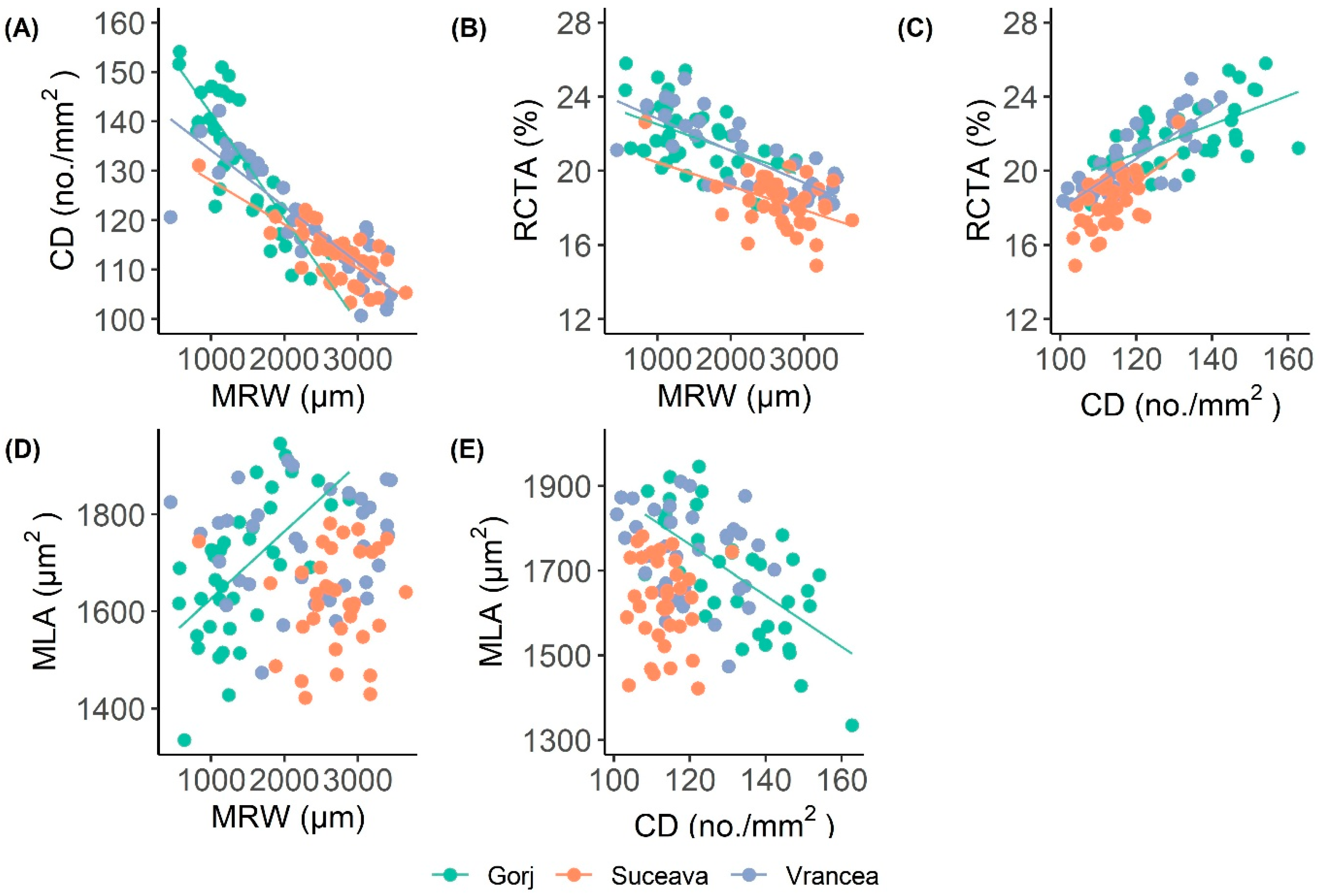
3.1. Differences in Wood-Anatomical Characteristics Between Sites
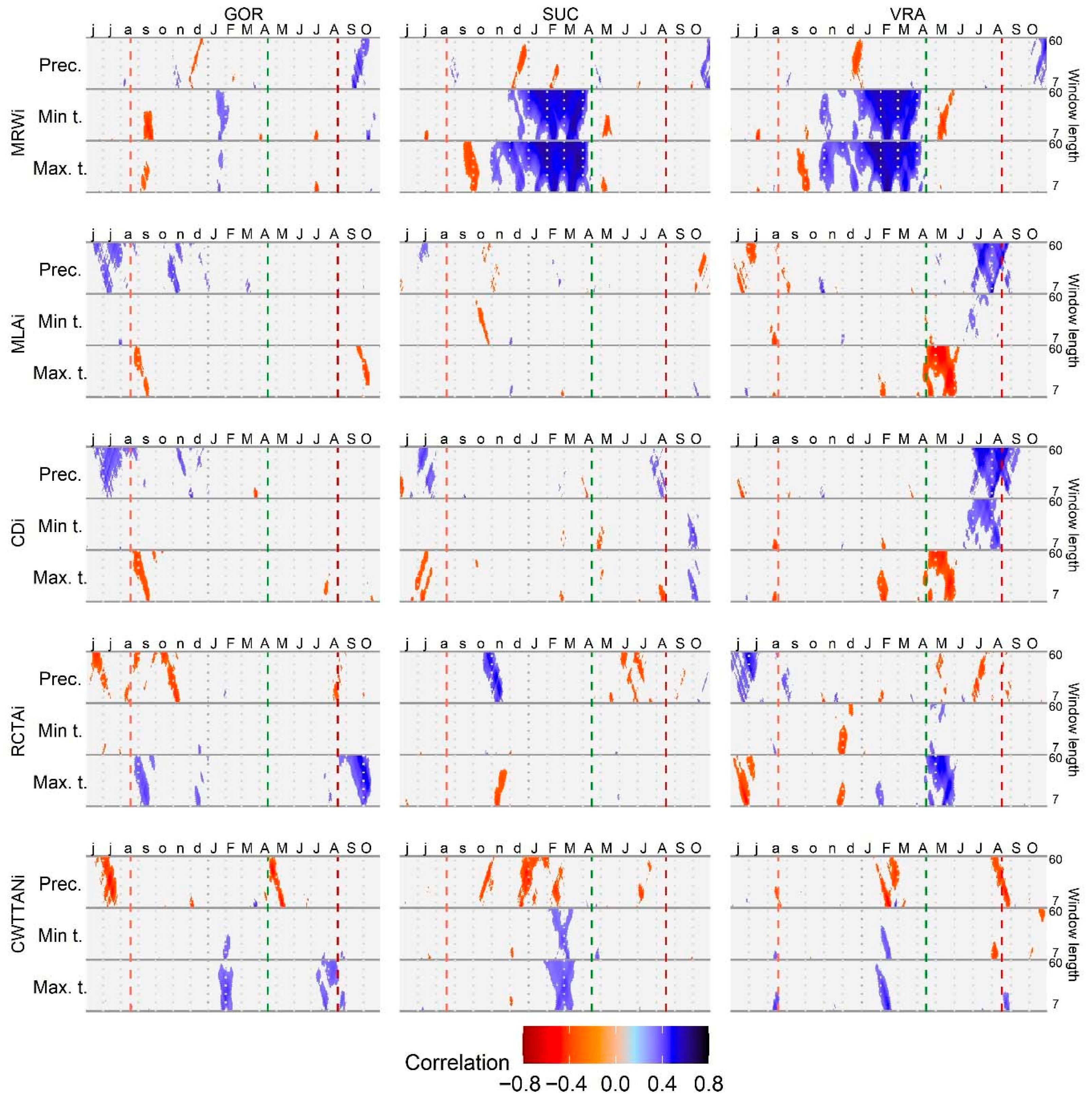
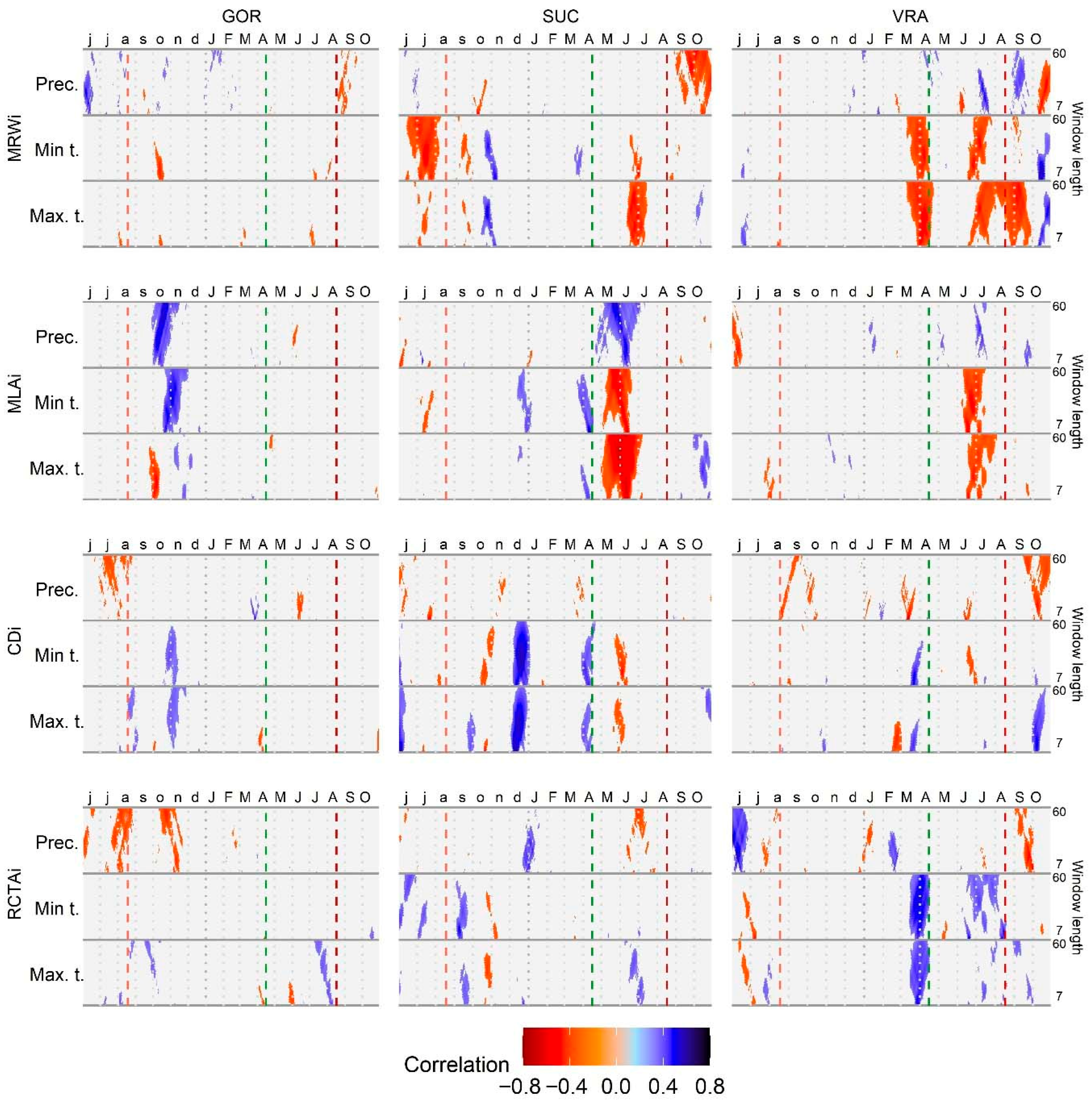
3.2. The Effect of Climate Variables on Tree-Ring and Vessel Parameters
4. Discussion
4.1. Wood-Anatomical Characteristics of Fir and Beech Differ Between the Selected Sites
4.2. Relationships Between Tree-Ring Widths and Wood-Anatomical Traits
4.3. Fir and Beech Respond Differently to Climatic Variables
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRW | Mean tree-ring width |
| MLA | Mean vessel/tracheid lumen area |
| CD | Cell (tracheid or vessel) density |
| RCTA | Relative conductive area |
| CWTTAN | Mean thickness of tangential cell walls |
| LMM | Linear mixed model |
| CGP | Canopy gap fraction |
| MAT | Mean annual air temperature |
| MAP | Mean annual precipitation |
| N | Number of trees |
| DBH | Mean diameter at breast height |
Appendix A
Appendix A.1
| Species | Tree-Ring Characteristic | Test (Statistic, df) | p-Value | Pairwise Comparison | Adjusted p | Significance |
|---|---|---|---|---|---|---|
| ABAL | MRW | Friedman (χ2 = 74, df = 2) | <0.0001 | SUC–VRA | <0.0001 | **** |
| SUC–GOR | <0.0001 | **** | ||||
| VRA–GOR | <0.0001 | **** | ||||
| CD | Friedman (χ2 = 31.3, df =2) | <0.0001 | SUC–VRA | <0.0001 | **** | |
| SUC–GOR | <0.01 | ** | ||||
| VRA–GOR | <0.0001 | **** | ||||
| RCTA | LMM (F = 30.47, df = 2, 72) | <0.0001 | SUC–VRA | <0.0001 | **** | |
| SUC–GOR | <0.0001 | **** | ||||
| VRA–GOR | 0.07 | ns | ||||
| MLA | LMM (F = 31.81, df = 2, 72) | <0.001 | SUC–VRA | <0.0001 | **** | |
| SUC–GOR | 1.00 | ns | ||||
| VRA–GOR | <0.0001 | **** | ||||
| CWTTAN | Friedman (χ2 = 32, df = 2) | <0.0001 | SUC–VRA | 1.00 | ns | |
| SUC–GOR | <0.0001 | **** | ||||
| VRA–GOR | <0.0001 | **** | ||||
| FASY | MRW | Friedman (χ2 = 26.32, df = 2) | <0.0001 | SUC–VRA | 0.023 | * |
| SUC–GOR | <0.0001 | **** | ||||
| VRA–GOR | <0.01 | ** | ||||
| CD | Friedman (χ2 = 19.68, df = 2) | <0.0001 | SUC–VRA | 0.009 | ** | |
| SUC–GOR | <0.0001 | **** | ||||
| VRA–GOR | 0.002 | ** | ||||
| RCTA | Friedman (χ2 = 42, df = 2) | <0.0001 | SUC–VRA | <0.0001 | **** | |
| SUC–GOR | <0.0001 | **** | ||||
| VRA–GOR | 0.042 | * | ||||
| MLA | LMM (F = 11.03, df = 2, 72) | <0.0001 | SUC–VRA | <0.0001 | **** | |
| SUC–GOR | 0.027 | * | ||||
| VRA–GOR | 0.149 | ns |
Appendix A.2
| Model | Term | Estimate | Std. Error | df | t Value | Pr (>|t|) | R2 Marginal | R2 Conditional |
|---|---|---|---|---|---|---|---|---|
| CD ~ MRW * site | (Intercept) | 854.19 | 39.46 | 97.49 | 21.647 | 0.000 | 0.47 | 0.73 |
| MRW | 0.01 | 0.03 | 91.70 | −0.372 | 0.711 | |||
| siteSuceava | 27.26 | 66.61 | 76.63 | 0.409 | 0.683 | |||
| siteVrancea | −57.82 | 47.89 | 72.11 | −1.207 | 0.231 | |||
| MRW:siteSuceava | 0.01 | 0.03 | 80.76 | 0.404 | 0.687 | |||
| MRW:siteVrancea | 0.09 | 0.03 | 78.88 | 2.662 | 0.009 | |||
| RCTA ~ MRW * site | (Intercept) | 41.68 | 1.23 | 98.88 | 33.800 | 0.000 | 0.27 | 0.55 |
| MRW | 0.00 | 0.00 | 95.47 | −0.973 | 0.333 | |||
| siteSuceava | −1.36 | 2.13 | 79.92 | −0.638 | 0.525 | |||
| siteVrancea | −1.72 | 1.54 | 74.91 | −1.118 | 0.267 | |||
| MRW:siteSuceava | 0.00 | 0.00 | 84.44 | 1.481 | 0.142 | |||
| MRW:siteVrancea | 0.00 | 0.00 | 82.32 | 0.881 | 0.381 | |||
| CWTTAN ~ MRW * site | (Intercept) | 5.95 | 0.13 | 98.40 | 45.240 | 0.000 | 0.39 | 0.64 |
| MRW | 0.00 | 0.00 | 94.33 | 2.151 | 0.034 | |||
| siteSuceava | 0.16 | 0.23 | 78.36 | 0.728 | 0.469 | |||
| siteVrancea | 0.54 | 0.16 | 73.35 | 3.332 | 0.001 | |||
| MRW:siteSuceava | 0.00 | 0.00 | 82.90 | −2.615 | 0.011 | |||
| MRW:siteVrancea | 0.00 | 0.00 | 80.78 | −4.160 | 0.000 | |||
| MLA ~ MRW * site | (Intercept) | 488.66 | 30.67 | 99.45 | 15.932 | 0.000 | 0.34 | 0.57 |
| MRW | 0.01 | 0.03 | 96.81 | 0.393 | 0.696 | |||
| siteSuceava | 53.70 | 53.54 | 81.23 | 1.003 | 0.319 | |||
| siteVrancea | 36.27 | 38.77 | 76.00 | 0.936 | 0.352 | |||
| MRW:siteSuceava | −0.02 | 0.03 | 85.91 | −0.613 | 0.542 | |||
| MRW:siteVrancea | −0.05 | 0.03 | 83.68 | −1.773 | 0.080 | |||
| RCTA ~ CD * site | (Intercept) | 55.20 | 4.19 | 92.09 | 13.167 | 0.000 | 0.42 | 0.68 |
