Activity of Flavanols Extracted from Prosopis juliflora Mesquite on Growth Inhibition of Wood-Decaying Fungi and Their Synergistic Effect with Tebuconazole †
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Materials
2.3. Extraction and Purification of Mesquitol
2.3.1. Maceration Extraction
2.3.2. Purification
2.4. FTIR Analysis
2.5. NMR Analysis
2.6. Microorganisms
2.7. Fungal Growth Tests
2.8. Synergistic and Antagonistic Tests
3. Results
3.1. Extraction and Purification of Mesquitol
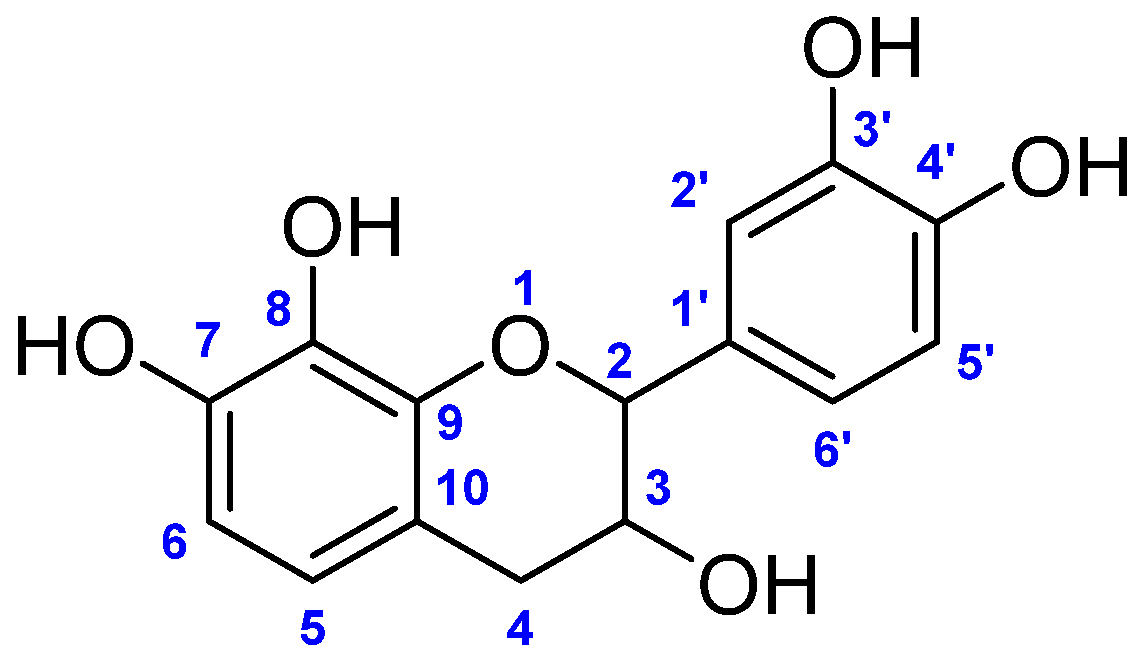
3.2. Characterization of Mesquitol
- 1H NMR (400 MHz, Acetone-d6) δ (ppm): 7.51 (1H, s, HOH), 7.48 (1H, s, HOH), 7.44 (1H, s, HOH), 7.31 (1H, s, HOH), 6.73–6.62 (3H, m, H2′, 5′, 6′), 6.27–6.25 (2H, m, H5, 6), 4.5 (1H, d, J 7.6 Hz, H2), 3.9 (1H, td, J 5.3 and 6.8 Hz, H3), 3.9 (1H, s, HOH), 2.8 (1H, dd, J 5.2 and 15.7 Hz, H4), 2.6 (1H, dd, J 8.5 and 15.7 Hz, H4).
- 13C NMR (400 MHz, Acetone-d6) δ (ppm): δ (ppm): 82.18 (C-2), 67.52 (C-3), 32.77 (C-4), 108.18 (C-5), 119.3 (C-6), 144.77.00 (C-7), 144.91 (C-8), 132.57 (C-9), 112.43 (C-10), 131.00 (C-1′), 119.05 (C-2′), 143.96 (C-3′), 142.66 (C-4′), 114.86 (C-5′), 114.35 (C-6′).
