Spatial Patterns of Stem Tissue Carbon Content in Fagaceae Species from Typical Forests in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of Study Sites
2.2. Sample Collection and Carbon Content Determination
2.3. Data Analysis
3. Results
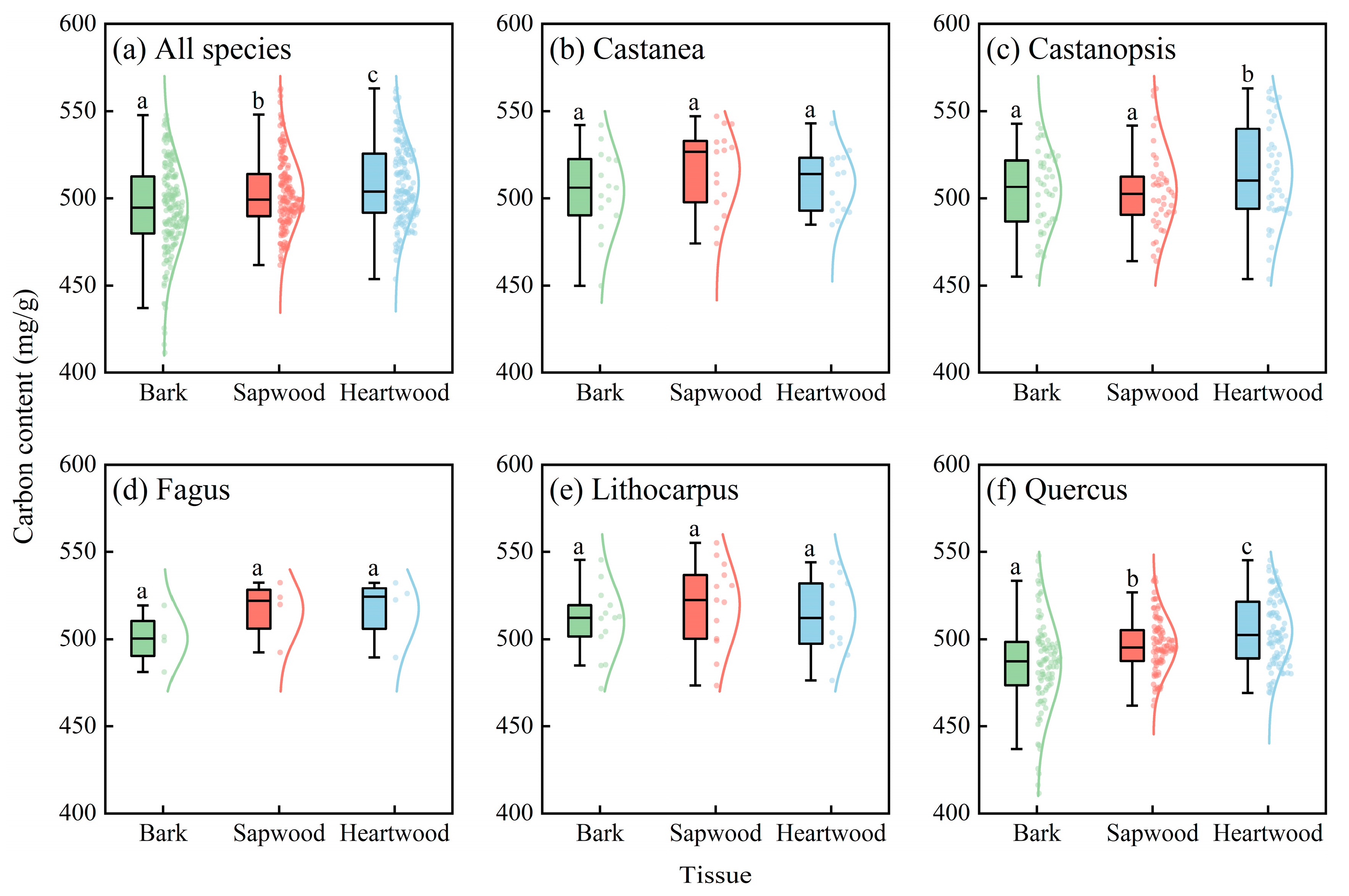
3.1. Basic Characteristics of Carbon Content in Different Stem Tissues
3.2. Comparison of Carbon Content in Different Stem Tissues
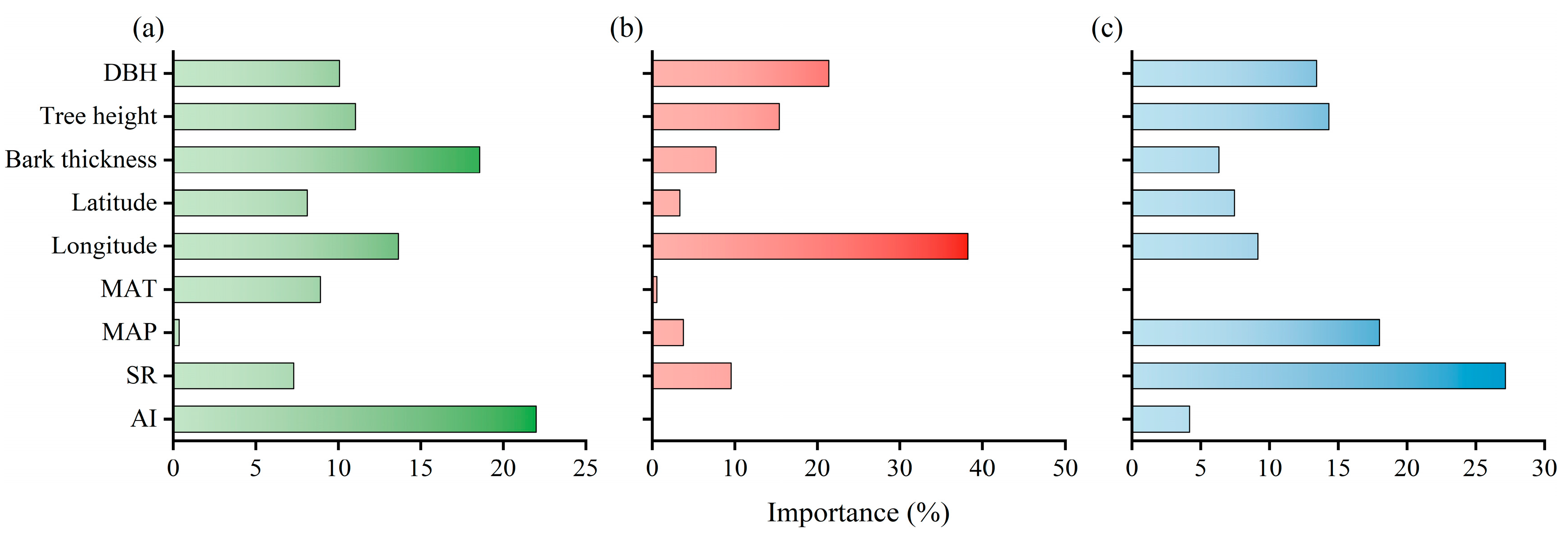
3.3. Geographical Patterns and Influencing Factors of Stem Tissues Carbon Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, A.R.; Doraisami, M.; Thomas, S.C. Global patterns in wood carbon concentration across the world’s trees and forests. Nat. Geosci. 2018, 11, 915–920. [Google Scholar] [CrossRef]
- Ma, S.; He, F.; Tian, D.; Zou, D.; Yan, Z.; Yang, Y.; Zhou, T.; Huang, K.; Shen, H.; Fang, J. Variations and determinants of carbon content in plants: A global synthesis. Biogeosciences 2018, 15, 693–702. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.; Wang, X.; Quan, X. Carbon concentration variability of 10 Chinese temperate tree species. For. Ecol. Manag. 2009, 258, 722–727. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Paroshy, N.J.; Doraisami, M.; Kish, R.; Martin, A.R. Carbon concentration in the world’s trees across climatic gradients. New Phytol. 2021, 232, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, J.; Wang, X.; Wang, X.; Wang, Q.; Liu, Y.; Liu, Y.; Diao, Y.; Quan, X.; Wang, C.; et al. Variations and determinants of tissue carbon concentration of 32 sympatric temperate tree species. J. For. Res. 2024, 35, 113. [Google Scholar] [CrossRef]
- Ma, S.; Eziz, A.; Tian, D.; Yan, Z.; Cai, Q.; Jiang, M.; Ji, C.; Fang, J. Size- and age-dependent increases in tree stem carbon concentration: Implications for forest carbon stock estimations. J. Plant Ecol. 2020, 13, 233–240. [Google Scholar] [CrossRef]
- Widagdo, F.R.A.; Li, F.; Xie, L.; Dong, L. Intra- and inter-species variations in carbon content of 14 major tree species in Northeast China. J. For. Res. 2021, 32, 2545–2556. [Google Scholar] [CrossRef]
- Doraisami, M.; Kish, R.; Paroshy, N.J.; Domke, G.M.; Thomas, S.C.; Martin, A.R. A global database of woody tissue carbon concentrations. Sci. Data 2022, 9, 284. [Google Scholar] [CrossRef]