| CD | −0.02 | 0.00 | 91.70 | −3.508 | 0.001 | |||
| siteSuceava | 8.50 | 5.85 | 78.28 | 1.453 | 0.150 | |||
| siteVrancea | −7.06 | 4.50 | 75.44 | −1.568 | 0.121 | |||
| CD:siteSuceava | −0.01 | 0.01 | 78.37 | −0.870 | 0.387 | |||
| CD:siteVrancea | 0.01 | 0.01 | 77.16 | 1.745 | 0.085 | |||
| MLA ~ CD * site | (Intercept) | 1142.87 | 52.17 | 92.24 | 21.905 | 0.000 | 0.87 | 0.92 |
| CD | −0.76 | 0.06 | 91.87 | −12.365 | 0.000 | |||
| siteSuceava | 126.68 | 72.97 | 77.99 | 1.736 | 0.086 | |||
| siteVrancea | −157.42 | 56.16 | 74.96 | −2.803 | 0.006 | |||
| CD:siteSuceava | −0.09 | 0.08 | 78.08 | −1.052 | 0.296 | |||
| CD:siteVrancea | 0.21 | 0.06 | 76.74 | 3.189 | 0.002 | |||
| CWTTAN ~ CD * site | (Intercept) | 6.50 | 0.54 | 99.75 | 12.075 | 0.000 | 0.40 | 0.52 |
| CD | 0.00 | 0.00 | 99.68 | −0.525 | 0.601 | |||
| siteSuceava | −0.79 | 0.78 | 88.07 | −1.016 | 0.312 | |||
| siteVrancea | 1.05 | 0.61 | 82.50 | 1.738 | 0.086 | |||
| CD:siteSuceava | 0.00 | 0.00 | 88.17 | 0.549 | 0.584 | |||
| CD:siteVrancea | 0.00 | 0.00 | 84.53 | −1.924 | 0.058 | |||
| CWTTAN ~ MLA * site | (Intercept) | 6.28 | 0.39 | 96.84 | 16.167 | 0.000 | 0.37 | 0.52 |
| MLA | 0.00 | 0.00 | 96.55 | −0.153 | 0.879 | |||
| siteSuceava | 0.66 | 0.52 | 90.21 | 1.273 | 0.206 | |||
| siteVrancea | −1.24 | 0.45 | 83.25 | −2.739 | 0.008 | |||
| MLA:siteSuceava | 0.00 | 0.00 | 90.46 | −1.963 | 0.053 | |||
| MLA:siteVrancea | 0.00 | 0.00 | 82.76 | 2.234 | 0.028 | |||
| CWTTAN ~ RCTA * site | (Intercept) | 8.28 | 0.72 | 101.94 | 11.442 | 0.000 | 0.56 | 0.57 |
| RCTA | −0.05 | 0.02 | 101.94 | −2.860 | 0.005 | |||
| siteSuceava | 1.23 | 0.91 | 96.24 | 1.357 | 0.178 | |||
| siteVrancea | 0.35 | 0.96 | 91.94 | 0.360 | 0.720 | |||
| RCTA:siteSuceava | −0.03 | 0.02 | 96.46 | −1.565 | 0.121 | |||
| RCTA:siteVrancea | −0.02 | 0.02 | 91.96 | −0.751 | 0.455 |
Appendix A.3
| Model | Term | Estimate | Std. Error | df | t Value | Pr (>|t|) | R2 Marginal | R2 Conditional |
|---|---|---|---|---|---|---|---|---|
| CD ~ MRW * site | (Intercept) | 162.74 | 2.72 | 101.85 | 59.839 | 0.000 | 0.80 | 0.83 |
| MRW | −0.02 | 0.00 | 101.51 | −12.086 | 0.000 | |||
| siteSuceava | −25.77 | 6.06 | 101.81 | −4.251 | 0.000 | |||
| siteVrancea | −17.19 | 4.07 | 96.27 | −4.223 | 0.000 | |||
| MRW:siteSuceava | 0.01 | 0.00 | 100.02 | 4.591 | 0.000 | |||
| MRW:siteVrancea | 0.01 | 0.00 | 91.75 | 4.470 | 0.000 | |||
| RCTA ~ MRW * site | (Intercept) | 23.91 | 0.63 | 102.00 | 37.840 | 0.000 | 0.63 | |
| MRW | 0.00 | 0.00 | 102.00 | −3.442 | 0.001 | |||
| siteSuceava | −2.16 | 1.40 | 102.00 | −1.548 | 0.125 | |||
| siteVrancea | 0.63 | 0.92 | 102.00 | 0.684 | 0.496 | |||
| MRW:siteSuceava | 0.00 | 0.00 | 102.00 | 0.185 | 0.853 | |||
| MRW:siteVrancea | 0.00 | 0.00 | 102.00 | −0.665 | 0.507 | |||
| MLA ~ MRW * site | (Intercept) | 1487.43 | 47.89 | 101.47 | 31.062 | 0.000 | 0.27 | 0.41 |
| MRW | 0.14 | 0.03 | 100.19 | 4.513 | 0.000 | |||
| siteSuceava | 103.95 | 107.06 | 101.97 | 0.971 | 0.334 | |||
| siteVrancea | 201.45 | 72.59 | 98.27 | 2.775 | 0.007 | |||
| MRW:siteSuceava | −0.13 | 0.05 | 101.20 | −2.676 | 0.009 | |||
| MRW:siteVrancea | −0.12 | 0.04 | 94.63 | −3.015 | 0.003 | |||
| RCTA ~ CD * site | (Intercept) | 11.61 | 1.96 | 100.83 | 5.924 | 0.000 | 0.69 | 0.72 |
| CD | 0.08 | 0.01 | 100.77 | 5.280 | 0.000 | |||
| siteSuceava | −10.24 | 4.55 | 101.87 | −2.249 | 0.027 | |||
| siteVrancea | −7.08 | 3.07 | 97.58 | −2.303 | 0.023 | |||
| CD:siteSuceava | 0.07 | 0.04 | 101.81 | 1.836 | 0.069 | |||
| CD:siteVrancea | 0.06 | 0.02 | 97.48 | 2.303 | 0.023 | |||
| MLA ~ CD * site | (Intercept) | 2486.75 | 159.75 | 99.07 | 15.567 | 0.000 | 0.31 | 0.41 |
| CD | −6.04 | 1.20 | 98.90 | −5.032 | 0.000 | |||
| siteSuceava | −726.43 | 371.74 | 100.74 | −1.954 | 0.053 | |||
| siteVrancea | −407.60 | 252.84 | 100.30 | −1.612 | 0.110 | |||
| CD:siteSuceava | 4.84 | 3.20 | 100.55 | 1.513 | 0.133 | |||
| CD:siteVrancea | 3.18 | 2.02 | 100.25 | 1.571 | 0.119 |
Appendix A.4
| Model | Type | Site/Contrast | Estimate | SE | df | Lower CL | Upper CL | t Ratio | p Value |
|---|---|---|---|---|---|---|---|---|---|
