Nutrient Variables Associated with Tapping Panel Dryness and Necrosis Syndromes in Rubber Tree Clones RRIM600 and RRIT251
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Evaluate the TPD and TPN Symptoms
2.3. Soil Property Analysis

2.4. Plant Sampling and Analysis
2.5. Yield, Yield Property, and Nutrient Concentration
2.6. Data Analysis
3. Results
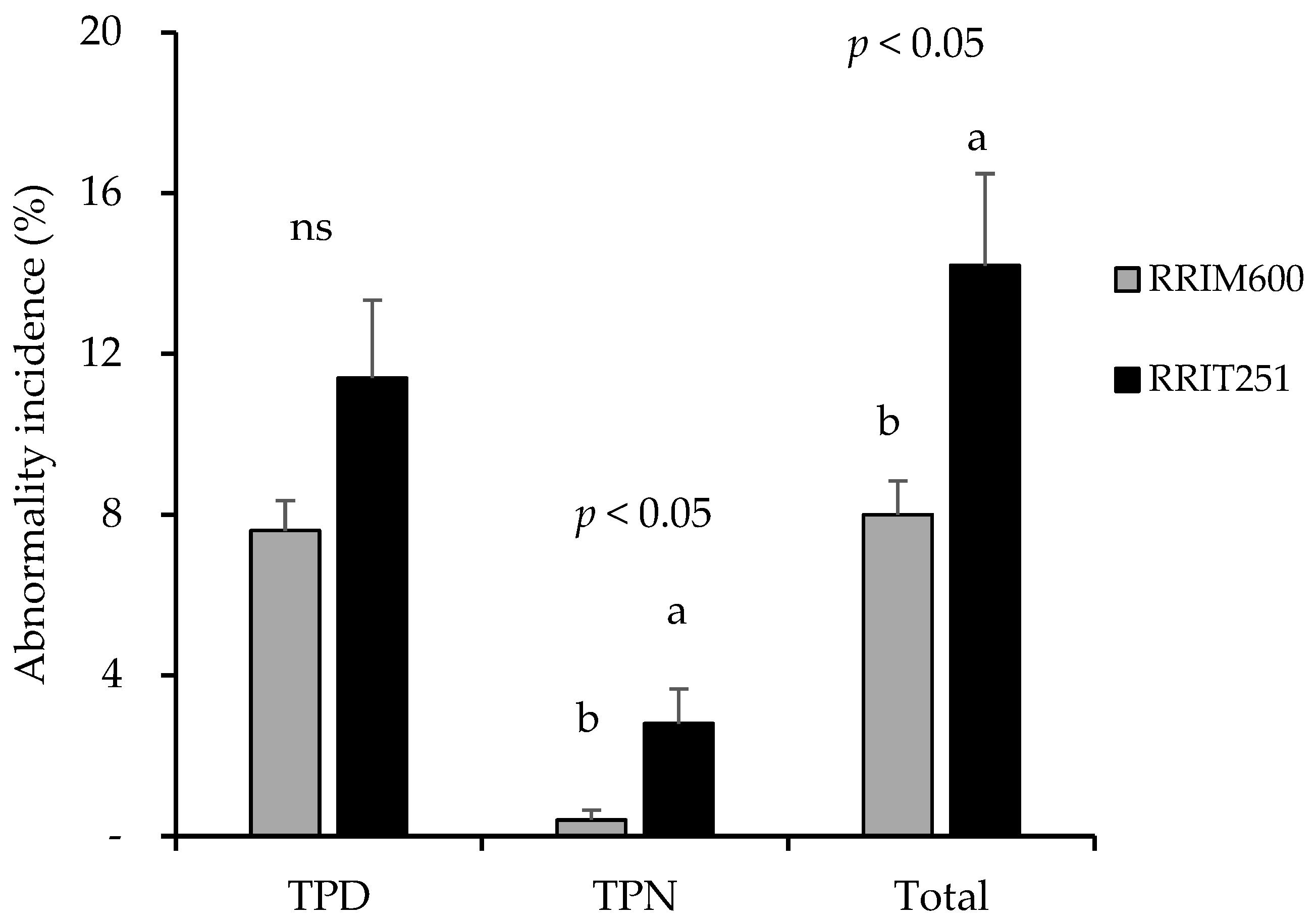
3.1. Expression of TPD and TPN Symptoms
3.2. Soil Properties
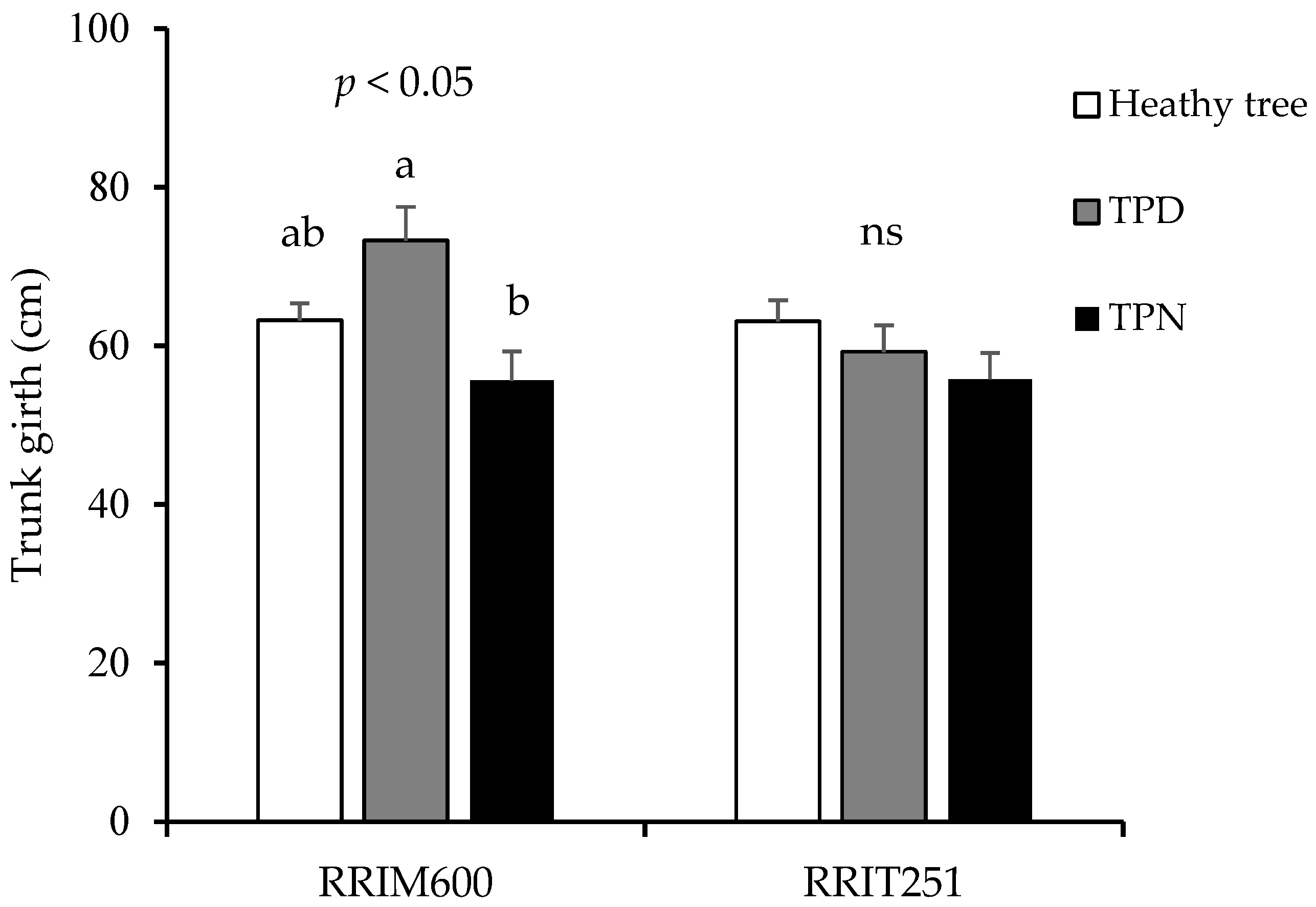
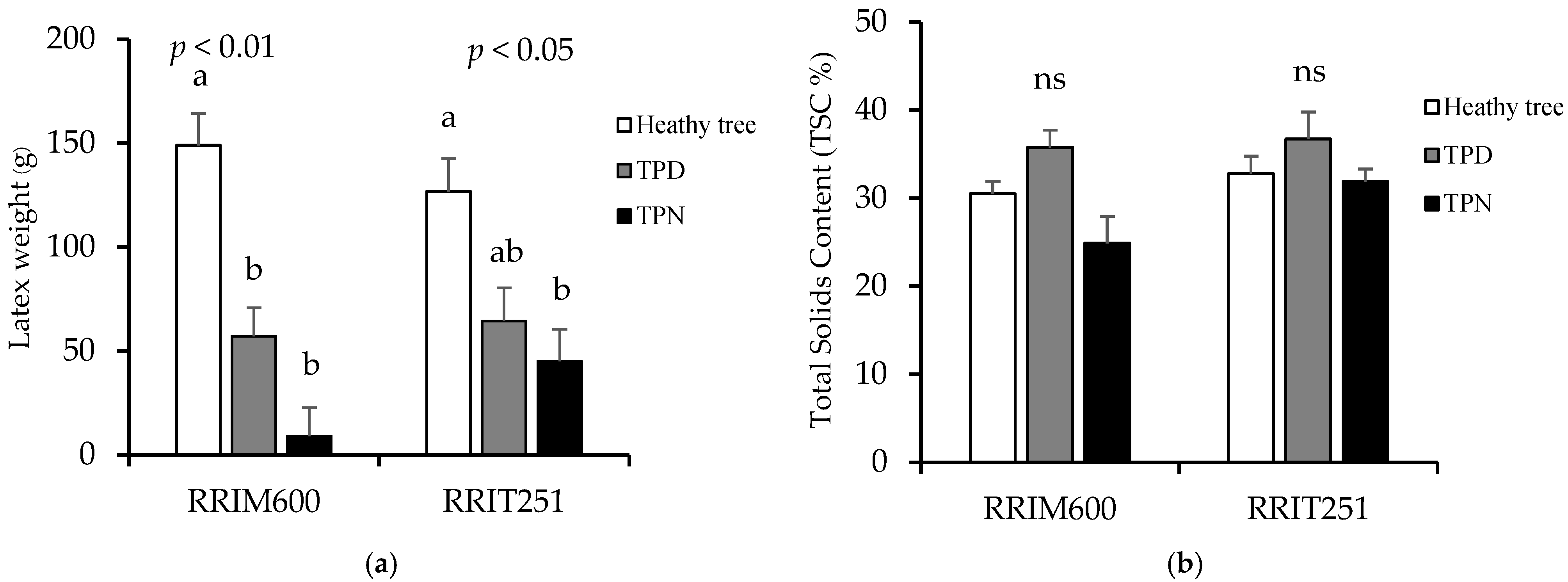
3.3. Growth and Yields
| Soil Depth | Symptom | pH | OM (%) | P (mg kg−1) | K (mg kg−1) | Ca (mg kg−1) | B (mg kg−1) |
|---|---|---|---|---|---|---|---|
| RRIM600 | |||||||
| 0–15 cm | Healthy tree | 3.79 ± 0.03 | 0.95 ± 0.06 | 25.21 ± 2.62 | 62.15± 3.73 | 57.25 ± 3.42 a | 1.49 ± 0.15 |
| TPD | 3.77 ± 0.03 | 0.88 ± 0.07 | 21.94 ± 1.62 | 59.87± 2.98 | 49.40 ± 1.63 b | 1.91 ± 0.18 | |
| TPN | 3.87 ± 0.03 | 0.64 ± 0.10 | 25.54 ± 6.95 | 62.25 ± 12.05 | 57.83 ± 1.51 a | 1.77 ± 0.33 | |
| F-test | ns | ns | ns | ns | ** | ns | |
| 15–30 cm | Healthy tree | 3.74 ± 0.04 | 0.83 ± 0.07 b | 7.85 ± 0.80 | 44.40± 2.78 | 44.64 ± 4.22 | 1.67 ± 0.24 |
| TPD | 3.72 ± 0.03 | 0.70 ± 0.04 b | 8.14 ± 0.69 | 38.83± 2.42 | 37.73 ± 2.20 | 1.75 ± 0.26 | |
| TPN | 3.81 ± 0.07 | 1.27 ± 0.27 a | 6.26 ± 0.48 | 45.07 ± 10.73 | 34.03 ± 3.21 | 2.00 ± 0.58 | |
| F-test | ns | ** | ns | ns | ns | ns | |
| RRIT251 | |||||||
| 0–15 cm | Healthy tree | 3.95 ± 0.03 | 1.15 ± 0.06 b | 46.78 ± 5.77 | 84.65 ± 4.74 | 59.24 ± 2.81 | 2.37 ± 0.20 b |
| TPD | 3.93 ± 0.05 | 1.36 ± 0.06 a | 46.06 ± 3.50 | 80.62 ± 4.15 | 59.34 ± 1.94 | 2.90 ± 0.20 a | |
| TPN | 3.90 ± 0.04 | 1.23 ± 0.11 b | 43.73 ± 7.30 | 74.99 ± 7.81 | 58.00 ± 3.95 | 1.94 ± 0.22 b | |
| F-test | ns | ** | ns | ns | ns | ** | |
| 15–30 cm | Healthy tree | 3.65 ± 0.02 | 0.67 ± 0.05 b | 20.55 ± 2.00 | 54.09 ± 3.02 | 34.84 ± 1.30 | 1.51 ± 0.23 |
| TPD | 3.63 ± 0.04 | 1.31 ± 0.19 a | 18.25 ± 1.50 | 57.07 ± 2.55 | 43.42 ± 3.35 | 1.84 ± 0.21 | |
