Analysing Causes of Carbon Density Dynamics in Subtropical Forests
Abstract
1. Introduction
2. Materials and Methods
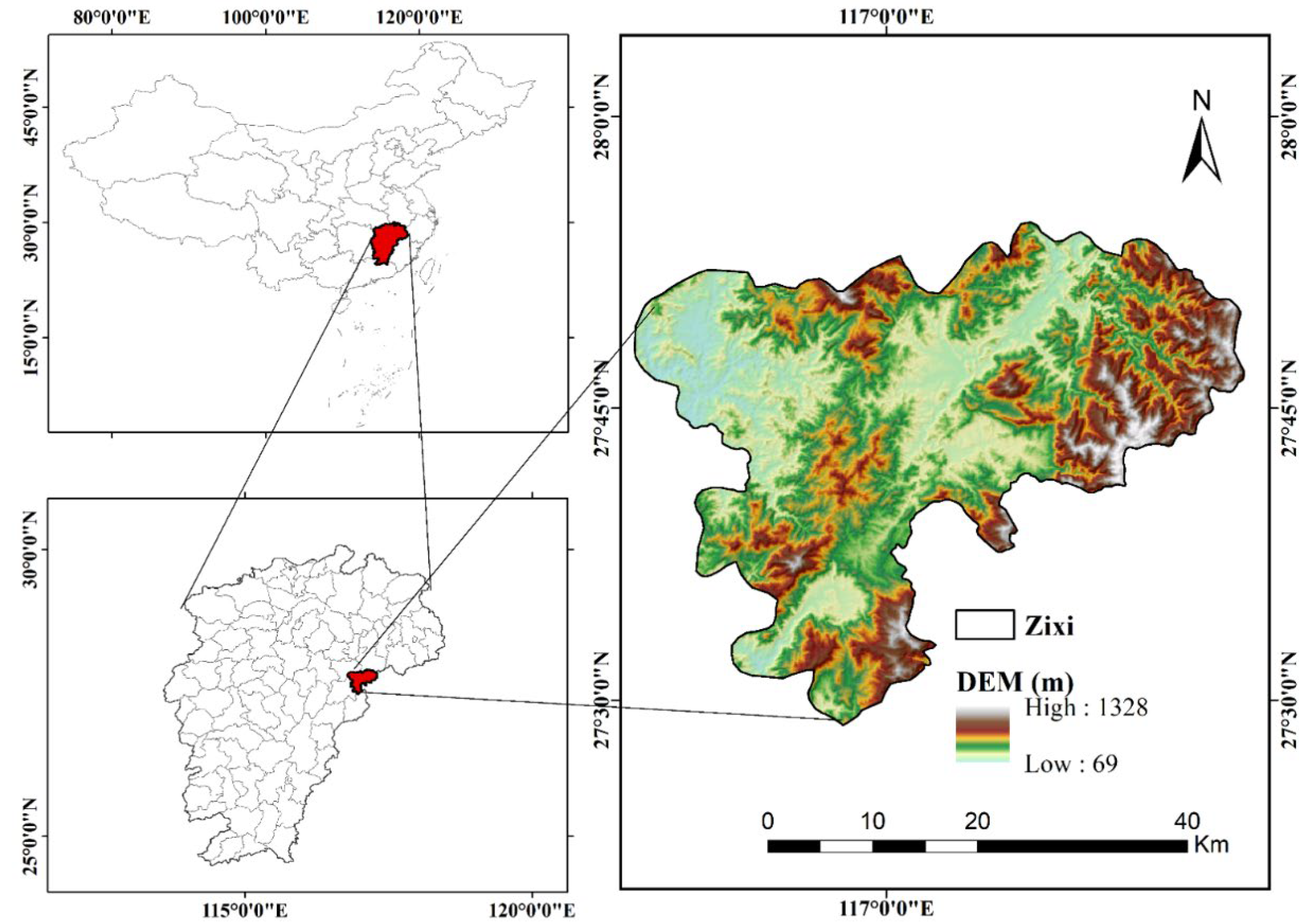
2.1. Data Acquisition
2.2. Species Richness, Diversity Index, and Functional Diversity
2.3. Method to Calculate Carbon Stocks
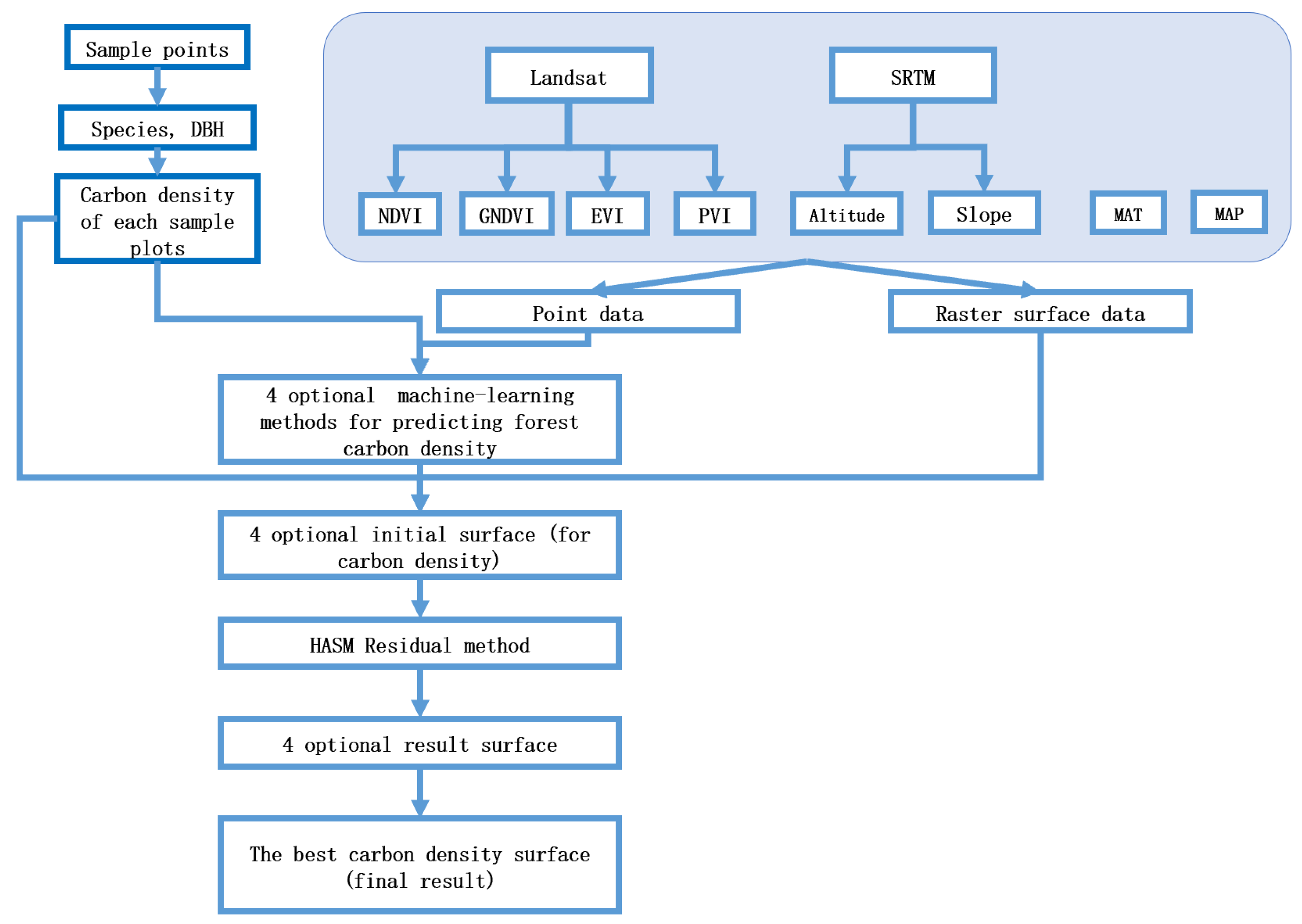
2.4. Spatial Simulation of Forest Aboveground Carbon Density
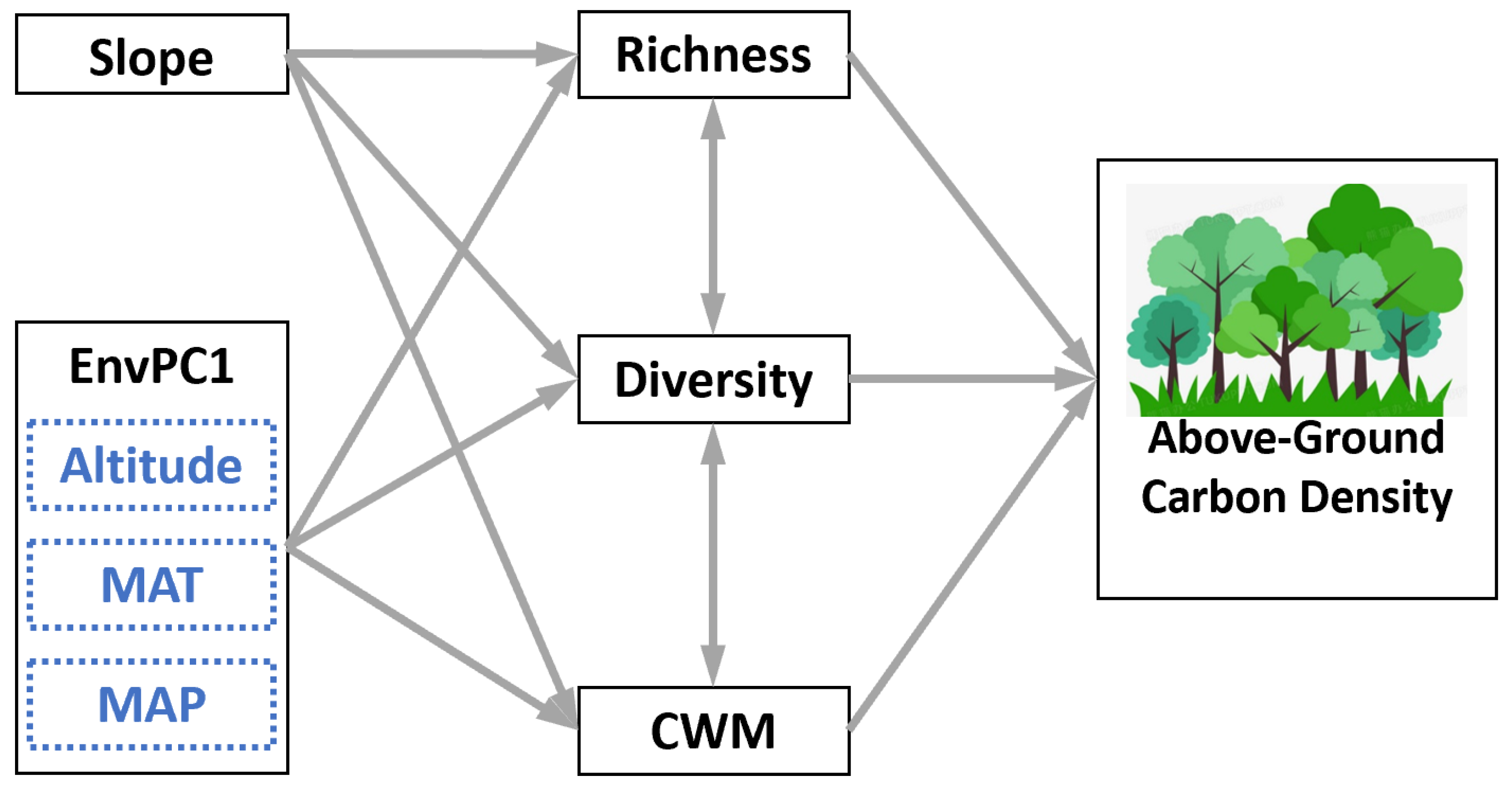
2.5. Structural Equation Modelling (SEM)
3. Results
3.1. Spatial Patterns and Temporal Dynamics of Carbon Density
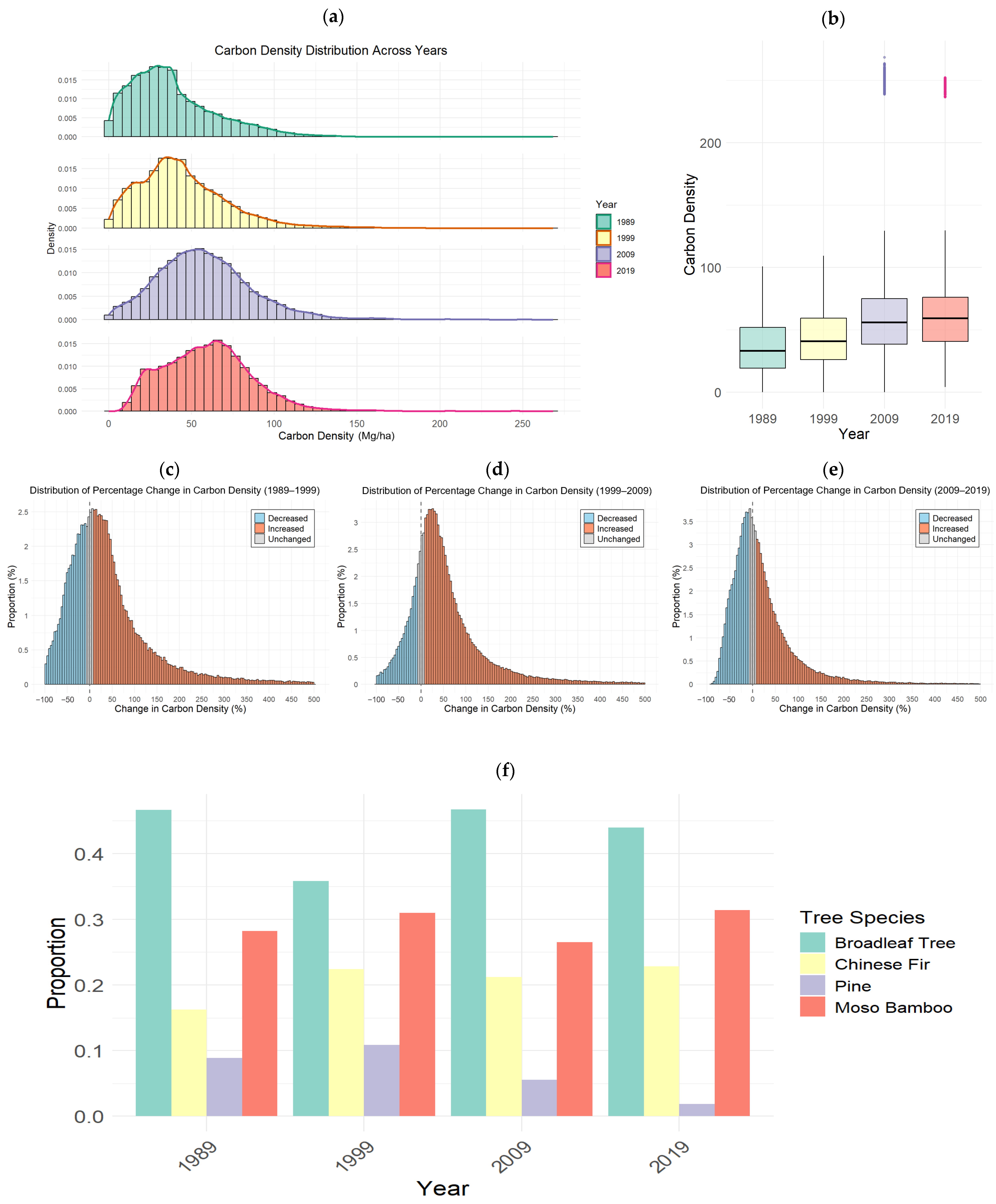
3.2. Temporal Trends and Distributional Analysis of Carbon Density
3.3. Effect of Environmental and Biotic Factors on Carbon Density over Time
| Model | p-Value (Chi-Square) | Degrees of Freedom | CFI | RMSEA | SRMR | GFI | |
|---|---|---|---|---|---|---|---|
| (a) | SEM1989 | 0.754 | 3 | 1.000 | 0.000 | 0.007 | 0.999 |
| (b) | SEM1999 | 0.532 | 3 | 1.000 | 0.000 | 0.025 | 0.997 |
| (c) | SEM2009 | 0.741 | 2 | 1.000 | 0.000 | 0.008 | 0.999 |
| (d) | SEM2019 | 0.896 | 3 | 1.000 | 0.000 | 0.011 | 0.999 |

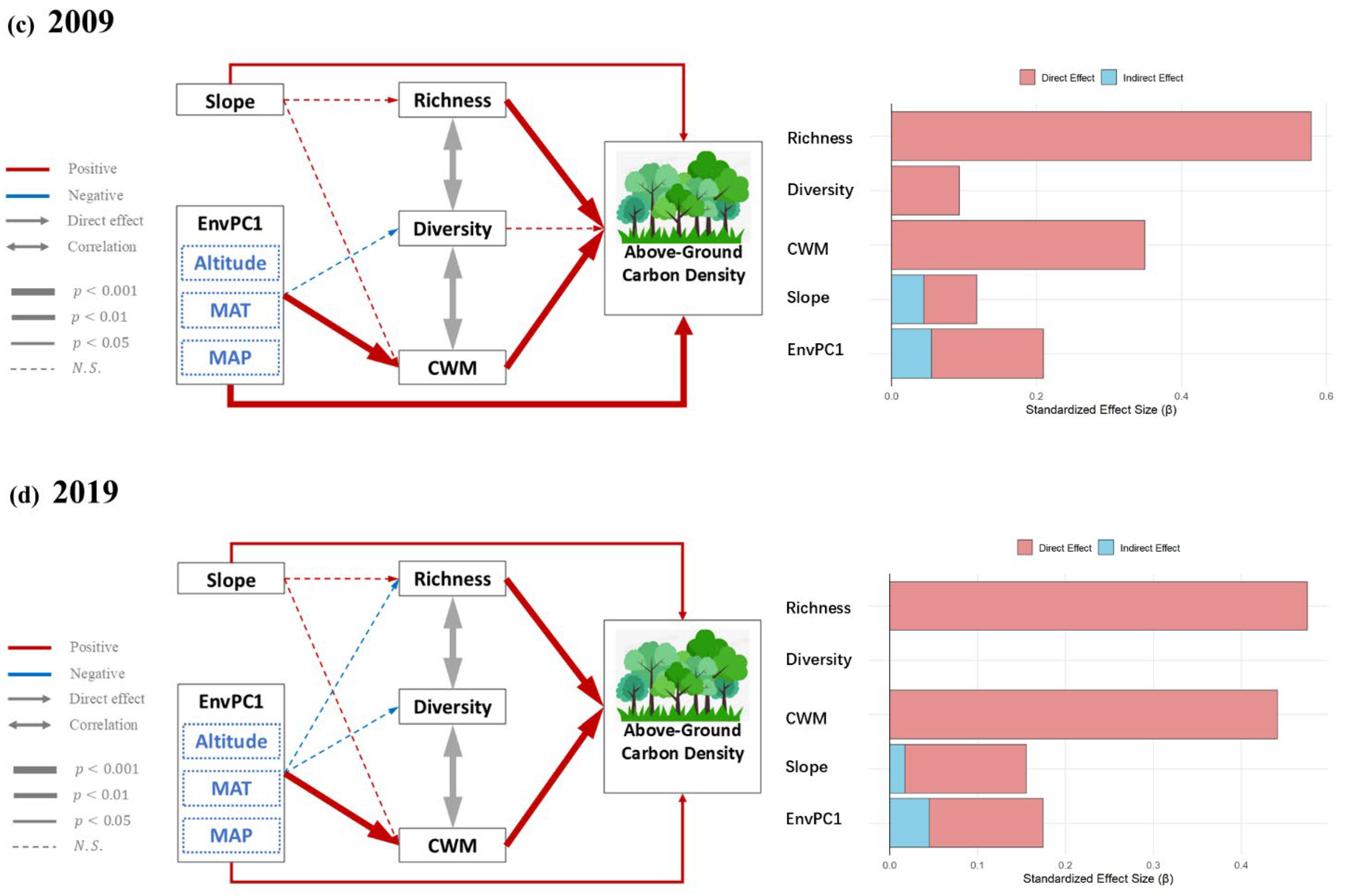
| Factor | Effect | 1989 | 1999 | 2009 | 2019 | ||||
|---|---|---|---|---|---|---|---|---|---|
| p-Value | p-Value | p-Value | p-Value | ||||||
| EnvPC1 | Direct | −0.171 | 0.000 | −0.156 | 0.000 | 0.154 | 0.000 | 0.130 | 0.022 |
| Indirect Richness | −0.285 | 0.000 | −0.211 | 0.000 | --- | --- | −0.078 | N.S. | |
| Indirect Diversity | −0.302 | 0.000 | −0.207 | 0.000 | −0.008 | N.S. | --- | --- | |
| Indirect CWM | −0.262 | 0.000 | −0.194 | 0.000 | 0.159 | 0.000 | 0.186 | 0.000 | |
| Slope | Direct | 0.079 | 0.049 | 0.086 | 0.011 | 0.073 | 0.035 | 0.138 | 0.016 |
| Indirect Richness | 0.121 | 0.016 | 0.077 | N.S. | 0.023 | N.S. | 0.037 | N.S. | |
| Indirect Diversity | --- | --- | 0.085 | N.S. | --- | --- | --- | --- | |
| Indirect CWM | --- | --- | --- | --- | 0.089 | N.S. | --- | --- | |
| Richness | Direct | 0.527 | 0.000 | 0.699 | 0.000 | 0.579 | 0.000 | 0.475 | 0.000 |
| Diversity | Direct | --- | --- | −0.110 | N.S. | 0.093 | N.S. | --- | --- |
| Indirect CWM | 0.185 | 0.001 | --- | --- | --- | --- | --- | --- | |
| CWM | Direct | 0.345 | 0.000 | 0.417 | 0.000 | 0.350 | 0.000 | 0.441 | 0.000 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike Information Criterion |
| BIC | Bayesian Information Criterion |
| CBEF | Continuous Biomass Expansion Factor |
