Does a Commercial Organic Fertilizer with Hydrogel or Biochar Guarantee the Quality of Eucalyptus Seedlings?
Abstract
1. Introduction
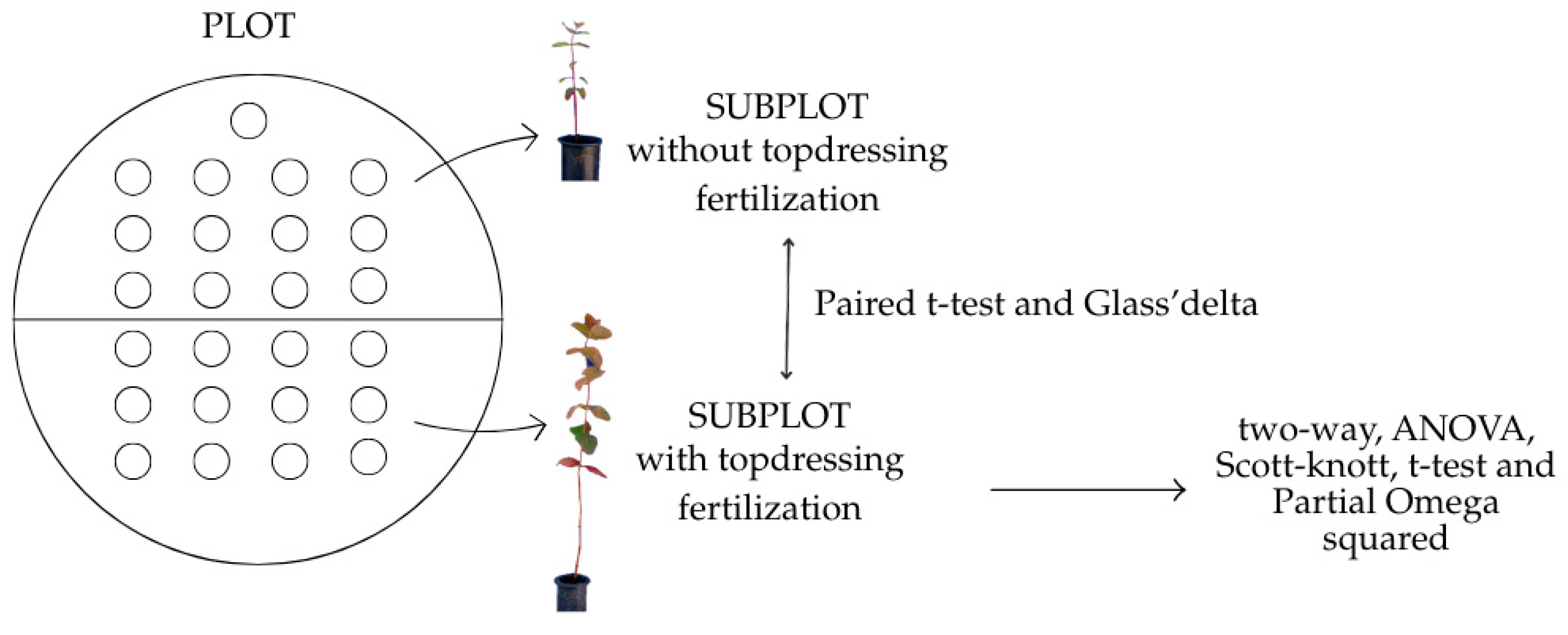
2. Materials and Methods
2.1. The Experiments
2.2. Evaluated Characteristics
2.3. Statistical Analyses
3. Results