| TPN | 3.67 ± 0.05 | 0.61 ± 0.06 b | 18.41 ± 2.18 | 60.25 ± 5.17 | 43.25 ± 4.28 | 1.51 ± 0.27 | |
| F-test | ns | ** | ns | ns | ns | ns | |
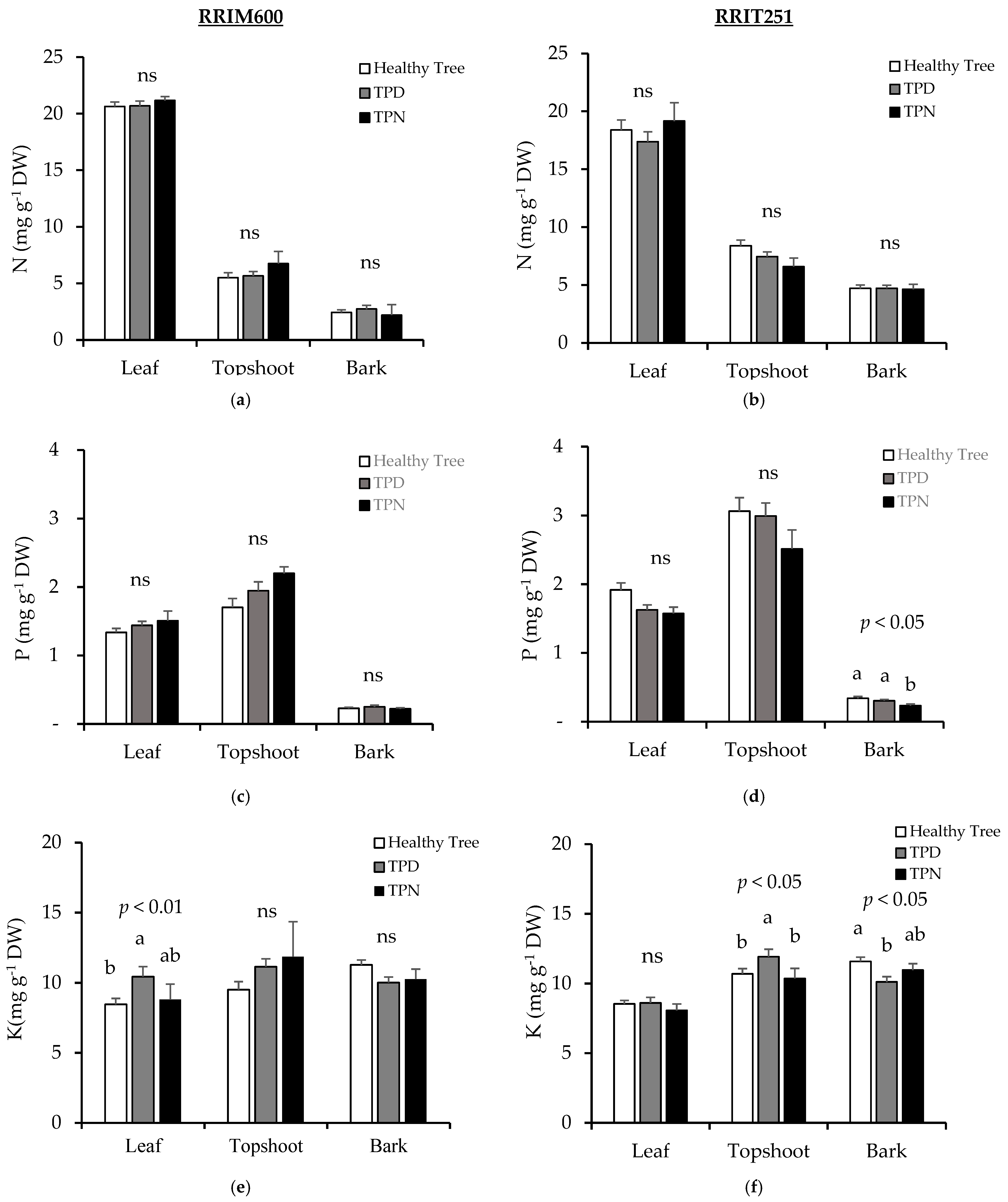
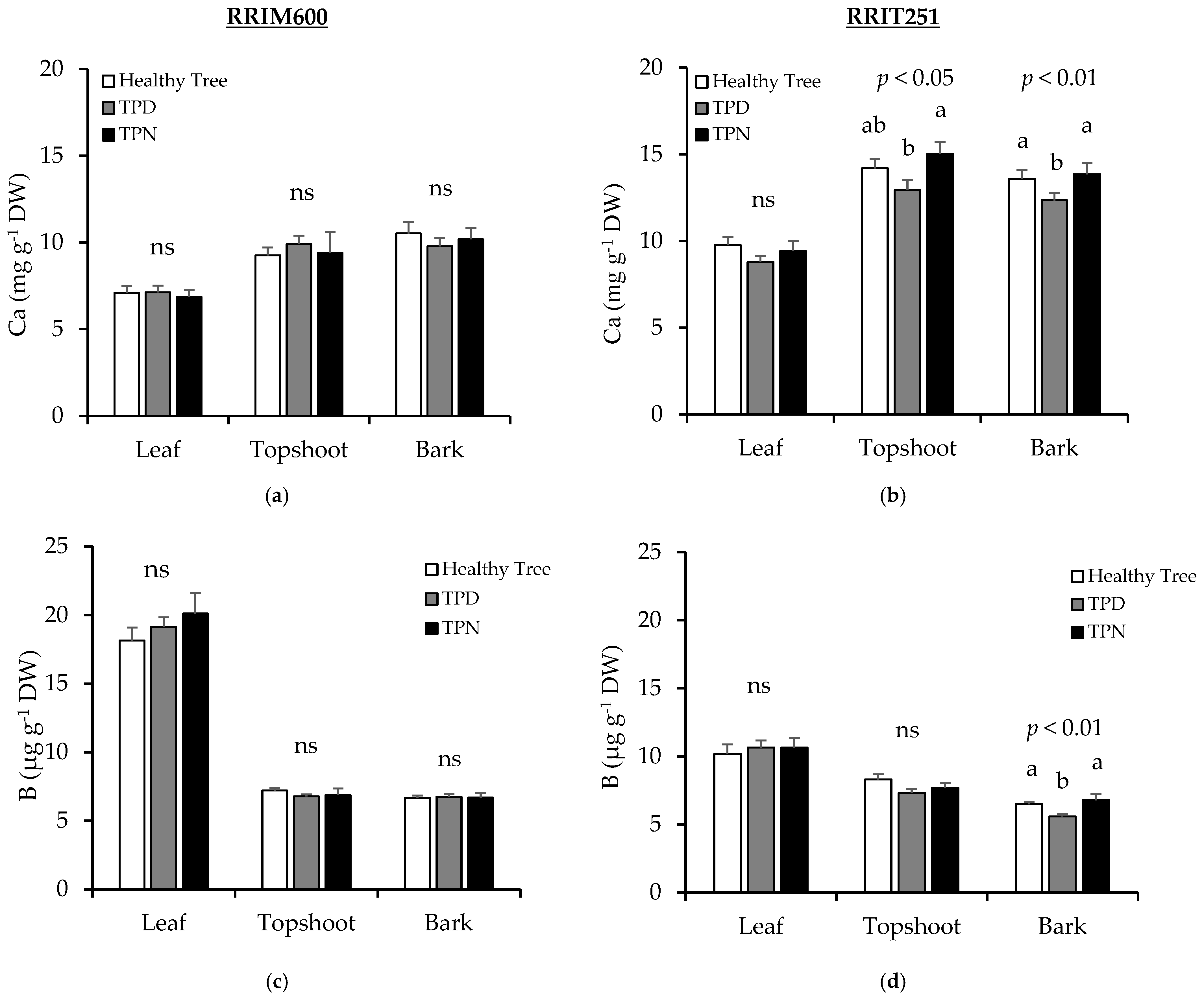
3.4. Nutrients in Plant Parts
3.5. Nutrients in Dry Rubber
4. Discussion
4.1. Level of Abnormal Expression
4.2. Soil Properties Associated with Bark Dryness
4.3. Bark Dryness on Growth and Yield
4.4. Bark Dryness on the Change in Nutrients in Plant Tissue
4.5. Bark Dryness on the Change in Nutrients in the Yield
4.6. Key Findings and Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, D.; Deng, Z.; Chen, C.; Xia, Z.; Wu, M.; He, P.; Chen, S. Identification and characterization of genes associated with tapping panel dryness from Hevea brasiliensis latex using suppression subtractive hybridization. BMC Plant Biol. 2010, 10, 140. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Online Statistical Service; United Nations Food and Agriculture Organization: Rome, Italy. Available online: http://faostat.fao.org (accessed on 1 July 2025).
- Watson, G.A. Climate and Soil. In Rubber; Webster, C.C., Baulkwill, W.J., Eds.; Longman Singapore Publishers (Pte) Ltd.: Singapore, 1989; pp. 125–164. [Google Scholar]
- Kositsup, B.; Montpied, P.; Kasemsap, P.; Thanisawanyangkura, S.; Marquier, A.; Dreyer, E. Photosynthetic capacity and temperature responses of photosynthesis of rubber trees (Hevea brasiliensis Müll. Arg.) acclimate to changes in ambient temperatures. Trees 2009, 23, 357–365. [Google Scholar] [CrossRef]
- Mangmeechai, A. Effects of Rubber Plantation Policy on Water Resources and Land Use Change in the Northeastern Region of Thailand. Geogr. Environ. Sustain. 2020, 13, 73–83. [Google Scholar] [CrossRef]
- Do, F.C.; Pierret, A.; Couteron, P.; Lesturgez, G.; Boithias, L.; Isarangkool Na Ayutthaya, S.; Junjittakarn, J.; Gonkhamdee, S.; Maeght, J.L.; Hartmann, C.; et al. Spatial Distribution of Hevea brasiliensis Trunk Phloem Necrosis within a Plot: Aggregation but No Evidence of Constraint on Cumulated Growth. For. Pathol. 2011, 41, 90–100. [Google Scholar] [CrossRef]