| CD ~ MRW * site | Estimated slope | Gorj | −0.013 | 0.035 | 92.17 | −0.081 | 0.056 | ||
| Suceava | 0.001 | 0.013 | 93.70 | −0.025 | 0.027 | ||||
| Vrancea | 0.078 | 0.014 | 94.60 | 0.050 | 0.106 | ||||
| Pairwise contrast | Gorj–Suceava | −0.014 | 0.034 | 81.62 | −0.401 | 0.915 | |||
| Gorj–Vrancea | −0.091 | 0.034 | 79.80 | −2.646 | 0.026 | ||||
| Suceava–Vrancea | −0.077 | 0.017 | 72.17 | −4.645 | 0.000 | ||||
| RCTA ~ MRW * site | Estimated slope | Gorj | −0.001 | 0.001 | 95.54 | −0.003 | 0.001 | ||
| Suceava | 0.001 | 0.000 | 96.87 | 0.000 | 0.001 | ||||
| Vrancea | 0.000 | 0.000 | 97.63 | −0.001 | 0.001 | ||||
| Pairwise contrast | Gorj–Suceava | −0.002 | 0.001 | 84.62 | −1.469 | 0.311 | |||
| Gorj–Vrancea | −0.001 | 0.001 | 82.52 | −0.875 | 0.658 | ||||
| Suceava–Vrancea | 0.001 | 0.001 | 73.79 | 1.222 | 0.444 | ||||
| CWTTAN ~ MRW * site | Estimated slope | Gorj | 0.000 | 0.000 | 94.77 | 0.000 | 0.000 | ||
| Suceava | 0.000 | 0.000 | 96.16 | 0.000 | 0.000 | ||||
| Vrancea | 0.000 | 0.000 | 96.97 | 0.000 | 0.000 | ||||
| Pairwise contrast | Gorj–Suceava | 0.000 | 0.000 | 83.87 | 2.594 | 0.030 | |||
| Gorj–Vrancea | 0.000 | 0.000 | 81.83 | 4.130 | 0.000 | ||||
| Suceava–Vrancea | 0.000 | 0.000 | 73.36 | 3.157 | 0.006 | ||||
| MLA ~ MRW * site | Estimated slope | Gorj | 0.011 | 0.027 | 96.86 | −0.043 | 0.064 | ||
| Suceava | −0.006 | 0.010 | 98.04 | −0.026 | 0.014 | ||||
| Vrancea | −0.038 | 0.011 | 98.71 | −0.060 | −0.016 | ||||
| Pairwise contrast | Gorj–Suceava | 0.017 | 0.028 | 86.04 | 0.60729 | 0.81653 | |||
| Gorj–Vrancea | 0.048 | 0.028 | 83.81 | 1.75874 | 0.18986 | ||||
| Suceava–Vrancea | 0.032 | 0.013 | 74.64 | 2.3563 | 0.05433 | ||||
| RCTA ~ CD * site | Estimated slope | Gorj | −0.017 | 0.005 | 91.92 | −0.027 | −0.007 | ||
| Suceava | −0.023 | 0.005 | 89.24 | −0.034 | −0.013 | ||||
| Vrancea | −0.008 | 0.003 | 92.26 | −0.014 | −0.003 | ||||
| Pairwise contrast | Gorj–Suceava | 0.006 | 0.007 | 78.79 | 0.86429 | 0.66428 | |||
| Gorj–Vrancea | −0.009 | 0.005 | 77.59 | −1.7357 | 0.19852 | ||||
| Suceava–Vrancea | −0.015 | 0.006 | 79.90 | −2.6893 | 0.02343 | ||||
| MLA ~ CD * site | Estimated slope | Gorj | −0.765 | 0.063 | 92.42 | −0.889 | −0.641 | ||
| Suceava | −0.853 | 0.065 | 89.77 | −0.983 | −0.724 | ||||
| Vrancea | −0.560 | 0.033 | 92.76 | −0.625 | −0.495 | ||||
| Pairwise contrast | Gorj–Suceava | 0.089 | 0.085 | 79.19 | 1.04513 | 0.55084 | |||
| Gorj–Vrancea | −0.205 | 0.065 | 77.90 | −3.1705 | 0.0061 | ||||
| Suceava–Vrancea | −0.294 | 0.069 | 80.32 | −4.258 | 0.00016 | ||||
| CWTTAN ~ CD * site | Estimated slope | Gorj | 0.000 | 0.001 | 99.64 | −0.002 | 0.001 | ||
| Suceava | 0.000 | 0.001 | 98.39 | −0.001 | 0.002 | ||||
| Vrancea | −0.002 | 0.000 | 99.79 | −0.002 | −0.001 | ||||
| Pairwise contrast | Gorj–Suceava | 0.000 | 0.001 | 87.95 | −0.543 | 0.85032 | |||
| Gorj–Vrancea | 0.001 | 0.001 | 84.26 | 1.90759 | 0.14282 | ||||
| Suceava–Vrancea | 0.002 | 0.001 | 89.16 | 2.47074 | 0.04039 | ||||
| CWTTAN ~ MLA * site | Estimated slope | Gorj | 0.000 | 0.001 | 96.80 | −0.002 | 0.001 | ||
| Suceava | −0.002 | 0.001 | 95.91 | −0.004 | −0.001 | ||||
| Vrancea | 0.002 | 0.001 | 97.18 | 0.001 | 0.003 | ||||
| Pairwise contrast | Gorj–Suceava | 0.002 | 0.001 | 90.96 | 1.93827 | 0.13387 | |||
| Gorj–Vrancea | −0.002 | 0.001 | 83.52 | −2.2133 | 0.07477 | ||||
| Suceava–Vrancea | −0.004 | 0.001 | 87.88 | −4.486 | 6.5 × 10−5 | ||||
| CWTTAN ~ RCTA * site | Estimated slope | Gorj | −0.051 | 0.018 | 101.94 | −0.087 | −0.015 | ||
| Suceava | −0.085 | 0.013 | 101.93 | −0.111 | −0.059 | ||||
| Vrancea | −0.069 | 0.016 | 101.95 | −0.101 | −0.037 | ||||
| Pairwise contrast | Gorj–Suceava | 0.034314 | 0.02226 | 96.3681 | 1.54184 | 0.27611 | |||
| Gorj–Vrancea | 0.017945 | 0.02422 | 91.7898 | 0.74098 | 0.73981 | ||||
| Suceava–Vrancea | −0.01637 | 0.02081 | 94.0712 | −0.7866 | 0.71217 |
Appendix A.5
| Model | Type | Site/Contrast | Estimate | SE | df | Lower CL | Upper CL | t Ratio | p Value |
|---|---|---|---|---|---|---|---|---|---|
| CD ~ MRW * site | Estimated slope | Gorj | −0.021 | 0.002 | 101.52 | −0.025 | −0.018 | ||
| Suceava | −0.009 | 0.002 | 100.88 | −0.013 | −0.005 | ||||