- Qiu, L.; Wu, X.; Liu, T. Spatial diversities and differences of all genera in Fagaceae of China. Acta Bot. Boreal-Occident Sin. 2016, 36, 2103–2108. [Google Scholar]
- Liu, M.; Hong, B. The distribution of Fagaceae in China and its relationship with climatic and geographic character. Acta Phytoecol. Sin. 1998, 22, 41–50. [Google Scholar]
- Martin, A.R.; Gezahegn, S.; Thomas, S.C. Variation in carbon and nitrogen concentration among major woody tissue types in temperate trees. Can. J. For. Res. 2015, 45, 744–757. [Google Scholar] [CrossRef]
- Doraisami, M.; Domke, G.M.; Martin, A.R. Improving wood carbon fractions for multiscale forest carbon estimation. Carbon Balance Manag. 2024, 19, 25. [Google Scholar] [CrossRef]
- Lin, Y.; Lai, Y.; Tang, S.; Qin, Z.; Liu, J.; Kang, F.; Kuang, Y. Climatic and edaphic variables determine leaf C, N, P stoichiometry of deciduous Quercus species. Plant Soil 2022, 474, 383–394. [Google Scholar] [CrossRef]
- Lamlom, S.H.; Savidge, R.A. A reassessment of carbon content in wood: Variation within and between 41 North American species. Biomass Bioenergy 2003, 25, 381–388. [Google Scholar]
- Wu, H.; Xiang, W.; Fang, X.; Lei, P.; Ouyang, S.; Deng, X. Tree functional types simplify forest carbon stock estimates induced by carbon concentration variations among species in a subtropical area. Sci. Rep. 2017, 7, 4992. [Google Scholar] [CrossRef]
- Li, B.; Fang, X.; Tian, D.; Xiang, W.; Yan, W.; Kang, W.; Deng, X. Studies on carbon concentration of main forest vegetation tree species in Hunan Province. J. Cent. South Univ. For. Technol. 2015, 35, 71–78. [Google Scholar]
- Castano-Santamaria, J.; Bravo, F. Variation in carbon concentration and basic density along stems of sessile oak (Quercus petraea (Matt.) Liebl.) and Pyrenean oak (Quercus pyrenaica Willd.) in the Cantabrian Range (NW Spain). Ann. For. Sci. 2012, 69, 663–672. [Google Scholar] [CrossRef]
- Herrero de Aza, C.; Turrión, M.B.; Pando, V.; Bravo, F. Carbon in heartwood, sapwood and bark along the stem profile in three Mediterranean Pinus species. Ann. For. Sci. 2011, 68, 1067. [Google Scholar] [CrossRef]
- Thomas, S.C.; Martin, A.R. Carbon content of tree tissues: A synthesis. Forests 2012, 3, 332–352. [Google Scholar] [CrossRef]