- Sangchanda, N.; Isarangkool Na Ayutthaya, S.; Meetha, S.; Songsri, P. The Influence of Rainfall on Growth of Rubber Trees in Marginal Area of Northeast Thailand. Adv. Mater. Res. 2014, 844, 7–10. [Google Scholar] [CrossRef]
- Sugebo, B.; Yebeyen, D.; Adugna, A. Latex Yield Variation among Hevea brasiliensis Clones Grown under the Agro-Climate of South-West Ethiopia. Trees For. People 2022, 9, 100285. [Google Scholar] [CrossRef]
- Peries, O.S.; Brohier, Y.E.M. A Virus as Causal Agent of Bark Cracking in Hevea brasiliensis. Nature 1965, 205, 624–625. [Google Scholar] [CrossRef]
- Peries, O.S. The Etiology of “Bark Cracking” Disease of Hevea brasiliensis. Plant Dis. Rep. 1977, 61, 946–948. [Google Scholar]
- Pellegrin, F.; Nandris, D.; Chrestin, H.; Duran-Vila, N. Rubber Tree (Hevea brasiliensis) Bark Necrosis Syndrome I: Still No Evidence of a Biotic Causal Agent. Plant Dis. 2004, 88, 1046. [Google Scholar] [CrossRef]
- Pellegrin, F.; Duran-Vila, N.; Van Munster, N.; Nandris, D. Rubber Tree (Hevea brasiliensis) Trunk Phloem Necrosis: Aetiological Investigations Failed to Confirm Any Biotic Causal Agent. For. Pathol. 2007, 37, 9–21. [Google Scholar] [CrossRef]
- Buakong, W.; Suwanmanee, P.; Ruttajorn, K.; Assawatreerathakul, K.; Leak, J.E. Improving Rubber Production and Tapping Panel Dryness in Hevea Tree Using Organic Fertiliser and Micronutrients. J. Sustain. Sci. Manag. 2023, 20, 1275–1292. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, D.M.; Abraham, T.; Mathew, J.; Ramachandran, P.; Malathi, V.G. Determination of biotic aetiology of tapping panel dryness (TPD) syndrome of rubber tree (Hevea brasiliensis) by return-polyacrylamide gel electrophoresis (R-PAGE) technique. Arch. Phytopathol. Plant Prot. 2013, 46, 710–720. [Google Scholar] [CrossRef]
- Nandris, D.; Nicole, M.; Geiger, J.P.; Fernandez, D. Bark Necrosis and Other Abnormal Syndromes of Hevea brasiliensis in Relation to Clonal Susceptibility, Latex Harvesting, and Environment. In Natural Rubber: Biology, Cultivation and Technology; Sethuraj, M.R., Mathew, N.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 326–349. [Google Scholar]