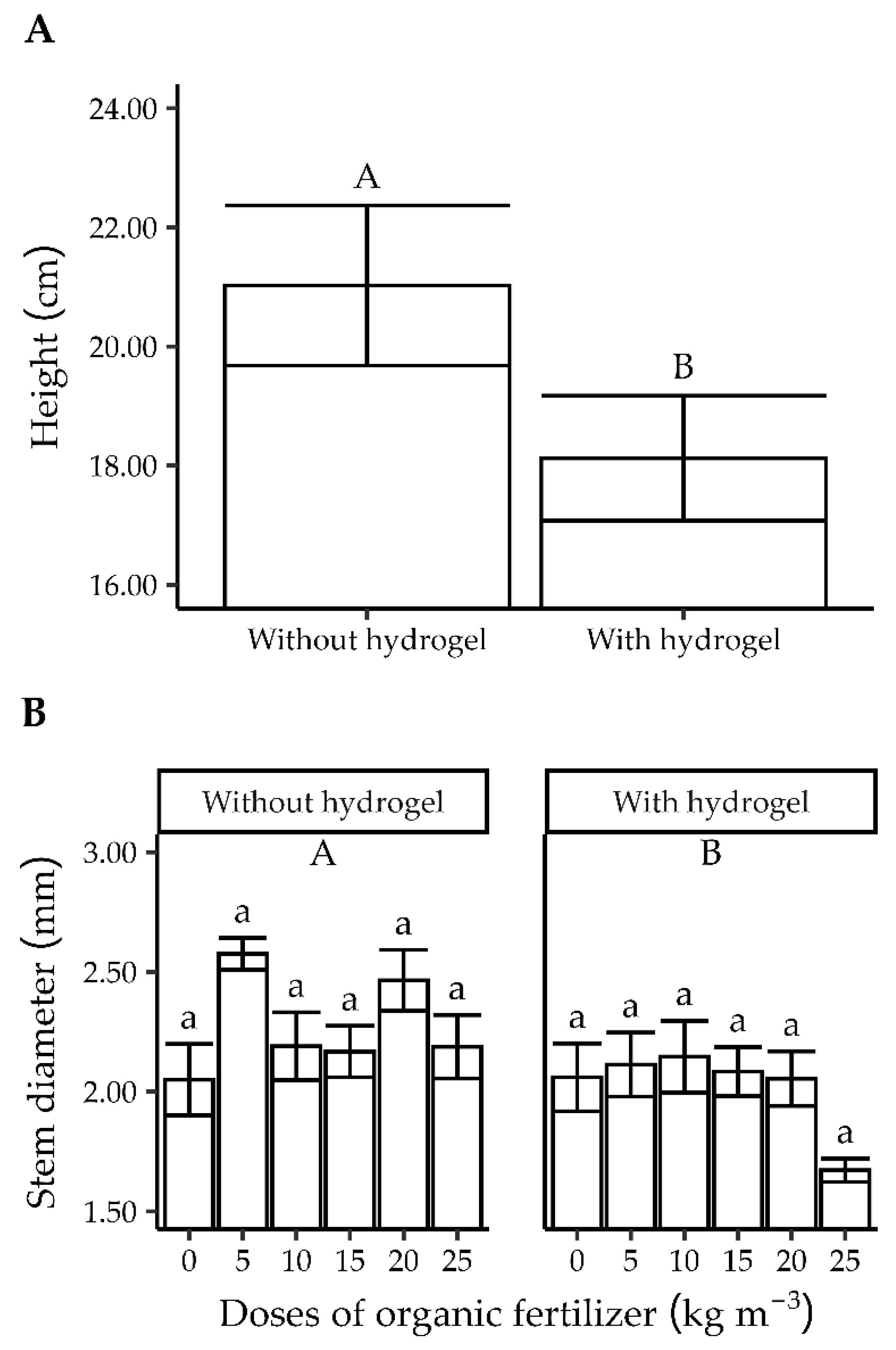
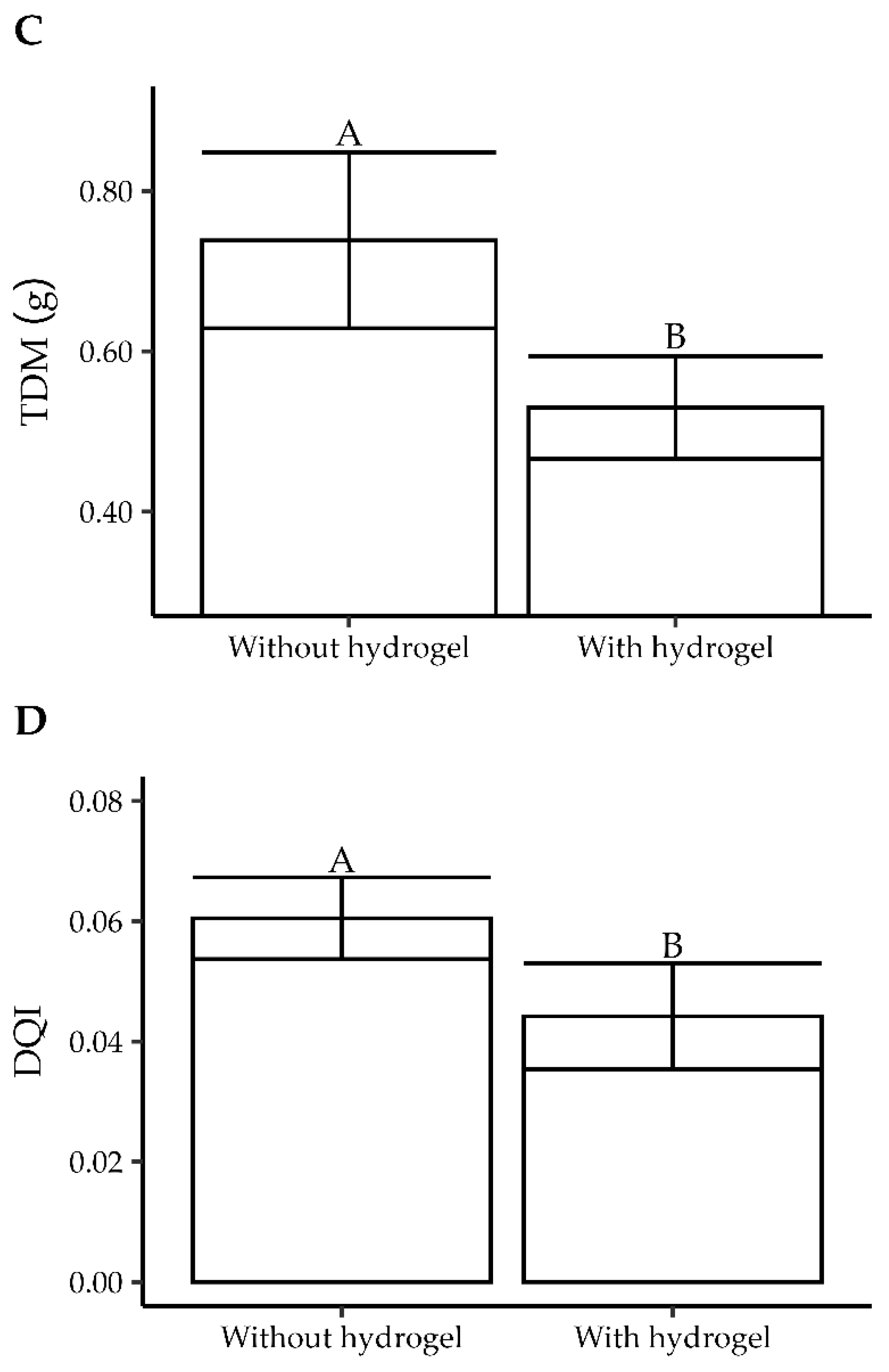
3.1. Experiment 1
3.1.1. Quality Characteristics
3.1.2. Root Morphological Characteristics
3.1.3. Physiological Characteristics
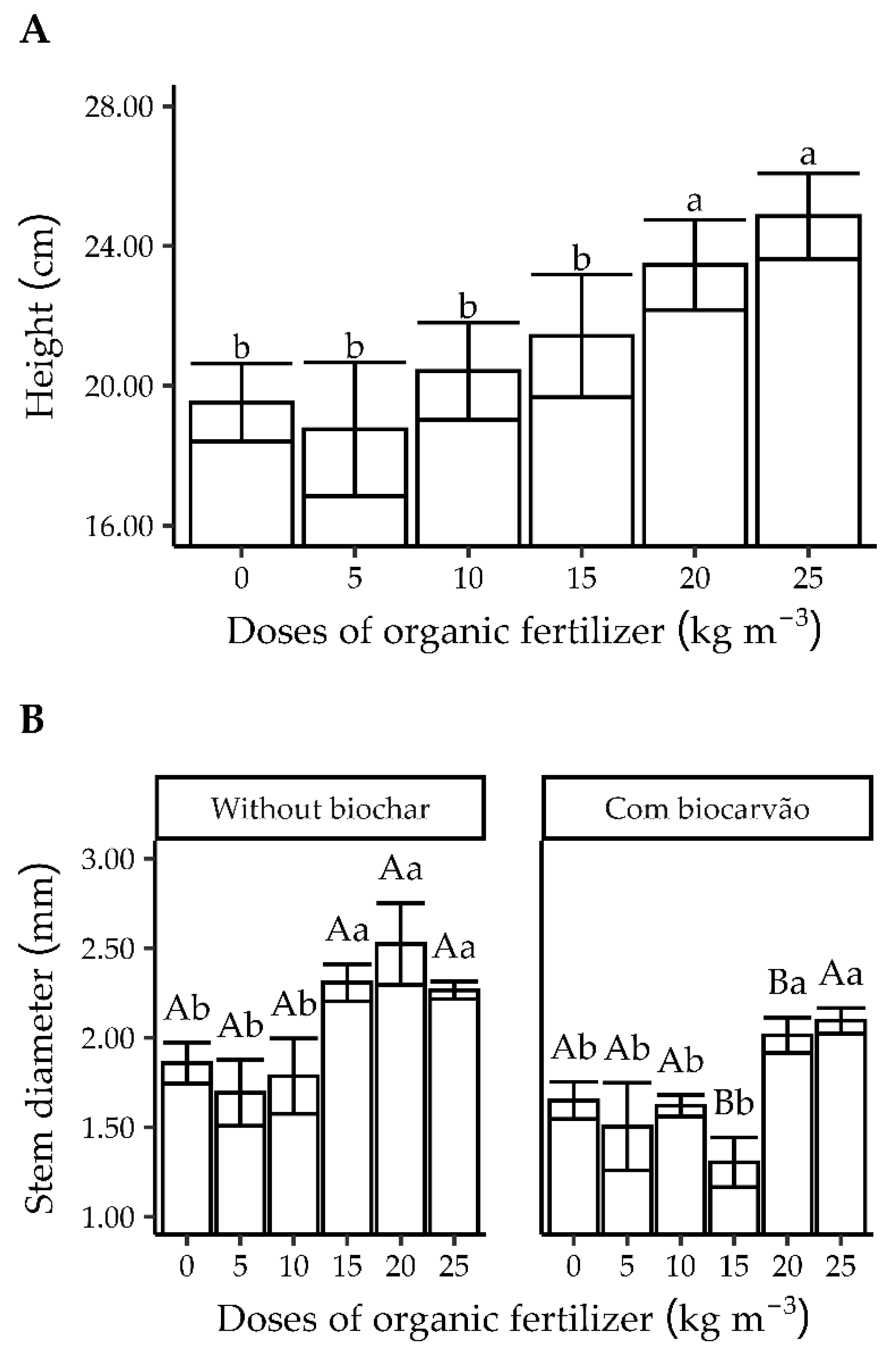
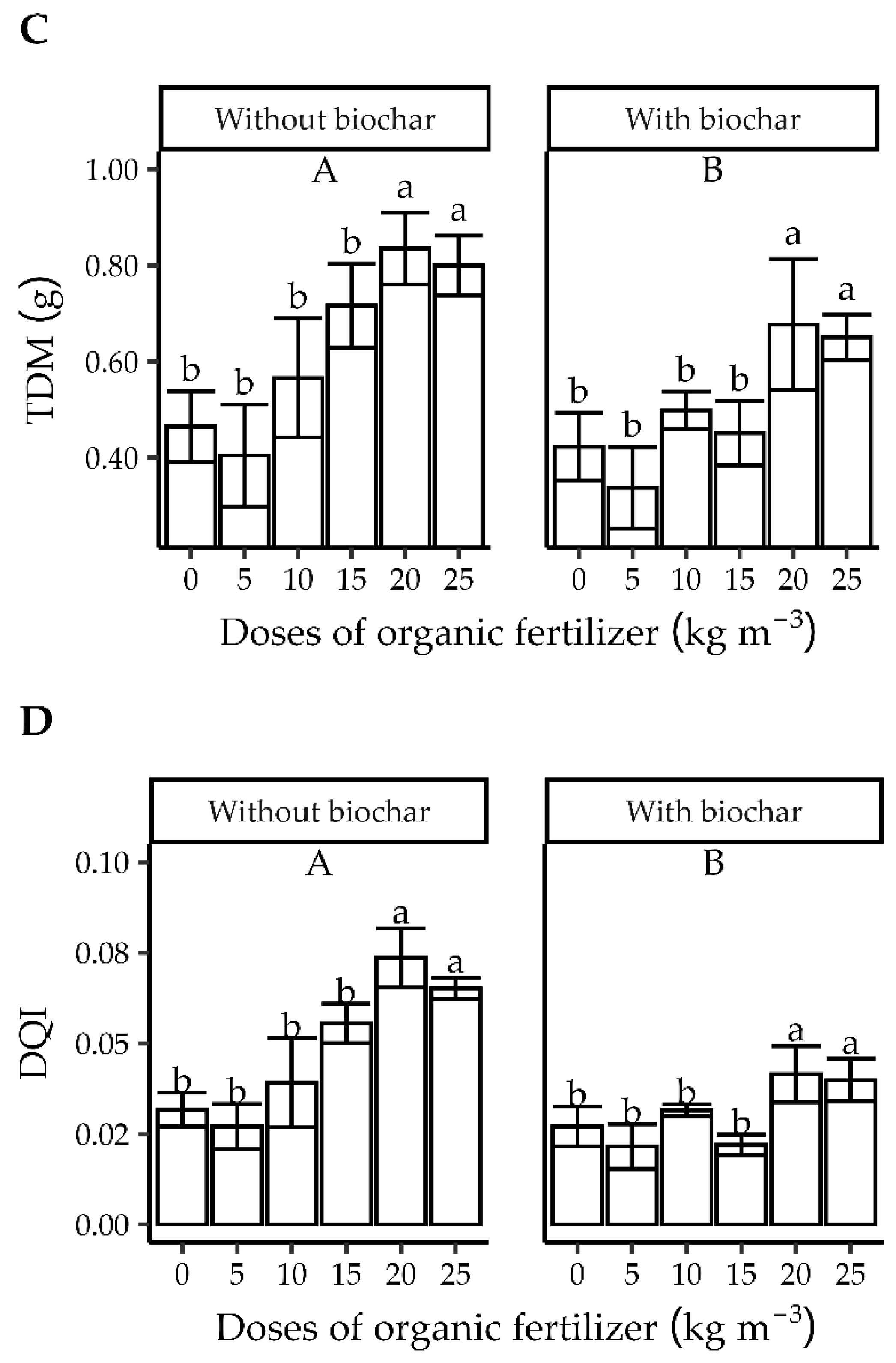
3.2. Experiment 2
3.2.1. Quality Characteristics
3.2.2. Root Morphological Characteristics
3.2.3. Physiological Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Indústria Brasileira de Árvores. Available online: https://iba.org/publicacoes/ (accessed on 11 September 2024).