| CD | Aboveground biomass carbon density |
| CFI | Comparative Fit Index |
| CWM | Community-Weighted Mean |
| DBH | Diameter at Breast Height |
| DEM | Digital Elevation Model |
| EnvPC1 | Composite environmental principal component 1 |
| EVI | Enhanced Vegetation Index |
| GFI | Goodness of Fit Index |
| GNDVI | Green Normalized Difference Vegetation Index |
| HASM | High Accuracy Surface Modelling |
| IQR | Interquartile Range |
| MAP | Mean Annual Precipitation |
| MAT | Mean Annual Temperature |
| NDVI | Normalized Difference Vegetation Index |
| PCA | Principal Component Analysis |
| PVI | Plant Variety Index |
| RF | Random Forest |
| RMSEA | Root Mean Square Error of Approximation |
| SEM | Structural Equation Modelling |
| SRMR | Standardized Root Mean Square Residual |
| SRTM | Shuttle Radar Topography Mission |
| VIF | Variance Inflation Factors |
| XGBoost | Gradient Boosting |
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Crockett, E.T.; Atkins, J.W.; Guo, Q.; Sun, G.; Potter, K.M.; Ollinger, S.; Silva, C.A.; Tang, H.; Woodall, C.W.; Holgerson, J. Structural and species diversity explain aboveground carbon storage in forests across the United States: Evidence from GEDI and forest inventory data. Remote Sens. Environ. 2023, 295, 113703. [Google Scholar] [CrossRef]
- Chen, K.; Li, T.; Yang, M.; Zhou, X.; Peng, C. The effects of environmental factors and plant diversity on forest carbon sequestration vary between eastern and western regions of China. J. Clean. Prod. 2024, 437, 140371. [Google Scholar] [CrossRef]
- Mitchard, E.T. The tropical forest carbon cycle and climate change. Nature 2018, 559, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Taylor, A.R.; Reich, P.B.; Hisano, M.; Chen, H.Y.H.; Chang, S.X. Tree diversity increases decadal forest soil carbon and nitrogen accrual. Nature 2023, 618, 94–101. [Google Scholar] [CrossRef]
- Singh, G.; Mishra, D.; Singh, K.; Shukla, S.; Choudhary, G. Geographical settings and tree diversity influenced soil carbon storage in different forest types in Rajasthan, India. Catena 2022, 209, 105856. [Google Scholar] [CrossRef]
- Lv, Z.; Duan, A.; Zhang, J. Influence of forest age, tree size, and climate factors on biomass and carbon storage allocation in Chinese fir forests. Ecol. Indic. 2024, 163, 112096. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Schmid, B. The species richness–productivity controversy. Trends Ecol. Evol. 2002, 17, 113–114. [Google Scholar] [CrossRef]