- de Fay, E.; Jacob, J.L. The Bark Dryness Disease (Brown-Bast) of Hevea: Symptomatology, Histological and Cytological Aspects. In Physiology of Rubber Tree Latex; Auzac, J., Jacob, J.L., Chrestin, H., Eds.; CRC Press: Boca Raton, FL, USA, 1989; pp. 407–430. [Google Scholar]
- Jacob, J.; Krishnakumar, R. Tapping Panel Dryness Syndrome: What We Know and What We Do Not Know. In Tapping Panel Dryness of Rubber Trees; James, J., Krishnakumar, R., Mathew, N.M., Eds.; Rubber Research Institute of India: Kottayam, India, 2006; pp. 1–27. [Google Scholar]
- Liyanage, A.S.; Peries, O.S.; Liyanage, N.I.S.; Irugalbandara, Z.E.; Wetta, S.J.L.P. Annual Review; Rubber Research Institute of Sri Lanka: Agalawatta, Sri Lanka, 1983; pp. 55–56. [Google Scholar]
- Qi, D.L.; Zhou, J.N.; Xie, G.S.; Wu, Z.X. Studies on Rubber (Hevea brasiliensis) Trees Exist Plant Type after Planting and Available Tapping Tree of Rubber Plantation in China. Am. J. Plant Sci. 2014, 5, 3017–3021. [Google Scholar] [CrossRef]
- Paranjothy, K.; Yeang, H.Y. A Consideration on the Nature and Control of Brown Bast. In Proceedings of the RRIM Planters Conference, Kuala Lumpur, Malaysia, 17–19 October 1977; pp. 74–90. [Google Scholar]
- Putranto, R.-A.; Herlinawati, E.; Rio, M.; Leclercq, J.; Piyatrakul, P.; Gohet, E.; Sanier, C.; Oktavia, F.; Pirrello, J.; Montoro, P. Involvement of Ethylene in the Latex Metabolism and Tapping Panel Dryness of Hevea brasiliensis. Int. J. Mol. Sci. 2015, 16, 17885–17908. [Google Scholar] [CrossRef]
- Zhang, Y.; Leclercq, J.; Montoro, P. Reactive Oxygen Species in Hevea brasiliensis Latex and Relevance to Tapping Panel Dryness. Tree Physiol. 2017, 37, 261–269. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Sulaiman, Z.; Samad, M.Y.A.; Karim, S.M.R.; Salisu, M.A. Effect of Deficiency-Adjusted Macronutrients to Cure Brown Bast Syndrome in Rubber Tree (Hevea brasiliensis). Pertanika J. Trop. Agric. Sci. 2023, 46, 1309–1326. [Google Scholar] [CrossRef]