| Vrancea | −0.011 | 0.001 | 101.66 | −0.014 | −0.009 | ||||
| Pairwise contrast | Gorj–Suceava | −0.012 | 0.003 | 100.05 | −4.517 | 0.000 | |||
| Gorj–Vrancea | −0.010 | 0.002 | 91.89 | −4.384 | 0.000 | ||||
| Suceava–Vrancea | 0.002 | 0.002 | 89.47 | 1.089 | 0.524 | ||||
| RCTA ~ MRW * site | Estimated slope | Gorj | −0.001 | 0.000 | 102.00 | −0.002 | −0.001 | ||
| Suceava | −0.001 | 0.000 | 102.00 | −0.002 | 0.000 | ||||
| Vrancea | −0.002 | 0.000 | 102.00 | −0.002 | −0.001 | ||||
| Pairwise contrast | Gorj–Suceava | 0.000 | 0.001 | 97.14 | −0.183 | 0.982 | |||
| Gorj–Vrancea | 0.000 | 0.001 | 84.13 | 0.653 | 0.791 | ||||
| Suceava–Vrancea | 0.000 | 0.001 | 94.74 | 0.815 | 0.694 | ||||
| MLA ~ MRW * site | Estimated slope | Gorj | 0.139 | 0.031 | 100.63 | 0.077 | 0.201 | ||
| Suceava | 0.012 | 0.035 | 99.16 | −0.057 | 0.081 | ||||
| Vrancea | 0.021 | 0.021 | 100.98 | −0.022 | 0.063 | ||||
| Pairwise contrast | Gorj–Suceava | 0.127 | 0.048 | 101.40 | 2.632 | 0.026 | |||
| Gorj–Vrancea | 0.118 | 0.040 | 96.35 | 2.957 | 0.011 | ||||
| Suceava–Vrancea | −0.009 | 0.039 | 86.14 | −0.220 | 0.974 | ||||
| RCTA ~ CD * site | Estimated slope | Gorj | 0.078 | 0.015 | 101.09 | 0.048 | 0.107 | ||
| Suceava | 0.150 | 0.037 | 100.65 | 0.077 | 0.222 | ||||
| Vrancea | 0.134 | 0.019 | 101.07 | 0.096 | 0.173 | ||||
| Pairwise contrast | Gorj–Suceava | −0.072 | 0.040 | 101.86 | −1.802 | 0.174 | |||
| Gorj–Vrancea | −0.057 | 0.025 | 98.64 | −2.255 | 0.067 | ||||
| Suceava–Vrancea | 0.015 | 0.041 | 98.66 | 0.369 | 0.928 | ||||
| MLA ~ CD * site | Estimated slope | Gorj | −6.044 | 1.220 | 99.85 | −8.464 | −3.624 | ||
| Suceava | −1.208 | 2.986 | 98.91 | −7.133 | 4.717 | ||||
| Vrancea | −2.869 | 1.583 | 99.80 | −6.009 | 0.272 | ||||
| Pairwise contrast | Gorj–Suceava | −4.836 | 3.253 | 101.00 | −1.487 | 0.302 | |||
| Gorj–Vrancea | −3.175 | 2.064 | 100.79 | −1.539 | 0.277 | ||||
| Suceava–Vrancea | 1.661 | 3.353 | 96.37 | 0.495 | 0.874 |
References
- Björklund, J.; Seftigen, K.; Schweingruber, F.; Fonti, P.; Von Arx, G.; Bryukhanova, M.V.; Cuny, H.E.; Carrer, M.; Castagneri, D.; Frank, D.C. Cell size and wall dimensions drive distinct variability of earlywood and latewood density in Northern Hemisphere conifers. N. Phytol. 2017, 216, 728–740. [Google Scholar] [CrossRef]
- Rathgeber, C.B.K. Conifer tree-ring density inter-annual variability—Anatomical, physiological and environmental determinants. N. Phytol. 2017, 216, 621–625. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Fonti, P.; von Arx, G.; Garcia-Gonzalez, I.; Eilmann, B.; Sass-Klaassen, U.; Gartner, H.; Eckstein, D. Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. N. Phytol. 2010, 185, 42–53. [Google Scholar] [CrossRef]
- Eilmann, B.; Sterck, F.; Wegner, L.; De Vries, S.M.; Von Arx, G.; Mohren, G.M.; Den Ouden, J.; Sass-Klaassen, U. Wood structural differences between northern and southern beech provenances growing at a moderate site. Tree Physiol. 2014, 34, 882–893. [Google Scholar] [CrossRef]
- Diaconu, D.; Stangler, D.F.; Kahle, H.P.; Spiecker, H. Vessel plasticity of European beech in response to thinning and aspect. Tree Physiol. 2016, 36, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Arnič, D.; Gričar, J.; Jevšenak, J.; Božič, G.; von Arx, G.; Prislan, P. Different Wood Anatomical and Growth Responses in European Beech (Fagus sylvatica L.) at Three Forest Sites in Slovenia. Front. Plant Sci. 2021, 12, 669229. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.C.; Calderaro, C.; Cocozza, C.; Lasserre, B.; Tognetti, R.; von Arx, G. Wood Anatomical Responses of European Beech to Elevation, Land Use Change, and Climate Variability in the Central Apennines, Italy. Front. Plant Sci. 2022, 13, 855741. [Google Scholar] [CrossRef] [PubMed]
- Camarero, J.J.; Olano, J.M.; Parras, A. Plastic bimodal xylogenesis in conifers from continental Mediterranean climates. N. Phytol. 2010, 185, 471–480. [Google Scholar] [CrossRef]
- Gruber, A.; Strobl, S.; Veit, B.; Oberhuber, W. Impact of drought on the temporal dynamics of wood formation in Pinus sylvestris. Tree Physiol. 2010, 30, 490–501. [Google Scholar] [CrossRef]