- Chase, J.M.; Leibold, M.A. Spatial scale dictates the productivity–biodiversity relationship. Nature 2002, 416, 427–430. [Google Scholar] [CrossRef]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Snäll, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Fröberg, M.; Stendahl, J.; Philipson, C.D. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 2013, 4, 1340. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Castro-Izaguirre, N.; Baruffol, M.; Brezzi, M.; Lang, A.; Li, Y.; Härdtle, W.; Von Oheimb, G.; Yang, X. Impacts of species richness on productivity in a large-scale subtropical forest experiment. Science 2018, 362, 80–83. [Google Scholar] [CrossRef]
- Mori, A.S. Environmental controls on the causes and functional consequences of tree species diversity. J. Ecol. 2018, 106, 113–125. [Google Scholar] [CrossRef]
- Song, J.; Wan, S.; Piao, S.; Xia, J.; Ning, Y.; Zheng, M.; Hui, D.; Ru, J.; Han, J.; Feng, J. Global change and China’s terrestrial carbon sink: A quantitative review of 30 years’ ecosystem manipulative experiments. Ecol. Monogr. 2025, 95, e70005. [Google Scholar] [CrossRef]
- Chiang, J.-M.; Spasojevic, M.J.; Muller-Landau, H.C.; Sun, I.-F.; Lin, Y.; Su, S.-H.; Chen, Z.-S.; Chen, C.-T.; Swenson, N.G.; McEwan, R.W. Functional composition drives ecosystem function through multiple mechanisms in a broadleaved subtropical forest. Oecologia 2016, 182, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Grime, J. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J. A review of processes behind diversity—Productivity relationships in forests. Curr. For. Rep. 2016, 2, 45–61. [Google Scholar] [CrossRef]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.-D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef]
- Poorter, L.; van der Sande, M.T.; Arets, E.J.M.M.; Ascarrunz, N.; Enquist, B.J.; Finegan, B.; Licona, J.C.; Martínez-Ramos, M.; Mazzei, L.; Meave, J.A.; et al. Biodiversity and climate determine the functioning of Neotropical forests. Glob. Ecol. Biogeogr. 2017, 26, 1423–1434. [Google Scholar] [CrossRef]
- Paquette, A.; Messier, C. The effect of biodiversity on tree productivity: From temperate to boreal forests. Glob. Ecol. Biogeogr. 2011, 20, 170–180. [Google Scholar] [CrossRef]