- Nandris, D.; Chrestin, H.; Geiger, J.P.; Nicole, M.; Thouvenel, J.C. Occurrence of a Phloem Necrosis on the Trunk of Rubber Tree. In Proceedings of the 75th Annual Conference of Rubber Research in Sri Lanka, Colombo, Sri Lanka, 17–19 September 1984; p. 59. [Google Scholar]
- Isarangkool Na Ayutthaya, S.; Do, F.C. Rubber Trees Affected by Necrotic Tapping Panel Dryness Exhibit Poor Transpiration Regulation under Atmospheric Drought. Adv. Mater. Res. 2014, 844, 3–6. [Google Scholar] [CrossRef]
- Suchartgul, S.; Maneepong, S.; Issarakrisila, M. Establishment of Standard Values for Nutritional Diagnosis in Soil and Leaves of Immature Rubber Tree. Rubber Thai J. 2012, 1, 19–31. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Chotiphan, R.; Vaysse, L.; Lacote, R.; Gohet, E.; Thaler, P.; Sajjaphan, K.; Bottier, C.; Char, C.; Liengprayoon, S.; Gay, F. Can Fertilization Be a Driver of Rubber Plantation Intensification? Ind. Crops Prod. 2019, 141, 111752. [Google Scholar] [CrossRef]
- Priyadarshan, P.M. Biology of Hevea Rubber; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Alle, J.Y.; Dick, E.A.; Soymahin, E.F.; Glaba, R.O.; Keli, J.Z.; Obouayeba, S. Effect of Mineral Fertilization on Agrophysiological Parameters and Economic Viability of Clone PB 235 of Hevea brasiliensis in the Region of GO in South Western Côte d’Ivoire. J. Anim. Plant Sci. 2015, 2, 3768–3780. [Google Scholar]
- Hashyati Nik, N.H.; Sulaiman, Z.; Salisu, M.A.; Samad, M.Y.A.; Mohamed, M.T.M.; Mokhtar, M.S.J.; Ghani, Z.A.B.; Adinan, A. Application of Different Rates and Frequencies of Rejuvenator for the Treatments of Brown Bast Syndrome on Hevea brasiliensis. Indian J. Agric. Res. 2022, 56, 469–473. [Google Scholar] [CrossRef]
- Nartvaranant, P.; Subhadrabandhu, S.; Whiley, A.W. Effect of Soil Boron Application on Gummosis and Leaf Boron Content of Mango (Mangifera indica L.) cvs. Khieo Sawoei and Nam Dok Mai. Acta Hortic. 2002, 575, 875–879. [Google Scholar] [CrossRef]