- Vieira, J.; Rossi, S.; Campelo, F.; Freitas, H.; Nabais, C. Seasonal and daily cycles of Pinus pinaster stem radial variation of in a drought-prone environment. Agric. For. Meteorol. 2013, 180, 173–181. [Google Scholar] [CrossRef]
- Ren, P.; Rossi, S.; Gričar, J.; Liang, E.; Čufar, K. Is precipitation a trigger for the onset of xylogenesis in Juniperus przewalskii on the north-eastern Tibetan Plateau? Ann. Bot. 2015, 119, 1011–1020. [Google Scholar] [CrossRef]
- Eilmann, B.; Zweifel, R.; Buchmann, N.; Graf Pannatier, E.; Rigling, A. Drought alters timing, quantity, and quality of wood formation in Scots pine. J. Exp. Bot. 2011, 62, 2763–2771. [Google Scholar] [CrossRef]
- De Micco, V.; Campelo, F.; De Luis, M.; Bräuning, A.; Grabner, M.; Battipaglia, G.; Cherubini, P. Intra-Annual Density Fluctuations in Tree Rings: How, When, Where, and Why? IAWA J. 2016, 37, 232–259. [Google Scholar] [CrossRef]
- Körner, C. Conservation of mountain biodiversity in the context of climate change. In Proceedings of the International Mountain Biodiversity Conference, ICIMOD 2009, Kathmandu, Nepal, 16–18 November 2008. [Google Scholar]
- Micu, D.M.; Birsan, M.V.; Cheval, S.; Dumitrescu, A. Climate of the Romanian Carpathians: Variability and Trends, 1st ed.; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Pachauri, R.K.; Reisinger, A. (Eds.) IPCC, 2007: Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2007; p. 104. [Google Scholar]
- Dobrowolska, D.; Boncina, A.; Klumpp, R. Ecology and silviculture of silver fir (Abies alba Mill.): A review. J. For. Res. Jpn. 2017, 22, 326–335. [Google Scholar] [CrossRef]
- Jump, A.S.; Hunt, J.M.; Martinez-Izquierdo, J.A.; Penuelas, J. Natural selection and climate change: Temperature-linked spatial and temporal trends in gene frequency in Fagus sylvatica. Mol. Ecol. 2006, 15, 3469–3480. [Google Scholar] [CrossRef]
- Hajek, P.; Kurjak, D.; von Wuhlisch, G.; Delzon, S.; Schuldt, B. Intraspecific Variation in Wood Anatomical, Hydraulic, and Foliar Traits in Ten European Beech Provenances Differing in Growth Yield. Front. Plant Sci. 2016, 7, 791. [Google Scholar] [CrossRef] [PubMed]
- Ellenberg, H. Coniferous woodland and mixed woods dominated by conifers. Veg. Ecol. Cent. Eur. 2009, 1, 191–242. [Google Scholar]
- Larysch, E.; Stangler, D.F.; Puhlmann, H.; Rathgeber, C.B.K.; Seifert, T.; Kahle, H.P. The 2018 hot drought pushed conifer wood formation to the limit of its plasticity: Consequences for woody biomass production and tree ring structure. Plant Biol. 2022, 24, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Montwé, D.; Spiecker, H.; Hamann, A. An experimentally controlled extreme drought in a Norway spruce forest reveals fast hydraulic response and subsequent recovery of growth rates. Trees 2014, 28, 891–900. [Google Scholar] [CrossRef]
- Nabais, C.; Campelo, F.; Vieira, J.; Cherubini, P. Climatic signals of tree-ring width and intra-annual density fluctuations in Pinus pinaster and Pinus pinea along a latitudinal gradient in Portugal. Forestry 2014, 87, 598–605. [Google Scholar] [CrossRef]
- Stangler, D.F.; Kahle, H.P.; Raden, M.; Larysch, E.; Seifert, T.; Spiecker, H. Effects of Intra-Seasonal Drought on Kinetics of Tracheid Differentiation and Seasonal Growth Dynamics of Norway Spruce along an Elevational Gradient. Forests 2021, 12, 274. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B. Xylogenesis: Coniferous Trees of Temperate Forests Are Listening to the Climate Tale during the Growing Season But Only Remember the Last Words! Plant Physiol. 2016, 171, 306–317. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.K.; Frank, D.; Fonti, P.; Fournier, M. Kinetics of tracheid development explain conifer tree-ring structure. N. Phytol. 2014, 203, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Ziaco, E.; Biondi, F.; Rossi, S.; Deslauriers, A. Climatic influences on wood anatomy and tree-ring features of Great Basin conifers at a new mountain observatory. Appl. Plant Sci. 2014, 2, 1400054. [Google Scholar] [CrossRef]