- Oberschelp, G.P.J.; Morales, L.L.; Montecchiarini, M.L.; Harrand, L.; Podestá, F.E.; Margarit, E. Harder, Better, Faster, Stronger: Frost Tolerance of Eucalyptus benthamii under Cold Acclimation. Plant Physiol. Biochem. 2022, 186, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Resquin, F.; Navarro-Cerrillo, R.M.; Rachid-Casnati, C.; Hirigoyen, A.; Carrasco-Letelier, L.; Duque-Lazo, J. Allometry, Growth and Survival of Three Eucalyptus Species (Eucalyptus benthamii Maiden and Cambage, E. dunnii Maiden and E. grandis Hill Ex. Maiden) in High-Density Plantations in Uruguay. Forests 2018, 9, 745. [Google Scholar] [CrossRef]
- Benin, C.C.; Lúcio, D.M.; Watzlawick, L.F.; Lima, V.A. Energy Properties of Eucalyptus benthamii Wood Based on Tree Age and Region in Guarapuava, Paraná State, Brazil. South. For. 2021, 83, 264–268. [Google Scholar] [CrossRef]
- Tomio, G.F.; Cunha, A.B.; Brand, M.A.; Córdova, U.A. Rendimento e Qualidade Da Madeira de Eucalyptus benthamii Maiden et Cambage de Rotação Longa No Processo de Desdobro. Sci. For. 2021, 49, e3674. [Google Scholar] [CrossRef]
- Teixeira, M.Z.; Terezo, R.F.; Corrêa, C.A.; Santos, S.S.; Vieira, H.C.; da Cunha, A.B. Brazilian Potential of Eucalyptus benthamii Maiden & Cambage for Cross-Laminated Timber Panels: Structural Analysis and Comparison with Pinus spp. and European Standards. Buildings 2025, 15, 2606. [Google Scholar] [CrossRef]
- Dovzhenko, A.P.; Yapryntseva, O.A.; Sinyashin, K.O.; Doolotkeldieva, T.; Zairov, R.R. Recent Progress in the Development of Encapsulated Fertilizers for Time-Controlled Release. Heliyon 2024, 10, e34895. [Google Scholar] [CrossRef]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release. Plants 2021, 10, 238. [Google Scholar] [CrossRef]
- Rocha, D.L.F.D.; Alencar, M.R.F.D.S.; Matos, L.F.; Luiza, M.; Souza, S.; Neto, O.C. Nourishing the earth and your pocket: Organic fertilizers as a low-cost solution. Rev. Multidiscip. Nord. Mineiro 2024, 9. [Google Scholar] [CrossRef]
- Guedes, M.J.F.; Ribeiro, T.F.F.; Cruz, D.B.; Evangelista, I.C.; Neto, O.C. Fertilizantes orgânicos: Uma alternativa sustentável. Rev. Multidiscip. Nord. Mineiro 2025, 8, 1–9. [Google Scholar] [CrossRef]
- Bhanwaria, R.; Singh, B.; Musarella, C.M. Effect of Organic Manure and Moisture Regimes on Soil Physiochemical Properties, Microbial Biomass Cmic:Nmic:Pmic Turnover and Yield of Mustard Grains in Arid Climate. Plants 2022, 11, 722. [Google Scholar] [CrossRef]
- Ikoyi, I.; Egeter, B.; Chaves, C.; Ahmed, M.; Fowler, A.; Schmalenberger, A. Responses of Soil Microbiota and Nematodes to Application of Organic and Inorganic Fertilizers in Grassland Columns. Biol. Fertil. Soils 2020, 56, 647–662. [Google Scholar] [CrossRef]
- Santos, J.B.; Cruz, J.O.; Matias, L.D.L.; Dias, E.G.; Geraldo, L.C.; Figueiredo, C.C.; Blum, L.E.B. Sour Passion Fruit (Passiflora edulis Sims) Seedlings in Response to Sewage Sludge-Derived Biochar and Compost. Org. Agric. 2024, 14, 467–479. [Google Scholar] [CrossRef]
- Ekinci, M.; Atamanalp, M.; Turan, M.; Alak, G.; Kul, R.; Kitir, N.; Yildirim, E. Integrated Use of Nitrogen Fertilizer and Fish Manure: Effects on the Growth and Chemical Composition of Spinach. Commun. Soil Sci. Plant Anal. 2019, 50, 1580–1590. [Google Scholar] [CrossRef]
- Wan, L.-J.; Tian, Y.; He, M.; Zheng, Y.-Q.; Lyu, Q.; Xie, R.-J.; Ma, Y.-Y.; Deng, L.; Yi, S.-L. Effects of Chemical Fertilizer Combined with Organic Fertilizer Application on Soil Properties, Citrus Growth Physiology, and Yield. Agriculture 2021, 11, 1207. [Google Scholar] [CrossRef]
- Silva, R.S.; Jalal, A.; Nascimento, R.E.N.; Elias, N.C.; Kawakami, K.C.; Abreu-Junior, C.H.; Oliveira, F.C.; Jani, A.D.; He, Z.; Zhao, F.; et al. Composted Sewage Sludge Application Reduces Mineral Fertilization Requirements and Improves Soil Fertility in Sugarcane Seedling Nurseries. Sustainability 2022, 14, 4684. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Navroski, M.C.; Araújo, M.M.; Cunha, F.S.; Berghetti, Á.P.; Pereira, M.O. Redução da adubação e melhoria das características do substrato com uso do hidrogel na produção de mudas de Eucalyptus dunnii Maiden. Ciênc. Florest. 2016, 26, 1155–1165. [Google Scholar] [CrossRef]
- Felippe, D.; Navroski, M.C.; Sampietro, J.A.; Frigotto, T.; Albuquerque, J.A.; Mota, C.S.; Pereira, M.O. Efeito do hidrogel no crescimento de mudas de Eucalyptus benthamii submetidas a diferentes frequências de irrigação. Floresta 2016, 46, 215. [Google Scholar] [CrossRef]
- IBI. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil (Aka IBI Biochar Standards); International Biochar Initiative: Norfolk, VA, USA, 2015. [Google Scholar]
- Meng, L.; Rahman, A.; Han, S.H.; Kim, S.B.; Cho, M.S.; Park, B.B. Growth of Zelkova serrata seedlings in a containerised production system treated with effective microorganisms and biochar. J. Trop. For. Sci. 2018, 30, 49–57. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does Biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Elad, Y.; Paudel, I.; Graber, E.R.; Cytryn, E.; Frenkel, O. Linking the belowground microbial composition, diversity and activity to soilborne disease suppression and growth promotion of tomato amended with biochar. Sci. Rep. 2017, 7, 44382. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: A meta-analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef]