- Ma, X.; Liu, X.; Xiang, P.; Qiu, S.; Yuan, X.; Yang, M. Effects of the Contents of Mineral Elements on Gummosis in Prunus salicina Lindl. HortScience 2021, 56, 568–571. [Google Scholar] [CrossRef]
- Thitithanakul, S.; Ma, N.; Sukkawong, S.; Jaikrajang, B. Determination of Nitrogen and Phosphorus Requirement of the RRIM 600 and RRIT 251 Young Rubber Trees. Walailak J. Sci. Technol. 2016, 14, 571–580. [Google Scholar]
- Puwaphut, R.; Nakkaew, A.; Phongdara, A. Diversity among Three Cultivars (RRIM600, RRIT251, and PB350) of Hevea brasiliensis and Secondary Metabolite Production. Songklanakarin J. Sci. Technol. 2016, 38, 15–22. [Google Scholar] [CrossRef]
- Herlinawati, E.; Montoro, P.; Ismawanto, S.; Syafaah, A.; Aji, M.; Giner, M.; Flori, A.; Gohet, E.; Oktavia, F. Dynamic Analysis of Tapping Panel Dryness in Hevea brasiliensis Reveals New Insights on This Physiological Syndrome Affecting Latex Production. Heliyon 2022, 8, e10920. [Google Scholar] [CrossRef]
- Walkley, A.J.; Black, I.A. Estimation of Soil Organic Carbon by the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Roper, W.R.; Robarge, W.P.; Osmond, D.L.; Heitman, J.L. Comparing Four Methods of Measuring Soil Organic Matter in North Carolina Soils. Soil Sci. Soc. Am. J. 2019, 83, 466–474. [Google Scholar] [CrossRef]
- Gupta, U.C. Boron, Molybdenum, and Selenium. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; CRC Press: Boca Raton, FL, USA, 1993; pp. 91–99. [Google Scholar]
- Mills, H.A.; Jones, J.B. Plant Analysis Handbook II: A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Publishing: Athens, GA, USA, 1996. [Google Scholar]
- Kayaphad, B.; Isarangkool Na Ayutthaya, S.; Hongpakdee, P.; Siriwong, C. Comparison of Two Methods for the Determination of Nitrogen in Leaf and Yield in Banana, Jujube and Rubber Tree. Acta Hortic. 2021, 1312, 417–422. [Google Scholar] [CrossRef]
- Gaines, T.P.; Mitchell, G.A. Boron Determination in Plant Tissue by the Azomethine H Method. Commun. Soil Sci. Plant Anal. 1979, 10, 1099–1108. [Google Scholar] [CrossRef]