- Thomas, S.C.; Malczewski, G. Wood carbon content of tree species in Eastern China: Interspecific variability and the importance of the volatile fraction. J. Environ. Manag. 2007, 85, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Thripob, P.; Fortunel, C.; Réjou-Méchain, M.; Nathalang, A.; Chanthorn, W. Size-dependent intraspecific variation in wood traits has little impact on aboveground carbon estimates in a tropical forest landscape. Funct. Ecol. 2022, 36, 2303–2316. [Google Scholar] [CrossRef]
- Jones, D.A.; O’Hara, K.L. Variation in carbon fraction, density, and carbon density in conifer tree tissues. Forests 2018, 9, 430. [Google Scholar] [CrossRef]
- Elias, M.; Potvin, C. Assessing inter- and intra-specific variation in trunk carbon concentration for 32 neotropical tree species. Can. J. For. Res. 2003, 33, 1039–1045. [Google Scholar] [CrossRef]
- Di Matteo, G.; Luzzi, G.; Basile, A.; Sposato, A.; Bertini, G.; Neri, U.; Pennelli, B.; Napoli, R.; Nardi, P. Carbon concentrations and carbon storage capacity of three old-growth forests in the Sila National Park, Southern Italy. J. For. Res. 2023, 34, 233–242. [Google Scholar] [CrossRef]


| No. | Site | No. of Species (No. of Trees) | Species (No. of Trees) | Lat. (°N) | Long. (°E) | Eel. (m) | MAT (°C) | PPT (mm) |
|---|---|---|---|---|---|---|---|---|
| 1 | Ganshiling | 4 (7) | Castanopsis hainanensis (4), Quercus edithiae (2), Castanopsis jucunda (2), Lithocarpus corneus (1) | 18.39 | 109.65 | 300–330 | 25 | 1800 |
| 2 | Wuzhishan | 6 (11) | Castanopsis tonkinensis (3), Quercus disciformis (2), Lithocarpus silvicolarum (2), Lithocarpus handelianus (2), Lithocarpus fenzelianus (1), Lithocarpus hancei (1) | 18.91 | 109.68 | 700–810 | 21 | 2400 |
| 3 | Qingyunshan | 9 (19) | Castanopsis kawakamii (4), Castanopsis faberi (4), Castanopsis hystrix (3), Castanopsis carlesii (2), Castanopsis fargesii (2), Lithocarpus haipinii (1), Castanopsis fissa (1), Quercus glauca (1), Quercus chungii (1) | 24.31 | 114.24 | 430–590 | 19.5 | 1638 |
| 4 | Dagangshan | 9 (17) | Castanopsis fargesii (3), Castanopsis sclerophylla (3), Castanopsis tibetana (1), Quercus glauca (4), Quercus gilva (2), Quercus myrsinifolia (2), Castanea mollissima (1), Lithocarpus harlandii (1) | 27.59 | 114.56 | 400–700 | 15.8 | 1591 |
| 5 | Fanjingshan | 7 (19) | Castanopsis fargesii (3), Castanea henryi (3), Castanea seguinii (3), Castanopsis tibetana (3), Fagus longipetiolata (3), Quercus acutissima (3), Quercus myrsinifolia (1) | 27.83 | 108.75 | 540–690 | 14 | 1800 |