- Li, W.; Tian, Y.; Liu, C.; Yang, R.; Jin, Y.; Li, T. Biochar Application: A Viable and Pyrolysis Temperature-Dependent Option for Enhancing Leaf Secondary Metabolites of Cyclocarya paliurus. Forests 2023, 14, 1298. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Carlos Navroski, M.; Machado Araujo, M.; Sidnei Fior, C.; da Silva Cunha, F.; Luís Pasquetti Berghetti, Á.; de Oliveira Pereira, M. Uso de Hidrogel Possibilita Redução Da Irrigação e Melhora o Crescimento Inicial de Mudas de Eucalyptus dunnii Maiden. Sci. For. 2015, 43, 467–476. [Google Scholar]
- Lopes, B.C. Uso de Biocarvão em Sistema de Produção de Mudas. Ph.D. Thesis, Universidade do Estado de Santa Catarina, Lages, Brazil, 2024. [Google Scholar]
- Cunha, F.L.; Silva, O.M.C.; Araujo, V.C.; Venturin, N.; Melo, L.A. Palha de Café Carbonizada Em Substratos Renováveis Para Produção de Mudas de Eucalyptus urophylla e Anadenanthera macrocarpa. Ciênc. Florest. 2022, 32, 548–572. [Google Scholar] [CrossRef]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality appraisal of white spruce and white pine seedling stock in nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Espírito Santo, H.; Daniel, F. Calcular e Apresentar Tamanhos Do Efeito Em Trabalhos Científicos (2): Guia Para Reportar a Força Das Relações. Rev. Port. Investig. Comport. Soc. 2017, 3, 53–64. [Google Scholar] [CrossRef]
- Berben, L.; Sereika, S.M.; Engberg, S. Effect size estimation: Methods and examples. Int. J. Nurs. Stud. 2012, 49, 1039–1047. [Google Scholar] [CrossRef]
- Kroes, A.D.A.; Finley, J.R. Demystifying Omega Squared: Practical Guidance for Effect Size in Common Analysis of Variance Designs. Psychol. Methods 2023, 30, 866–887. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R. Available online: http://www.posit.co/downloads/ (accessed on 9 August 2025).
- Sawilowsky, S.S. New effect size rules of thumb. J. Mod. Appl. Stat. Methods 2009, 8, 597–599. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, X.; Hou, H.; Ji, J.; Liu, X.; Lv, Z. Multifaceted Ability of Organic Fertilizers to Improve Crop Productivity and Abiotic Stress Tolerance: Review and Perspectives. Agronomy 2024, 14, 1141. [Google Scholar] [CrossRef]
- Bergstrand, K.-J.; Löfkvist, K.; Asp, H. Dynamics of nutrient availability in tomato production with organic fertilisers. Biol. Agric. Hortic. 2020, 36, 200–212. [Google Scholar] [CrossRef]
- Cannavo, P.; Recous, S.; Valé, M.; Bresch, S.; Paillat, L.; Benbrahim, M.; Guénon, R. Organic Fertilization of Growing Media: Response of N Mineralization to Temperature and Moisture. Horticulturae 2022, 8, 152. [Google Scholar] [CrossRef]
- Allam, M.; Radicetti, E.; Quintarelli, V.; Petroselli, V.; Marinari, S.; Mancinelli, R. Influence of Organic and Mineral Fertilizers on Soil Organic Carbon and Crop Productivity under Different Tillage Systems: A Meta-Analysis. Agriculture 2022, 12, 464. [Google Scholar] [CrossRef]
- Wendling, I.; Ferreira, L.; Mônica, D.; Gabira, M.; Vieira, L.M.; Degenhardt Foto, J.; Buhrer, R. Produção de Mudas de Eucalipto. In O Eucalipto e a Embrapa: Quatro Décadas de Pesquisa e Desenvolvimento; Oliveira, E.B., Pinto Junior, J.E., Eds.; Embrapa: Brasília, Brazil, 2021; pp. 823–857. ISBN 9786587380049. [Google Scholar]
- Cruz, G.H.M.; Cunha, F.F.; Souza, E.J.; Silva, A.J.; Filgueiras, R. Irrigation frequencies for Eucalyptus grandis seedlings. Rev. Eng. Agric. 2020, 28, 364–374. [Google Scholar] [CrossRef]
- Araujo, M.M.; Navroski, M.C.; Schorn, L.A. Caracterização e análise de atributos morfológicos e fisiológicos indicadores da qualidade de mudas em viveiro florestal. In Produção de Sementes e Mudas: Um Enfoque à Silvicultura; Araújo, M.M., Navroski, M.C., Schorn, L.A., Eds.; Editora UFSM: Santa Maria, Brazil, 2018; p. 446. ISBN 9788573913156. [Google Scholar]
- Hunt, G.A. Effect of Styroblock Design and Copper Treatment on Morphology of Conifer Seedlings. In Target Seedling Symposium, Proceedings of the Combined Meeting of the Western Forest Nursery Associations, Roseburg, OR, USA, 13–17 August 1990; Rose, R., Campbell, S.J., Landis, T.D., Eds.; USDA Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1990; pp. 218–222. [Google Scholar]
- Kratz, D.; Wendling, I.; Nogueira, A.C.; Vitor, P.; De Souza, D. Renewable substrates in the seedling production of Eucalyptus benthamii. Ciênc. Florest. 2013, 23, 607–621. [Google Scholar] [CrossRef]
- Kratz, D.; Wendling, I.; Nogueira, A.C.; Souza, P.V.D. Utilização de Resíduos Urbanos e Agroflorestais Para Produção de Mudas de Eucalyptus benthamii e Mimosa scabrella. Floresta Ambiente 2013, 20, 530–537. [Google Scholar] [CrossRef][Green Version]
- Iannucci, A.; Amato, M. Root morphology and shoot growth in seedlings of chia (Salvia hispanica L.). Genet. Resour. Crop Evol. 2021, 68, 3205–3217. [Google Scholar] [CrossRef]
- Luera, P.; Wahl-Villarreal, K.; Christoffersen, B.O.; Treviño, A.; Soti, P.; Gabler, C.A. Effects of Scarification, Phytohormones, Soil Type, and Warming on the Germination and/or Seedling Performance of Three Tamaulipan Thornscrub Forest Species. Plants 2021, 10, 1489. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.M.; Oliveira, C.A.; Andrade, D.L.; Carvalho, C.G.; Ribeiro, V.P.; Pastina, M.M.; Marriel, I.E.; Paula Lana, U.G.; Gomes, E.A. Tropical Bacillus Strains Inoculation Enhances Maize Root Surface Area, Dry Weight, Nutrient Uptake and Grain Yield. J. Plant Growth Regul. 2021, 40, 867–877. [Google Scholar] [CrossRef]
- Aguilar, M.V.M.; Kuinchtner, C.C.; Senhor, D.F.; Birck, T.P.; Lima, C.S.; Kulmann, M.S.S.; Araujo, M.M.; Berghetti, Á.L.P.; Brunetto, G.; Tabaldi, L.A. Selecting Eucalyptus spp. Clones to Enable Higher Phosphorus Uptake Efficiency. J. Plant Growth Regul. 2024, 43, 854–870. [Google Scholar] [CrossRef]