- Bruelheide, H.; Dengler, J.; Purschke, O.; Lenoir, J.; Jiménez-Alfaro, B.; Hennekens, S.M.; Botta-Dukát, Z.; Chytrý, M.; Field, R.; Jansen, F. Global trait–environment relationships of plant communities. Nat. Ecol. Evol. 2018, 2, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, D.R.; Tahiry, H.; Sharma, P.; Pala, N.A.; Kumar, D.; Kumar, A. Bharti. Influence of Aspect and Elevational Gradient on Vegetation Pattern, Tree Characteristics and Ecosystem Carbon Density in Northwestern Himalayas. Land 2021, 10, 1109. [Google Scholar] [CrossRef]
- Cheng, Z.; Aakala, T.; Larjavaara, M. Elevation, aspect, and slope influence woody vegetation structure and composition but not species richness in a human-influenced landscape in northwestern Yunnan, China. Front. For. Glob. Chang. 2023, 6, 1187724. [Google Scholar] [CrossRef]
- Kohyama, T.I.; Sheil, D.; Sun, I.-F.; Niiyama, K.; Suzuki, E.; Hiura, T.; Nishimura, N.; Hoshizaki, K.; Wu, S.-H.; Chao, W.-C. Contribution of tree community structure to forest productivity across a thermal gradient in eastern Asia. Nat. Commun. 2023, 14, 1113. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, W.; Fu, J. Vegetation response to precipitation anomalies under different climatic and biogeographical conditions in China. Sci. Rep. 2020, 10, 830. [Google Scholar] [CrossRef]
- Poorter, L.; van der Sande, M.T.; Thompson, J.; Arets, E.J.M.M.; Alarcón, A.; Álvarez-Sánchez, J.; Ascarrunz, N.; Balvanera, P.; Barajas-Guzmán, G.; Boit, A.; et al. Diversity enhances carbon storage in tropical forests. Glob. Ecol. Biogeogr. 2015, 24, 1314–1328. [Google Scholar] [CrossRef]
- Madrigal-González, J.; Calatayud, J.; Ballesteros-Cánovas, J.A.; Escudero, A.; Cayuela, L.; Rueda, M.; Ruiz-Benito, P.; Herrero, A.; Aponte, C.; Sagardia, R. Climate reverses directionality in the richness–abundance relationship across the World’s main forest biomes. Nat. Commun. 2020, 11, 5635. [Google Scholar] [CrossRef]
- Dyola, N.; Sigdel, S.R.; Liang, E.; Babst, F.; Camarero, J.J.; Aryal, S.; Chettri, N.; Gao, S.; Lu, X.; Sun, J. Species richness is a strong driver of forest biomass along broad bioclimatic gradients in the Himalayas. Ecosphere 2022, 13, e4107. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, H.; Ding, Y.; Cao, Q.; Zhang, Y.; Huang, K. Carbon storage dynamics of subtropical forests estimated with multi-period forest inventories at a regional scale: The case of Jiangxi forests. J. For. Res. 2020, 31, 1247–1254. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y.; Reich, P.B. Forest productivity increases with evenness, species richness and trait variation: A global meta-analysis. J. Ecol. 2012, 100, 742–749. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; García-Palacios, P.; Bradford, M.A.; Eldridge, D.J.; Berdugo, M.; Sáez-Sandino, T.; Liu, Y.-R.; Alfaro, F.; Abades, S.; Bamigboye, A.R. Biogenic factors explain soil carbon in paired urban and natural ecosystems worldwide. Nat. Clim. Chang. 2023, 13, 450–455. [Google Scholar] [CrossRef]
- Fang, J.; Guo, Z.; Piao, S.; Chen, A. Terrestrial vegetation carbon sinks in China, 1981–2000. Sci. China Ser. D Earth Sci. 2007, 50, 1341–1350. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; Jiang, H.; Yu, S.; Fu, J.; Li, W.; Wang, W.; Ma, Z.; Peng, C. Carbon sequestration by Chinese bamboo forests and their ecological benefits: Assessment of potential, problems, and future challenges. Environ. Rev. 2011, 19, 418–428. [Google Scholar] [CrossRef]
- Bollen, K.A. Structural Equations with Latent Variables; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Li, Y.; Schmid, B.; Schuldt, A.; Li, S.; Wang, M.-Q.; Fornoff, F.; Staab, M.; Guo, P.-F.; Anttonen, P.; Chesters, D.; et al. Multitrophic arthropod diversity mediates tree diversity effects on primary productivity. Nat. Ecol. Evol. 2023, 7, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.P.; Wen, W.S.; Xu, L.; Chen, G.S.; Zhou, G.M.; Ji, B.Y.; Zhou, Y.F.; Zhu, G.L.; Shi, Y.J. Vegetation Carbon Accumulation Driven by Stand Characteristics and Climatic Factors in Subtropical Forests of Southeastern China. J. Sustain. For. 2022, 41, 941–958. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, C.; An, T.; Yue, T. Characteristics of Climate Change in Poyang Lake Basin and Its Impact on Net Primary Productivity. Sustainability 2024, 16, 9420. [Google Scholar] [CrossRef]
- Odum, E.P. The Strategy of Ecosystem Development: An understanding of ecological succession provides a basis for resolving man’s conflict with nature. Science 1969, 164, 262–270. [Google Scholar] [CrossRef]
- Hamilton, A.J. Species diversity or biodiversity? J. Environ. Manag. 2005, 75, 89–92. [Google Scholar] [CrossRef]
- Koellner, T.; Hersperger, A.M.; Wohlgemuth, T. Rarefaction method for assessing plant species diversity on a regional scale. Ecography 2004, 27, 532–544. [Google Scholar] [CrossRef]