- Castagneri, D.; Fonti, P.; von Arx, G.; Carrer, M. How does climate influence xylem morphogenesis over the growing season? Insights from long-term intra-ring anatomy in Picea abies. Ann. Bot. 2017, 119, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Bui, V. Wood anatomy of Scots pine and Norway spruce under warming and elevated CO2. In Proceedings of the Botany 2015: Science and Plants for People, Edmonton, AB, Canada, 25–29 July 2015. [Google Scholar]
- Gričar, J.; Jevšenak, J.; Giagli, K.; Eler, K.; Tsalagkas, D.; Gryc, V.; Vavrcik, H.; Čufar, K.; Prislan, P. Temporal and spatial variability of phloem structure in Picea abies and Fagus sylvatica and its link to climate. Plant Cell Environ. 2024, 47, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Adamič, P.C.; Levanič, T.; Hanzu, M.; Čater, M. Growth response of European beech (Fagus sylvatica L.) and silver fir (Abies alba Mill.) to climate factors along the Carpathian massive. Forests 2023, 14, 1318. [Google Scholar] [CrossRef]
- Čater, M.; Adamič, P.C.; Darenova, E. Response of beech and fir to different light intensities along the Carpathian and Dinaric Mountains. Front. Plant Sci. 2024, 15, 1380275. [Google Scholar] [CrossRef]
- Dařenová, E.; Adamič, P.C.; Čater, M. Effect of temperature, water availability, and soil properties on soil CO2 efflux in beech-fir forests along the Carpathian Mts. Catena 2024, 240, 107974. [Google Scholar] [CrossRef]
- Kašpar, J.; Král, K.; Levanič, T.; Adamič, P.C.; Čater, M. Climate growth limitations of European beech and silver fir along the Carpathian arc—The recent state and future prospects. Agric. For. Meteorol. 2025, 361, 110323. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Mirek, Z.; Piekos-Mirkowa, H. Flora and vegetation of the polish Tatra mountains. Mt. Res. Dev. 1992, 12, 147–173. [Google Scholar] [CrossRef]
- Vološčuk, I. The National Parks and Biosphere Reserves in Carpathians: The Last Nature Paradises; Association of the Carpathian National Parks and Biosphere Reserves: Tatranska Lomnica, Slovakia, 1999; p. 244. [Google Scholar]
- Rădulescu, D.P.; Săndulescu, M. The plate-tectonics concept and the geological structure of the Carpathians. Tectonophysics 1973, 16, 155–161. [Google Scholar] [CrossRef]
- Golonka, J.; Pietsch, K.; Marzec, P. The north European platform suture zone in Poland. Geol. Geophys. Environ. 2018, 44, 5–16. [Google Scholar] [CrossRef]
- Cornes, R.C.; Van Der Schrier, G.; Van Den Besselaar, E.J.M.; Jones, P.D. An ensemble version of the E-OBS temperature and precipitation data sets. J. Geophys. Res. Atmos. 2018, 123, 9391–9409. [Google Scholar] [CrossRef]
- Levanič, T. ATRICS—A new system for image acquisition in dendrochronology. Tree-Ring Res. 2007, 63, 117–122. [Google Scholar] [CrossRef]
- Buras, A. A comment on the expressed population signal. Dendrochronologia 2017, 44, 130–132. [Google Scholar] [CrossRef]
- von Arx, G.; Crivellaro, A.; Prendin, A.L.; Cufar, K.; Carrer, M. Quantitative Wood Anatomy-Practical Guidelines. Front. Plant Sci. 2016, 7, 781. [Google Scholar] [CrossRef]
- Gärtner, H.; Lucchinetti, S.; Schweingruber, F.H. A new sledge microtome to combine wood anatomy and tree-ring ecology. IAWA J. 2015, 36, 452–459. [Google Scholar] [CrossRef]
- Prislan, P.; Gričar, J.; De Luis, M.; Smith, K.T.; Čufar, K. Phenological variation in xylem and phloem formation in Fagus sylvatica from two contrasting sites. Agric. For. Meteorol. 2013, 180, 142–151. [Google Scholar] [CrossRef]
- Prendin, A.L.; Petit, G.; Carrer, M.; Fonti, P.; Bjorklund, J.; von Arx, G. New research perspectives from a novel approach to quantify tracheid wall thickness. Tree Physiol. 2017, 37, 976–983. [Google Scholar] [CrossRef]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Jevšenak, J.; Levanič, T. dendroTools: R package for studying linear and nonlinear responses between tree-rings and daily environmental data. Dendrochronologia 2018, 48, 32–39. [Google Scholar] [CrossRef]
- Olson, M.E.; Pace, M.R.; Anfodillo, T. The vulnerability to drought-induced embolism-conduit diameter link: Breaching the anatomy-physiology divide. IAWA J. 2023, 44, 335–354. [Google Scholar] [CrossRef]