- Souza Kulmann, M.S.; Arruda, W.S.; Vitto, B.B.; Souza, R.O.S.; Berghetti, Á.L.P.; Tarouco, C.P.; Araujo, M.M.; Nicoloso, F.T.; Schumacher, M.V.; Brunetto, G. Morphological and physiological parameters influence the use efficiency of nitrogen and phosphorus by Eucalyptus seedlings. New For. 2022, 53, 431–448. [Google Scholar] [CrossRef]
- Busch, F.A.; Ainsworth, E.A.; Amtmann, A.; Cavanagh, A.P.; Driever, S.M.; Ferguson, J.N.; Kromdijk, J.; Lawson, T.; Leakey, A.D.B.; Matthews, J.S.A.; et al. A guide to photosynthetic gas exchange measurements: Fundamental principles, best practice and potential pitfalls. Plant Cell Environ. 2024, 47, 3344–3364. [Google Scholar] [CrossRef]
- Wong, S.C.; Cowan, I.R.; Farquhar, G.D. Stomatal conductance correlates with photosynthetic capacity. Nature 1979, 282, 424–426. [Google Scholar] [CrossRef]
- Gadi, V.K.; Hussain, R.; Bordoloi, S.; Hossain, S.; Singh, S.R.; Garg, A.; Sekharan, S.; Karangat, R.; Lingaraj, S. Relating stomatal conductance and surface area with evapotranspiration induced suction in a heterogeneous grass cover. J. Hydrol. 2019, 568, 867–876. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal Size, Speed, and Responsiveness Impact on Photosynthesis and Water Use Efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Silva, P.; Campoe, O.; Paula, R.; Lee, D. Seedling Growth and Physiological Responses of Sixteen Eucalypt Taxa under Controlled Water Regime. Forests 2016, 7, 110. [Google Scholar] [CrossRef]
- Kirk, R.E. Practical significance: A concept whose time has come. Educ. Psychol. Meas. 1996, 56, 746–759. [Google Scholar] [CrossRef]
- Mazumder, P.; PM, A.; Jyoti; Khwairakpam, M.; Mishra, U.; Kalamdhad, A.S. Enhancement of soil physico-chemical properties post compost application: Optimization using response surface methodology comprehending central composite design. J. Environ. Manag. 2021, 289, 112461. [Google Scholar] [CrossRef]
- Kebede, T.; Diriba, D.; Boki, A. The Effect of Organic Solid Waste Compost on Soil Properties, Growth, and Yield of Swiss Chard Crop (Beta vulgaris L.). Sci. World J. 2023, 2023, 6175746. [Google Scholar] [CrossRef]
- Cui, X.; Lu, H.; Lu, Y.; Gao, P.; Peng, F. Replacing 30% chemical fertilizer with organic fertilizer increases the fertilizer efficiency, yield and quality of cabbage in intensive open-field production. Ciênc. Rural 2022, 52, e20210186. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Jiang, P.; Wang, R.; Guo, J.; Xiao, H.; Wu, J.; Shaaban, M.; Li, Y.; Huang, M. Organic fertilizer substituting 20% chemical N increases wheat productivity and soil fertility but reduces soil nitrate-N residue in drought-prone regions. Front. Plant Sci. 2024, 15, 1379485. [Google Scholar] [CrossRef]
- Ali, K.; Asad, Z.; Agbna, G.H.D.; Saud, A.; Khan, A.; Zaidi, S.J. Progress and Innovations in Hydrogels for Sustainable Agriculture. Agronomy 2024, 14, 2815. [Google Scholar] [CrossRef]
- Santos, C.C.; Beltramin, F.A.; Silva, W.C.; Silverio, J.M.; Scalon, S.P.Q.; Souza, F.H.; Holsbaque, V.G.; Janse, R.A.L. Hydrogel alleviates the stressful effect drought in Schinus terebinthifolia and helps with post-stress recovery. Braz. J. Biol. 2025, 85, e289691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Niu, W.; Luo, H. Effect of Biochar Amendment on the Growth and Photosynthetic Traits of Plants Under Drought Stress: A Meta-Analysis. Agronomy 2024, 14, 2952. [Google Scholar] [CrossRef]
- Köster, E.; Pumpanen, J.; Palviainen, M.; Zhou, X.; Köster, K. Effect of biochar amendment on the properties of growing media and growth of containerized Norway spruce, Scots pine, and silver birch seedlings. Can. J. For. Res. 2021, 51, 31–40. [Google Scholar] [CrossRef]
- Reyes Moreno, G.; Elena Fernández, M.; Darghan Contreras, E. Balanced mixture of biochar and synthetic fertilizer increases seedling quality of Acacia mangium. J. Saudi Soc. Agric. Sci. 2021, 20, 371–378. [Google Scholar] [CrossRef]
- Rakib, M.H.; Chowdhury, M.I.H.; Hossain, T.; Sadnan, M.W.M.; Hossain, S.M.S. Biochar as a drought mitigation tool to enhance growth and water retention in Pinus caribaea M. seedlings. Ceylon J. Sci. 2025, 54, 467–473. [Google Scholar] [CrossRef]
- Barbosa, B.S.; Shibata, M.; Costa dos Santos, C.R.; Lopes, B.C.; Campos, M.L.; Martins, W.B.R.; Wood, D.; Castro, J.P. Characterization of Biochar from Orange Tree Residues and Its Effect on the Growth of Handroanthus impetiginosus (Mart. Ex DC.) Mattos Seedlings. J. Plant Nutr. 2025, 48, 1604–1618. [Google Scholar] [CrossRef]
- Sahmat, S.S.; Rafii, M.Y.; Oladosu, Y.; Jusoh, M.; Hakiman, M.; Mohidin, H. A Systematic Review of the Potential of a Dynamic Hydrogel as a Substrate for Sustainable Agriculture. Horticulturae 2022, 8, 1026. [Google Scholar] [CrossRef]
- Natalli, L.H.; Hillig, E.; Lombardi, K.C.; Godinho, M.; Nuñez, R.P. Use of Biochar as a Component of Substrates in Horticulture and Forestry: A Review. Rev. Bras. Ciênc. Solo 2024, 48, e0240027. [Google Scholar] [CrossRef]
- Marouelli, W.A.; Oliveira, Á.S.; Coelho, E.F.; Nogueira, L.C.; Souza, V.F. Manejo Da Água de Irrigação. In Irrigação e Fertirrigação em Frutíferas e Hortaliças; Souza, V.F., Marquelli, W.A., Coelho, E.F., Pinto, J.M., Coelho Filho, M.A., Eds.; Embrapa: Brasília, Brazil, 2011. [Google Scholar]
- Konzen, E.R.; Navroski, M.C.; Friederichs, G.; Ferrari, L.H.; Pereira, M.O.; Felippe, D. The use of hydrogel combined with appropriate substrate and fertilizer improves quality and growth performance of Mimosa scabrella Benth. seedlings. Cerne 2017, 23, 473–482. [Google Scholar] [CrossRef]
- Instituto Nacional de Meteorologia—INMET (Normais Climatológicas). Available online: https://portal.inmet.gov.br/normais (accessed on 11 September 2025).