| 6 | Fengyangshan | 10 (18) | Castanopsis eyrei (4), Castanopsis carlesii (1), Castanea henryi (1), Fagus longipetiolata (1), Quercus stewardiana (5), Quercus multinervis (1), Quercus serrata (1), Lithocarpus brevicaudatus (1), Lithocarpus polystachyus (2), Lithocarpus hancei (1) | 27.89 | 119.16 | 1350–1460 | 12.3 | 2400 |
| 7 | Baotianman | 4 (19) | Castanea mollissima (4), Quercus aliena var. acutiserrata (6), Quercus serrata (4), Quercus variabilis (5) | 33.49 | 111.93 | 1130–1380 | 10.3 | 936 |
| 8 | Xianrendong | 6 (23) | Castanea mollissima (3), Quercus acutissima (5), Quercus aliena (2), Quercus aliena var. acuteserrata (1), Quercus dentata (3), Quercus mongolica (6), Quercus variabilis (3) | 39.98 | 122.96 | 150–200 | 8.7 | 799 |
| 9 | Daqingshan | 1 (3) | Quercus wutaishanica (3) | 40.96 | 111.67 | 1580 | 4 | 360 |
| 10 | Qingyuan | 1 (14) | Quercus mongolica (14) | 41.85 | 124.94 | 750–850 | 5.9 | 794 |
| 11 | Muling | 1 (12) | Quercus mongolica (12) | 43.49 | 129.45 | 540–690 | 3.8 | 530 |
| 12 | Maoershan | 1 (3) | Quercus mongolica (3) | 45.39 | 127.63 | 440–460 | 2.8 | 773 |
| 13 | Heihe | 1 (3) | Quercus mongolica (3) | 49.56 | 126.92 | 360–380 | −2 | 500 |
| Species | N | Bark | Sapwood | Heartwood |
|---|---|---|---|---|
| Castanea henryi | 4 | 496 (32) | 515 (19) | 513 (13) |
| Castanea mollissima | 8 | 500 (21) | 508 (22) | 499 (14) |
| Castanea seguinii | 3 | 526 (7) | 544 (2) | 531 (11) |
| Castanopsis carlesii | 3 | 512 (4) | 492 (18) | 508 (9) |
| Castanopsis eyrei | 4 | 484 (15) | 507 (7) | 520 (22) |
| Castanopsis fargesii | 8 | 520 (6) | 513 (15) | 529 (19) |
| Castanopsis fissa | 1 | 469 | 475 | 482 |
| Castanopsis sclerophylla | 3 | 532 (6) | 503 (9) | 555 (2) |
| Castanopsis tibetana | 4 | 497 (11) | 524 (23) | 530 (13) |
| Castanopsis tonkinensis | 3 | 540 (4) | 561 (2) | 561 (3) |
| Castanopsis faberi | 4 | 491 (15) | 490 (20) | 499 (7) |
| Castanopsis hainanensis | 3 | 503 (11) | 485 (31) | 464 (9) |
| Castanopsis hystrix | 3 | 490 (11) | 489 (4) | 493 (1) |
| Castanopsis jucunda | 2 | 478 (15) | 503 (1) | 491 (14) |
| Castanopsis kawakamii | 4 | 494 (33) | 489 (7) | 494 (11) |
| Fagus longipetiolata | 4 | 500 (16) | 517 (17) | 517 (19) |
| Lithocarpus corneus | 1 | 504 | 543 | 501 |
| Lithocarpus fenzelianus | 1 | 519 | 530 | 531 |