- Oladi, R.; Bräuning, A.; Pourtahmasi, K. “Plastic” and “static” behavior of vessel-anatomical features in Oriental beech (Fagus orientalis Lipsky) in view of xylem hydraulic conductivity. Trees Struct. Funct. 2014, 28, 493–502. [Google Scholar] [CrossRef]
- Prislan, P.; Čufar, K.; De Luis, M.; Gričar, J. Precipitation is not limiting for xylem formation dynamics and vessel development in European beech from two temperate forest sites. Tree Physiol. 2018, 38, 186–197. [Google Scholar] [CrossRef]
- Gasson, P. Automatic measurement of vessel lumen area and diameter with particular reference to pedunculate oak and common beech. IAWA J. 1985, 6, 219–237. [Google Scholar] [CrossRef]
- Rao, R.V.; Aebischer, D.P.; Denne, M.P. Latewood density in relation to wood fibre diameter, wall thickness, and fibre and vessel percentages in Quercus robur L. IAWA J. 1997, 18, 127–138. [Google Scholar] [CrossRef]
- Carrer, M.; von Arx, G.; Castagneri, D.; Petit, G. Distilling allometric and environmental information from time series of conduit size: The standardization issue and its relationship to tree hydraulic architecture. Tree Physiol. 2015, 35, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, J. Heritabilities and Additive Genetic Coefficients of Variation in Forest Trees. Can. J. For. Res. 1994, 24, 372–379. [Google Scholar] [CrossRef]
- Zobel, B.J.; Jett, J.B. Genetics of Wood Production; Springer: Berlin, Germany, 1995; p. 337. [Google Scholar]
- Piovesan, G.; Adams, J.M. Masting behaviour in beech: Linking reproduction and climatic variation. Can. J. Bot. 2001, 79, 1039–1047. [Google Scholar] [CrossRef]
- Köse, N.; Güner, H.T. The effect of temperature and precipitation on the intra-annual radial growth of Fagus orientalis Lipsky in Artvin, Turkey. Turk. J. Agric. For. 2012, 36, 501–509. [Google Scholar] [CrossRef]
- Aguilar-Rodrίguez, S.; Terrazas, T.; Lόpez-Mata, L. Anatomical wood variation of Buddleja cordata (Buddlejaceae) along its natural range in Mexico. Trees 2006, 20, 253–261. [Google Scholar] [CrossRef]
- Schume, H.; Grabner, M.; Eckmüllner, O. The influence of an altered groundwater regime on vessel properties of hybrid poplar. Trees Struct. Funct. 2004, 18, 184–194. [Google Scholar] [CrossRef]
- Oquist, G.; Huner, N.P.A. Photosynthesis of Overwintering Evergreen Plants. Annu. Rev. Plant Biol. 2003, 54, 329–355. [Google Scholar] [CrossRef]
- Mihai, G.; Barsan, M.V.; Dumitrescu, A.; Alexandru, A.; Mirancea, I.; Ivanov, P.; Stuparu, E.; Teodosiu, M.; Daia, M. Adaptive genetic potential of European silver fir in Romania in the context of climate change. Ann. For. Res. 2018, 61, 95–108. [Google Scholar] [CrossRef]
- Sass, U.; Eckstein, D. The Variability of Vessel Size in Beech (Fagus sylvatica L.) and Its Ecophysiological Interpretation. Trees Struct. Funct. 1995, 9, 247–252. [Google Scholar] [CrossRef]
- Durak, T. Long-term trends in vegetation changes of managed versus unmanaged Eastern Carpathian beech forests. For. Ecol. Manag. 2010, 260, 1333–1344. [Google Scholar] [CrossRef]
- Kucbel, S.; Saniga, M.; Jaloviar, P.; Vencurik, J. Stand structure and temporal variability in old-growth beech-dominated forests of the northwestern Carpathians: A 40-years perspective. For. Ecol. Manag. 2012, 264, 125–133. [Google Scholar] [CrossRef]
- Martinez del Castillo, E.; Zang, C.S.; Buras, A.; Hacket-Pain, A.; Esper, J.; Serrano-Notivoli, R.; Hartl, C.; Weigel, R.; Klesse, S.; Resco de Dios, V.; et al. Climate-change-driven growth decline of European beech forests. Commun. Biol. 2022, 5, 163. [Google Scholar] [CrossRef]
- Klesse, S.; Peters, R.L.; Alfaro-Sanchez, R.; Badeau, V.; Baittinger, C.; Battipaglia, G.; Bert, D.; Biondi, F.; Bosela, M.; Budeanu, M.; et al. No Future Growth Enhancement Expected at the Northern Edge for European Beech due to Continued Water Limitation. Glob. Change Biol. 2024, 30, e17546. [Google Scholar] [CrossRef] [PubMed]