- Paiva, I.O.; Morais, E.G.; Silva, C.A. Biochar and Ammonium Nitrate Synergies: Enhancing Nitrogen Availability and Maize Growth in Oxisols. Agronomy 2025, 15, 633. [Google Scholar] [CrossRef]
- Alharbi, H.A.; Alotaibi, K.D.; EL-Saeid, M.H.; Giesy, J.P. Polycyclic Aromatic Hydrocarbons (PAHs) and Metals in Diverse Biochar Products: Effect of Feedstock Type and Pyrolysis Temperature. Toxics 2023, 11, 96. [Google Scholar] [CrossRef] [PubMed]
| Product | Total Organic Carbon | Total Nitrogen | pH | Cation Exchange Capacity | C/N Ratio |
|---|---|---|---|---|---|
| % | - | mmolc kg−1 | - | ||
| Organic fertilizer | 15.0 | 0.5 | 7.0 | 75 | 20 |
| Biochar 1 | 12.8 | 0.1 | 7.9 | - | - |
| Treatments | Experiment 1 | Experiment 2 | ||
|---|---|---|---|---|
| Organic Fertilizer Doses | Hydrogel Levels | Organic Fertilizer Doses | Biochar Levels | |
| kg m−3 | kg m−3 | kg m−3 | % | |
| 1 | 0 | 0 | 0 | 0 |
| 2 | 5 | 5 | ||
| 3 | 10 | 10 | ||
| 4 | 15 | 15 | ||
| 5 | 20 | 20 | ||
| 6 | 25 | 25 | ||
| 7 | 0 | 3 | 0 | 30 |
| 8 | 5 | 5 | ||
| 9 | 10 | 10 | ||
| 10 | 15 | 15 | ||
| 11 | 20 | 20 | ||
| 12 | 25 | 25 | ||
| Variable | Hydrogel Levels | Doses of Organic Fertilizer (kg m−3) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | ||
| Average diameter (mm) | Without hydrogel | 0.70 ns | 0.65 | 0.66 | 0.62 | 0.75 | 0.69 |
| With hydrogel | 0.64 | 0.67 | 0.60 | 0.65 | 0.67 | 0.62 | |
| Length (cm) | Without hydrogel | 412.37 Aa | 301.30 Ab | 235.81 Ab | 239.86 Ab | 261.74 Ab | 249.85 Ab |
| With hydrogel | 220.97 Bb | 354.46 Aa | 310.71 Aa | 230.34 Ab | 257.89 Ab | 300.18 Aa | |
| Surface area (cm2) | Without hydrogel | 91.03 Aa | 60.44 Ab | 48.53 Ab | 46.89 Ab | 62.05 Ab | 53.31 Ab |
| With hydrogel | 44.20 Ba | 74.40 Aa | 58.49 Aa | 47.08 Aa | 53.59 Aa | 59.14 Aa | |
| Volume (cm3) | Without hydrogel | 1.61 Aa | 0.98 Ab | 0.80 Ab | 0.74 Ab | 1.18 Ab | 0.95 Ab |
| With hydrogel | 0.71 Ba | 1.25 Aa | 0.88 Aa | 0.77 Aa | 0.89 Aa | 0.95 Aa | |
| Tips | Without hydrogel | 1037.75 Aa | 850.75 Aa | 609.25 Bb | 682.50 Ab | 634.00 Ab | 648.75 Ab |
| With hydrogel | 544.75 Bb | 1087.50 Aa | 928.50 Aa | 600.25 Ab | 705.50 Ab | 812.00 Aa | |
| Crossings | Without hydrogel | 1509.00 Aa | 819.75 Ab | 585.50 Ab | 572.75 Ab | 818.50 Ab | 620.50 Ab |
| With hydrogel | 537.75 Ba | 1089.50 Aa | 906.00 Aa | 599.50 Aa | 679.75 Aa | 909.75 Aa | |
| Crossings | Without hydrogel | 666.00 Aa | 257.50 Ab | 178.00 Ab | 157.75 Ab | 286.50 Ab | 192.25 Ab |
| With hydrogel | 143.50 Ba | 370.50 Aa | 287.00 Aa | 159.50 Aa | 216.50 Aa | 328.25 Aa | |
| A | Without hydrogel | 3.63 ns | 4.19 | 2.68 | 3.84 | 3.70 | 4.10 |
| With hydrogel | 2.67 | 3.20 | 3.71 | 3.81 | 5.34 | 4.11 | |
| gs | Without hydrogel | 0.21 ns | 0.15 | 0.34 | 0.23 | 0.19 | 0.18 |
| With hydrogel | 0.21 | 0.24 | 0.32 | 0.20 | 0.30 | 0.18 | |
| E | Without hydrogel | 3.22 ns | 2.67 | 3.42 | 3.52 | 2.95 | 3.13 |
| With hydrogel | 3.33 | 3.30 | 3.87 | 3.22 | 3.78 | 2.85 | |
| Ci | Without hydrogel | 243.28 ns | 224.07 | 263.34 | 248.75 | 233.23 | 237.18 |
| With hydrogel | 256.69 | 251.77 | 250.18 | 240.10 | 237.14 | 224.76 | |
| Ci/Ca | Without hydrogel | 0.86 ns | 0.80 | 0.92 | 0.88 | 0.83 | 0.84 |
| With hydrogel | 0.88 | 0.88 | 0.89 | 0.85 | 0.85 | 0.80 | |
| A/Ci | Without hydrogel | 0.02 ns | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 |
| With hydrogel | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | |
| A/E | Without hydrogel | 1.16 ns | 1.59 | 0.79 | 1.05 | 1.37 | 1.30 |
| With hydrogel | 0.90 | 0.99 | 1.03 | 1.26 | 1.46 | 1.60 | |
| Variable | Biochar Levels | Doses of Organic Fertilizer (kg m−3) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | ||
| Average diameter (mm) | Without biochar | 0.60 ns | 0.67 | 0.70 | 0.68 | 0.65 | 0.66 |