| Lithocarpus hancei | 2 | 509 (52) | 516 (22) | 515 (25) |
| Lithocarpus handelianus | 2 | 514 (2) | 552 (5) | 544 |
| Lithocarpus harlandii | 1 | 513 | 499 | 491 |
| Lithocarpus silvicolarum | 2 | 531 (8) | 529 (11) | 530 (12) |
| Lithocarpus brevicaudatus | 1 | 485 | 522 | 496 |
| Lithocarpus haipinii | 1 | 502 | 473 | 476 |
| Lithocarpus polystachyus | 2 | 499 (19) | 499 (18) | 508 (6) |
| Quercus acutissima | 8 | 499 (15) | 514 (12) | 532 (8) |
| Quercus aliena | 2 | 492 (11) | 516 (28) | 513 (21) |
| Quercus aliena var. acutiserrata | 7 | 477 (25) | 484 (16) | 480 (6) |
| Quercus chungii | 1 | 472 | 487 | 470 |
| Quercus dentata | 3 | 457 (15) | 528 (6) | 527 (10) |
| Quercus disciformis | 2 | 538 (15) | 520 (18) | 533 (2) |
| Quercus gilva | 2 | 470 (10) | 492 (18) | 538 (6) |
| Quercus glauca | 5 | 489 (14) | 501 (8) | 515 (19) |
| Quercus myrsinifolia | 3 | 519 (16) | 511 (16) | 514 (16) |
| Quercus serrata | 5 | 490 (3) | 492 (8) | 498 (13) |
| Quercus variabilis | 8 | 522 (17) | 491 (11) | 495 (23) |
| Quercus wutaishanica | 3 | 473 (16) | 487 (10) | 500 (12) |
| Quercus edithiae | 1 | 499 | 490 | 497 |
| Quercus mongolica | 38 | 474 (25) | 493 (13) | 500 (14) |
| Quercus multinervis | 1 | 495 | 485 | 515 |
| Quercus stewardiana | 5 | 496 (9) | 500 (11) | 503 (19) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, C.; Liu, Y.; Zhang, L.; Liu, Z.; Zhao, H.; Li, W.; Chao, X.; Wang, X. Spatial Patterns of Stem Tissue Carbon Content in Fagaceae Species from Typical Forests in China. Forests 2025, 16, 1478. https://doi.org/10.3390/f16091478
Dong C, Liu Y, Zhang L, Liu Z, Zhao H, Li W, Chao X, Wang X. Spatial Patterns of Stem Tissue Carbon Content in Fagaceae Species from Typical Forests in China. Forests. 2025; 16(9):1478. https://doi.org/10.3390/f16091478
Chicago/Turabian StyleDong, Chengke, Yulong Liu, Luna Zhang, Zhecheng Liu, Huabin Zhao, Wenjing Li, Xiaoyi Chao, and Xingchang Wang. 2025. "Spatial Patterns of Stem Tissue Carbon Content in Fagaceae Species from Typical Forests in China" Forests 16, no. 9: 1478. https://doi.org/10.3390/f16091478
APA StyleDong, C., Liu, Y., Zhang, L., Liu, Z., Zhao, H., Li, W., Chao, X., & Wang, X. (2025). Spatial Patterns of Stem Tissue Carbon Content in Fagaceae Species from Typical Forests in China. Forests, 16(9), 1478. https://doi.org/10.3390/f16091478