- Yue, T.X.; Ma, S.N.; Wu, S.X.; Zhan, J.Y. Comparative analyses of the scaling diversity index and its applicability. Int. J. Remote Sens. 2007, 28, 1611–1623. [Google Scholar] [CrossRef]
- Yue, T.X.; Li, Q.Q. Relationship between species diversity and ecotope diversity. Ann. N. Y. Acad. Sci. 2010, 1195, E40–E51. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, J. Which species? What kind of diversity? Which ecosystem function? Some problems in studies of relations between biodiversity and ecosystem function. Appl. Soil Ecol. 1998, 10, 191–199. [Google Scholar] [CrossRef]
- Violle, C.; Enquist, B.J.; McGill, B.J.; Jiang, L.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The return of the variance: Intraspecific variability in community ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef]
- Li, H.; Lei, Y. Estimation and Evaluation of Forest Biomass Carbon Storage in China; China Forestry Publishing House: Beijing, China, 2010. [Google Scholar]
- Yue, T.; Wu, C.; Liu, Y.; Du, Z.; Zhao, N.; Jiao, Y.; Xu, Z.; Shi, W. HASM quantum machine learning. Sci. China Earth Sci. 2023, 66, 1937–1945. [Google Scholar] [CrossRef]
- Ponsar, S.; Luyten, P.; Dulière, V. Data assimilation with the ensemble Kalman filter in a numerical model of the North Sea. Ocean Dyn. 2016, 66, 955–971. [Google Scholar] [CrossRef]
- Yue, T.X.; Wang, Y.F.; Du, Z.P.; Zhao, M.W.; Zhang, L.L.; Zhao, N.; Lu, M.; Larocque, G.R.; Wilson, J.P. Analysing the uncertainty of estimating forest carbon stocks in China. Biogeosciences 2016, 13, 3991–4004. [Google Scholar] [CrossRef]
- Brill, K.F.; Uccellini, L.W.; Manobianco, J.; Kocin, P.J.; Homan, J.H. The use of successive dynamic initialization by nudging to simulate cyclogenesis during GALE IOP 1. Meteorol. Atmos. Phys. 1991, 45, 15–40. [Google Scholar] [CrossRef]
- Phillips, J.D. Global and local factors in earth surface systems. Ecol. Model. 2002, 149, 257–272. [Google Scholar] [CrossRef]
- Chiesi, M.; Fibbi, L.; Genesio, L.; Gioli, B.; Magno, R.; Maselli, F.; Moriondo, M.; Vaccari, F.P. Integration of ground and satellite data to model Mediterranean forest processes. Int. J. Appl. Earth Obs. Geoinf. 2011, 13, 504–515. [Google Scholar] [CrossRef]
- Yue, T.; Zhao, N.; Liu, Y.; Wang, Y.; Zhang, B.; Du, Z.; Fan, Z.; Shi, W.; Chen, C.; Zhao, M. A fundamental theorem for eco-environmental surface modelling and its applications. Sci. China Earth Sci. 2020, 63, 1092–1112. [Google Scholar] [CrossRef]