| With biochar | 0.65 | 0.63 | 0.65 | 0.62 | 0.68 | 0.70 | |
| Length (cm) | Without biochar | 220.09 Ba | 320.38 Aa | 304.49 Ba | 259.79 Aa | 247.18 Aa | 264.61 Aa |
| With biochar | 383.32 Aa | 271.52 Ab | 425.89 Aa | 240.03 Ab | 204.78 Ab | 266.45 Ab | |
| Surface area (cm2) | Without biochar | 41.78 Ba | 68.93 Aa | 65.98 Aa | 55.50 Aa | 49.96 Aa | 54.82 Aa |
| With biochar | 77.35 Aa | 53.31 Ab | 86.28 Aa | 45.98 Ab | 43.08 Ab | 55.13 Ab | |
| Volume (cm3) | Without biochar | 0.64 Ba | 1.19 Aa | 1.15 Aa | 0.95 Aa | 0.81 Aa | 0.90 Aa |
| With biochar | 1.24 Aa | 0.83 Ab | 1.39 Aa | 0.71 Ab | 0.72 Ab | 0.95 Ab | |
| Tips | Without biochar | 734.25 | 1118.25 | 1330.50 | 621.50 | 774.00 | 723.50 |
| With biochar | 1008.50 | 898.75 | 1469.25 | 685.00 | 703.50 | 1093.50 | |
| Crossings | Without biochar | 514.75 Bb | 1018.00 Aa | 837.75 Ba | 547.00 Ab | 562.25 Ab | 611.00 Ab |
| With biochar | 1271.25 Aa | 606.50 Bb | 1307.75 Aa | 514.50 Ab | 475.25 Ab | 616.00 Ab | |
| Crossings | Without biochar | 146.00 Bb | 376.25 Aa | 257.25 Ba | 149.25 Ab | 163.75 Ab | 173.50 Ab |
| With biochar | 480.50 Aa | 198.00 Bb | 492.25 Aa | 152.25 Ab | 145.75 Ab | 203.00 Ab | |
| A | Without biochar | 8.90 ns | 7.27 | 9.71 | 7.35 | 8.59 | 6.44 |
| With biochar | 6.94 | 5.48 | 7.27 | 6.92 | 7.80 | 8.59 | |
| gs | Without biochar | 0.50 * A | 0.41 | 0.60 | 0.39 | 0.56 | 0.32 |
| With biochar | 0.34 B | 0.27 | 0.32 | 0.30 | 0.44 | 0.42 | |
| E | Without biochar | 3.91 ns | 3.59 | 3.99 | 3.57 | 3.96 | 3.43 |
| With biochar | 3.57 | 3.08 | 3.55 | 3.01 | 3.93 | 3.29 | |
| Ci | Without biochar | 252.53 ns | 254.13 | 254.95 | 250.76 | 258.12 | 249.56 |
| With biochar | 248.62 | 254.65 | 242.05 | 236.56 | 252.86 | 239.28 | |
| Ci/Ca | Without biochar | 0.88 ns | 0.88 | 0.89 | 0.87 | 0.90 | 0.86 |
| With biochar | 0.86 | 0.87 | 0.84 | 0.81 | 0.88 | 0.83 | |
| A/Ci | Without biochar | 0.04 ns | 0.03 | 0.04 | 0.03 | 0.03 | 0.03 |
| With biochar | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 | 0.04 | |
| A/E | Without biochar | 2.32 ns | 2.04 | 2.45 | 2.06 | 2.14 | 1.87 |
| With biochar | 1.95 | 1.76 | 2.05 | 2.38 | 1.99 | 2.59 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filho, D.P.d.S.; Costa, K.J.S.d.; Schilisting, T.; Sá, A.C.S.; Silva, V.M.d.; Andrade, R.S.d.; Nascimento, B.; Azevedo, I.M.B.d.; Moraes, C.; Pereira, M.d.O.; et al. Does a Commercial Organic Fertilizer with Hydrogel or Biochar Guarantee the Quality of Eucalyptus Seedlings? Forests 2025, 16, 1489. https://doi.org/10.3390/f16091489
Filho DPdS, Costa KJSd, Schilisting T, Sá ACS, Silva VMd, Andrade RSd, Nascimento B, Azevedo IMBd, Moraes C, Pereira MdO, et al. Does a Commercial Organic Fertilizer with Hydrogel or Biochar Guarantee the Quality of Eucalyptus Seedlings? Forests. 2025; 16(9):1489. https://doi.org/10.3390/f16091489
Chicago/Turabian StyleFilho, Daniel Pereira da Silva, Karla Juliana Silva da Costa, Thalia Schilisting, Alexandra Cristina Schatz Sá, Valeria Martel da Silva, Ramon Silveira de Andrade, Bruno Nascimento, Izabelle Maria Barboza de Azevedo, Carolina Moraes, Mariane de Oliveira Pereira, and et al. 2025. "Does a Commercial Organic Fertilizer with Hydrogel or Biochar Guarantee the Quality of Eucalyptus Seedlings?" Forests 16, no. 9: 1489. https://doi.org/10.3390/f16091489
APA StyleFilho, D. P. d. S., Costa, K. J. S. d., Schilisting, T., Sá, A. C. S., Silva, V. M. d., Andrade, R. S. d., Nascimento, B., Azevedo, I. M. B. d., Moraes, C., Pereira, M. d. O., Gama, M. A. P., & Navroski, M. C. (2025). Does a Commercial Organic Fertilizer with Hydrogel or Biochar Guarantee the Quality of Eucalyptus Seedlings? Forests, 16(9), 1489. https://doi.org/10.3390/f16091489