- Heilman, K.A.; Trouet, V.M.; Belmecheri, S.; Pederson, N.; Berke, M.A.; McLachlan, J.S. Increased water use efficiency leads to decreased precipitation sensitivity of tree growth, but is offset by high temperatures. Oecologia 2021, 197, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Prigoliti, M.; Chiofalo, M.T.; Petruzzellis, F.; Lo Gullo, M.A.; Trifilò, P. Ecophysiological Behavior of Fagus sylvatica L. Growing at Its Southern Distribution Limit: Insights for Understanding the Fate of the European Beech under Warmer and Dryer Growth Conditions. Forests 2023, 14, 2058. [Google Scholar] [CrossRef]
- Kašpar, J.; Tumajer, J.; Altman, J.; Altmanová, N.; Čada, V.; Čihák, T.; Doležal, J.; Fibich, P.; Janda, P.; Kaczka, R.; et al. Major tree species of Central European forests differ in their proportion of positive, negative, and nonstationary growth trends. Glob. Change Biol. 2024, 30, 17146. [Google Scholar] [CrossRef] [PubMed]
- Brang, P.; Spathelf, P.; Larsen, J.B.; Bauhus, J.; Boncìna, A.; Chauvin, C.; Drössler, L.; García-Güemes, C.; Heiri, C.; Kerr, G.; et al. Suitability of close-to-nature silviculture for adapting temperate European forests to climate change. Forestry 2014, 87, 492–503. [Google Scholar] [CrossRef]
- Švik, M.; Brovkina, O.; Veljanovski, T.; Čater, M. Phenological trends of European beech stands along the Carpathian arc: A 20-year MOD13Q1/MYD13Q1 based analysis. Eur. J. Remote Sens. 2025, 58, 2506576. [Google Scholar] [CrossRef]
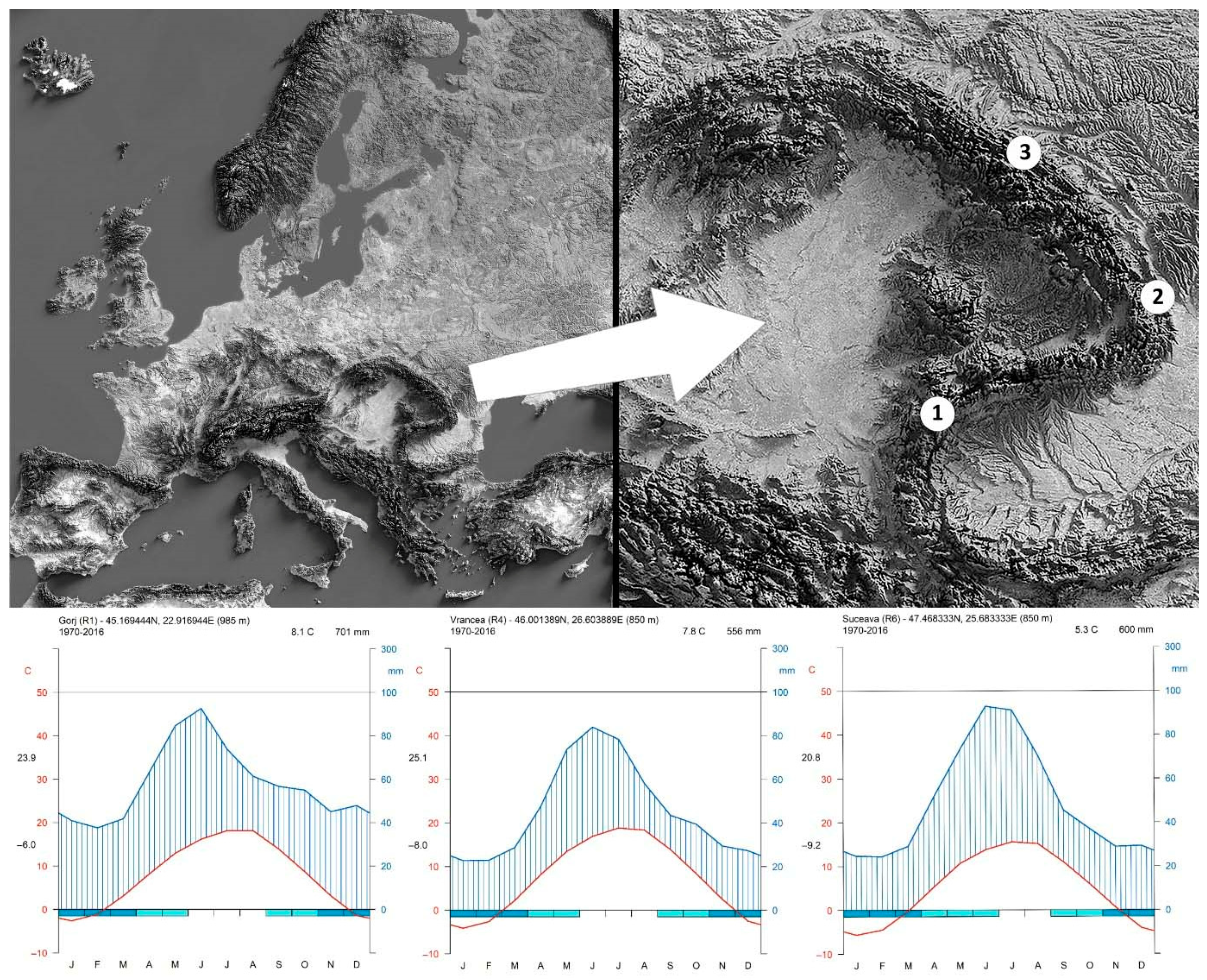



| County | Plot | Elevation (m) | Latitude | Longitude | Slope Inclination (%) | Exposure | CGP (%) * | MAT (°C) | MAP (mm) | N A. alba F. sylva. | Average DBH (cm) A. alba F. sylva. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gorj | Tismana | 985 | 45°10′10″ | 22°55′1″ | 10–15 | E-SE | 7.04 | 8.1 | 701 | 15 | 15 | 63.9 | 63.7 |
| Vrancea | Soveja | 830 | 46°0′5″ | 26°36′14″ | 20–25 | SE | 8.75 | 7.8 | 556 | 16 | 15 | 56.9 | 54.2 |
| Suceava | Frumosu | 850 | 47°28′6″ | 25°40′60″ | 15–20 | SW | 7.18 | 5.3 | 600 | 16 | 15 | 78.9 | 54.0 |
| Species | Site | Wood-Anatomical Characteristics | Mean | ±SD | AC1 |
|---|---|---|---|---|---|
| Abies alba | Suceava | MRW | 4654.9 | 916.3 | 0.706 |
| RCTA | 43.3 | 2.8 | 0.556 | ||
| CD | 882.9 | 59.3 | 0.510 | ||
| MLA | 515.5 | 56.1 | 0.568 | ||
| CWTTAN | 5.8 | 0.3 | 0.520 | ||
| Vrancea | MRW | 2262.8 | 913.0 | 0.764 | |
| RCTA | 39.5 | 3.5 | 0.485 | ||
| CD | 1017.1 | 121.3 | 0.680 | ||
| MLA | 417.5 | 69.2 | 0.583 | ||
| CWTTAN | 5.8 | 0.4 | 0.535 | ||
| Gorj | MRW | 1159.4 | 322.5 | 0.751 | |
| RCTA | 41.7 | 2.8 | 0.365 | ||
| CD | 836.5 | 59.4 | 0.548 | ||
| MLA | 517.1 | 59.5 | 0.475 | ||
| CWTTAN | 6.1 | 0.3 | 0.329 | ||
| Fagus sylvatica | Suceava | MRW | 2638.5 | 518.1 | 0.421 |
| RCTA | 18.3 | 1.4 | 0.493 | ||
| CD | 113.1 | 5.8 | 0.344 | ||
| MLA | 1627.4 | 98.8 | 0.439 | ||
| Vrancea | MRW | 2301.6 | 780.9 | 0.662 | |
| RCTA | 20.5 | 1.8 | 0.532 | ||
| CD | 119.4 | 10.3 | 0.545 | ||
| MLA | 1726.8 | 102.7 | 0.396 | ||
| Gorj | MRW | 1340.8 | 600.1 | 0.698 | |
| RCTA | 22.3 | 2.1 | 0.577 | ||
| CD | 134.8 | 15.3 | 0.581 | ||
| MLA | 1689.5 | 135.5 | 0.461 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamič, P.C.; Prislan, P.; Levanič, T.; Jevšenak, J.; Kašpar, J.; Čater, M. The Impact of Climate Change on Anatomical Characteristics of Silver Fir and European Beech Wood from Three Sites in the Carpathians, Romania. Forests 2025, 16, 1497. https://doi.org/10.3390/f16091497
Adamič PC, Prislan P, Levanič T, Jevšenak J, Kašpar J, Čater M. The Impact of Climate Change on Anatomical Characteristics of Silver Fir and European Beech Wood from Three Sites in the Carpathians, Romania. Forests. 2025; 16(9):1497. https://doi.org/10.3390/f16091497
Chicago/Turabian StyleAdamič, Pia Caroline, Peter Prislan, Tom Levanič, Jernej Jevšenak, Jakub Kašpar, and Matjaž Čater. 2025. "The Impact of Climate Change on Anatomical Characteristics of Silver Fir and European Beech Wood from Three Sites in the Carpathians, Romania" Forests 16, no. 9: 1497. https://doi.org/10.3390/f16091497
APA StyleAdamič, P. C., Prislan, P., Levanič, T., Jevšenak, J., Kašpar, J., & Čater, M. (2025). The Impact of Climate Change on Anatomical Characteristics of Silver Fir and European Beech Wood from Three Sites in the Carpathians, Romania. Forests, 16(9), 1497. https://doi.org/10.3390/f16091497