- Junaidi, N.; Nuringtyas, T.R.; Clément-Vidal, A.; Flori, A.; Syafaah, A.; Oktavia, F.; Ismawanto, S.; Aji, M.; Subandiyah, S.; Montoro, P. Analysis of Reduced and Oxidized Antioxidants in Hevea brasiliensis Latex Reveals New Insights into the Regulation of Antioxidants in Response to Harvesting Stress and Tapping Panel Dryness. Heliyon 2022, 8, e09840. [Google Scholar] [CrossRef]
- Nhean, S.; Isarangkool Na Ayutthaya, S.; Rathanawong, R.; Do, F.C. Immature Growth Performance of Three Important Rubber Tree (Hevea brasiliensis) Clones in a Drought-Prone Area. Aust. J. Crop Sci. 2020, 14, 469–474. [Google Scholar] [CrossRef]
- Onthong, J.; Khawmee, K.; Keawmano, C. Growth of Immature Rubber Trees Planted in Abandoned Paddy Field and Upland Areas in Relation to Soil Properties and Leaf Nutrients. Songklanakarin J. Sci. Technol. 2017, 39, 675–683. [Google Scholar]
- Perron, T.; Mareschal, L.; Laclau, J.-P.; Deffontaines, L.; Deleporte, P.; Masson, A.; Cauchy, T.; Gay, F. Dynamics of Biomass and Nutrient Accumulation in Rubber (Hevea brasiliensis) Plantations Established on Two Soil Types: Implications for Nutrient Management over the Immature Phase. Ind. Crops Prod. 2021, 159, 113073. [Google Scholar] [CrossRef]
- Chen, B.; Cao, J.; Wang, J.; Wu, Z.; Xie, G. Development and Implementation of Site-Specific Fertilizer Recommendation Model Based on Nutrient Balance for Rubber Plantation. Agron. J. 2011, 103, 464–471. [Google Scholar] [CrossRef]
- Hytonen, J.; Nurmi, J.; Kaakkurivaara, N.; Kaakkurivaara, T. Rubber Tree (Hevea brasiliensis) Biomass, Nutrient Content, and Heating Values in Southern Thailand. Forests 2019, 10, 638. [Google Scholar] [CrossRef]
- Njukeng, J.N.; Ehabe, E.E.; Nkeng, G.E.; Schick, J.; Kratz, S.; Schnug, E. Investigations on the Nutritional Status of Hevea brasiliensis Plantations in the Humid Forest Zone of Cameroon. Part 2: Establishment of Macronutrient Norms. J. Kult. 2013, 65, 376–384. [Google Scholar]