- Yue, T.-X.; Du, Z.-P.; Song, D.-J.; Gong, Y. A new method of surface modeling and its application to DEM construction. Geomorphology 2007, 91, 161–172. [Google Scholar] [CrossRef]
- Yue, T.-X. Surface Modeling: High Accuracy and High Speed Methods; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Hoerl, A.E.; Kennard, R.W. Ridge regression: Biased estimation for nonorthogonal problems. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B Stat. Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM Sigkdd International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Crowther, T.W.; Glick, H.B.; Covey, K.R.; Bettigole, C.; Maynard, D.S.; Thomas, S.M.; Smith, J.R.; Hintler, G.; Duguid, M.C.; Amatulli, G. Mapping tree density at a global scale. Nature 2015, 525, 201–205. [Google Scholar] [CrossRef]
- Zhou, J.; Du, J.; Bonifácio, L.; Yin, W.; Huang, L.; Ning, J.; Han, D.; Hu, J.; Song, W.; Zhao, L. Vulnerability of Global Pine Forestry’s Carbon Sink to an Invasive Pathogen–Vector System. Glob. Change Biol. 2024, 30, e17614. [Google Scholar] [CrossRef]
- Xu, Q.-F.; Jiang, P.-K.; Wu, J.-S.; Zhou, G.-M.; Shen, R.-F.; Fuhrmann, J.J. Bamboo invasion of native broadleaf forest modified soil microbial communities and diversity. Biol. Invasions 2015, 17, 433–444. [Google Scholar] [CrossRef]
- Yang, C.; Ni, H.; Zhong, Z.; Zhang, X.; Bian, F. Changes in soil carbon pools and components induced by replacing secondary evergreen broadleaf forest with Moso bamboo plantations in subtropical China. Catena 2019, 180, 309–319. [Google Scholar] [CrossRef]
- Shao, S.; He, H.; Liang, C.; Chen, J.; Qin, H.; Wang, S.; Wang, Z.; Li, Y.; Jia, W.; Zheng, X. Moso bamboo expansion into a broadleaved forest alters the dominant soil organic carbon source. Eur. J. Soil Sci. 2023, 74, e13366. [Google Scholar] [CrossRef]
- Zu, K.; Wang, Z.; Zhu, X.; Lenoir, J.; Shrestha, N.; Lyu, T.; Luo, A.; Li, Y.; Ji, C.; Peng, S. Upward shift and elevational range contractions of subtropical mountain plants in response to climate change. Sci. Total Environ. 2021, 783, 146896. [Google Scholar] [CrossRef]
- Wang, Q.; Punchi-Manage, R.; Lu, Z.; Franklin, S.B.; Wang, Z.; Li, Y.; Chi, X.; Bao, D.; Guo, Y.; Lu, J. Effects of topography on structuring species assemblages in a subtropical forest. J. Plant Ecol. 2017, 10, 440–449. [Google Scholar] [CrossRef]
- Rudolph, G.; Schmidt, M.; Schmidt, M. Differential Geometry and Mathematical Physics; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]


| Forest Type | |||
|---|---|---|---|
| Abies sp. | 0.5519 | 48.861 | 0.4999 |
| Picea sp. | 0.5519 | 48.861 | 0.5208 |
| Tsuga sp. | 0.3491 | 39.816 | 0.5022 |
| Cryptomeria sp. | 0.3491 | 39.816 | 0.5235 |
| Keteleeria sp. | 0.3491 | 39.816 | 0.4997 |
| Larix sp. | 0.6096 | 33.806 | 0.5211 |
| Pinus koraiensis | 0.5723 | 16.489 | 0.5113 |
| Pinus sylvestris var. mongolica | 1.112 | 2.6951 | 0.5223 |
| Pinus tabuliformis | 0.869 | 9.1212 | 0.5207 |
| Pinus armandii | 0.5856 | 18.744 | 0.5225 |
| Pinus massoniana | 0.5034 | 20.547 | 0.4596 |
| Pinus yunnanensis | 0.5034 | 20.547 | 0.5113 |
| Cunninghamia lanceolata | 0.4652 | 19.141 | 0.5201 |
| Cupressus sp. | 0.8893 | 7.3965 | 0.5034 |
| Quercus sp. | 1.1453 | 8.5473 | 0.5004 |
| Betula sp. | 1.0687 | 10.237 | 0.4914 |
| Broad-leaved mixed forests | 0.9788 | 5.3764 | 0.4900 |
| Cinnamomum sp. | 0.9788 | 5.3764 | 0.4916 |
| Casuarina sp. | 0.7441 | 3.2377 | 0.4980 |
| Populus sp. | 0.4969 | 26.973 | 0.4956 |
| Mixed hardwood forests | 1.1783 | 2.5585 | 0.4834 |
| Mixed softwood forests | 0.6255 | 91.001 | 0.4956 |
| Mixed coniferous and broad-leaved forests | 0.8136 | 18.466 | 0.4978 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Yue, T.; Wang, Y.; Zhao, N.; Yang, Y.; Du, Z.; Shao, W.; Zhang, X.; Li, Z.; Pan, J.; et al. Analysing Causes of Carbon Density Dynamics in Subtropical Forests. Forests 2025, 16, 1496. https://doi.org/10.3390/f16091496
Wu C, Yue T, Wang Y, Zhao N, Yang Y, Du Z, Shao W, Zhang X, Li Z, Pan J, et al. Analysing Causes of Carbon Density Dynamics in Subtropical Forests. Forests. 2025; 16(9):1496. https://doi.org/10.3390/f16091496
Chicago/Turabian StyleWu, Chenchen, Tianxiang Yue, Yifu Wang, Na Zhao, Yang Yang, Zhengping Du, Wei Shao, Xin Zhang, Zishen Li, Jie Pan, and et al. 2025. "Analysing Causes of Carbon Density Dynamics in Subtropical Forests" Forests 16, no. 9: 1496. https://doi.org/10.3390/f16091496
APA StyleWu, C., Yue, T., Wang, Y., Zhao, N., Yang, Y., Du, Z., Shao, W., Zhang, X., Li, Z., Pan, J., Liu, B., & Peng, Y. (2025). Analysing Causes of Carbon Density Dynamics in Subtropical Forests. Forests, 16(9), 1496. https://doi.org/10.3390/f16091496