- Chen, S.; Peng, S.; Huang, G.; Wu, K.; Fu, X.; Chen, Z. Association of Decreased Expression of a Myb Transcription Factor with the TPD (Tapping Panel Dryness) Syndrome in Hevea brasiliensis. Plant Mol. Biol. 2003, 51, 51–58. [Google Scholar] [CrossRef]
| Clone | Symptom | N (mg g−1 DW) | P (mg g−1 DW) | K (mg g−1 DW) | Ca (mg g−1 DW) | B (μg g−1 DW) |
|---|---|---|---|---|---|---|
| RRIM600 | Healthy tree | 7.34 ± 0.85 ab | 2.31 ± 0.28 b | 1.97 ± 0.18 | 0.34 ± 0.03 a | 1.44 ± 0.05 b |
| TPD | 5.60 ± 1.34 b | 1.88 ± 0.33 b | 1.26 ± 0.30 | 0.19 ± 0.07 b | 1.31 ± 0.11 b | |
| TPN | 12.49 ± 3.22 a | 4.42 ± 1.06 a | 2.46 ± 0.48 | 0.40 ± 0.10 a | 1.74 ± 0.04 a | |
| F-test | * | * | ns | * | * | |
| RRIT251 | Healthy tree | 9.66 ± 0.72 a | 4.15 ± 0.43 | 1.72 ± 0.17 | 0.30 ± 0.03 | 1.35 ± 0.05 |
| TPD | 7.21 ± 0.70 ab | 3.42 ± 0.56 | 1.93 ± 0.29 | 0.29 ± 0.06 | 1.44 ± 0.13 | |
| TPN | 6.21 ± 1.47 b | 2.93 ± 0.66 | 1.19 ± 0.21 | 0.22 ± 0.04 | 1.30 ± 0.04 | |
| F-test | * | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriubon, S.; Wongcharoen, A.; Meetha, S.; Isarangkool Na Ayutthaya, S. Nutrient Variables Associated with Tapping Panel Dryness and Necrosis Syndromes in Rubber Tree Clones RRIM600 and RRIT251. Forests 2025, 16, 1477. https://doi.org/10.3390/f16091477
Sriubon S, Wongcharoen A, Meetha S, Isarangkool Na Ayutthaya S. Nutrient Variables Associated with Tapping Panel Dryness and Necrosis Syndromes in Rubber Tree Clones RRIM600 and RRIT251. Forests. 2025; 16(9):1477. https://doi.org/10.3390/f16091477
Chicago/Turabian StyleSriubon, Sujittra, Anan Wongcharoen, Somyot Meetha, and Supat Isarangkool Na Ayutthaya. 2025. "Nutrient Variables Associated with Tapping Panel Dryness and Necrosis Syndromes in Rubber Tree Clones RRIM600 and RRIT251" Forests 16, no. 9: 1477. https://doi.org/10.3390/f16091477
APA StyleSriubon, S., Wongcharoen, A., Meetha, S., & Isarangkool Na Ayutthaya, S. (2025). Nutrient Variables Associated with Tapping Panel Dryness and Necrosis Syndromes in Rubber Tree Clones RRIM600 and RRIT251. Forests, 16(9), 1477. https://doi.org/10.3390/f16091477