- FTIR (υ max cm−1): 3500–3100 (O-H stretch), 2950–2850 (C-H stretch, aliphatic), 1610, 1691 (C=C stretch, aromatic).
3.3. Fungal Growth Inhibition
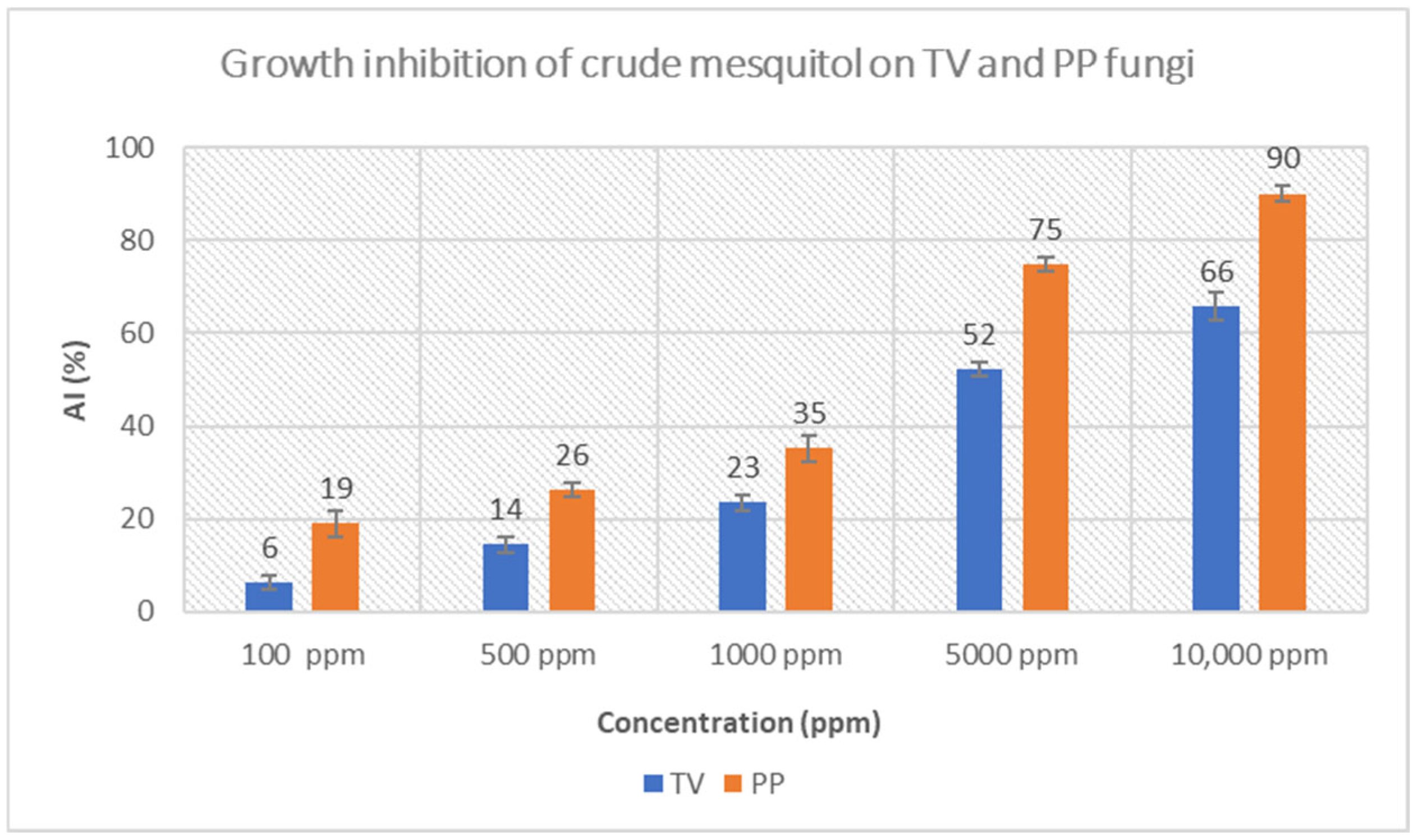
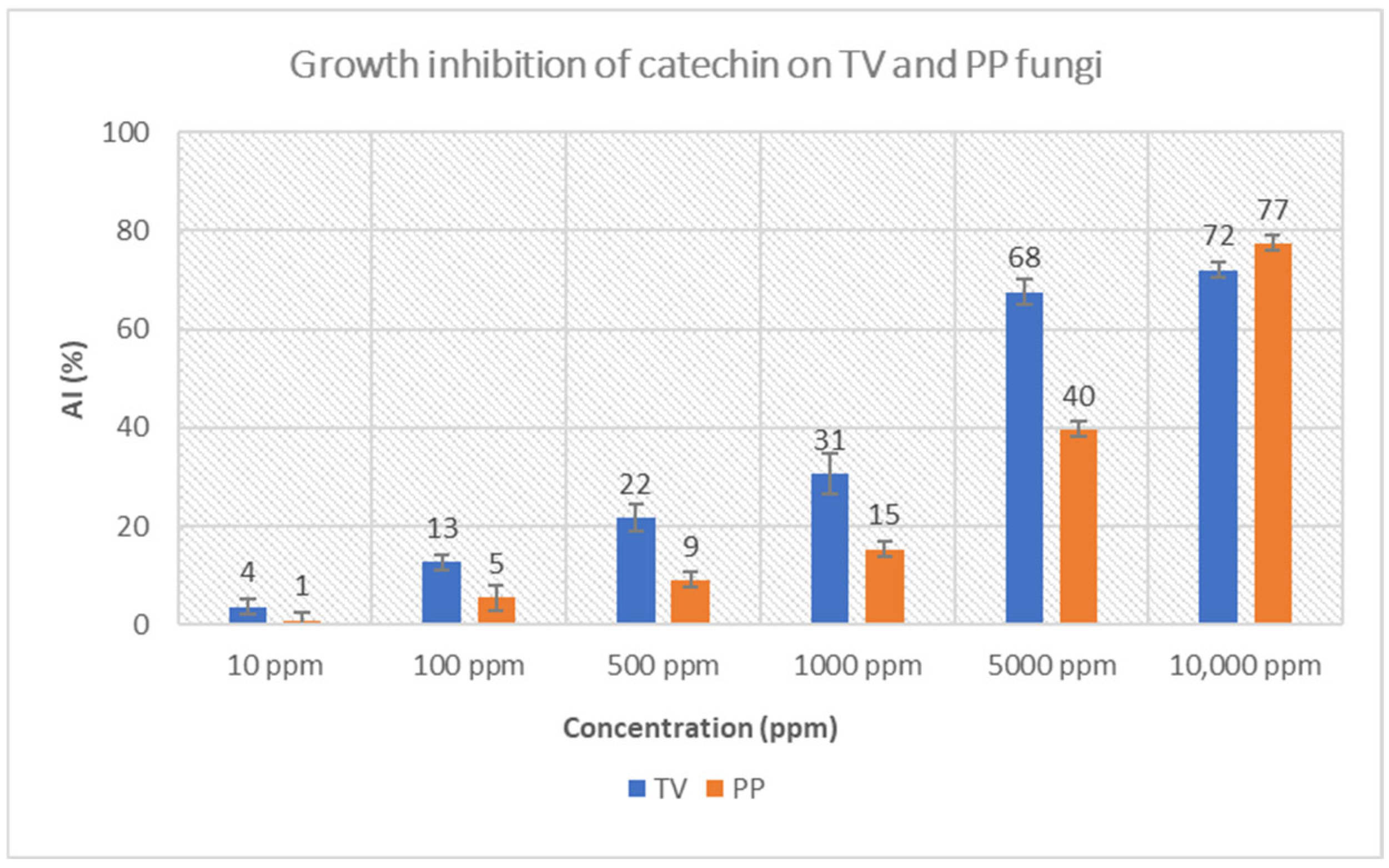
3.3.1. Crude Mesquitol, Pure Mesquitol and Catechin Growth Inhibition of Fungi
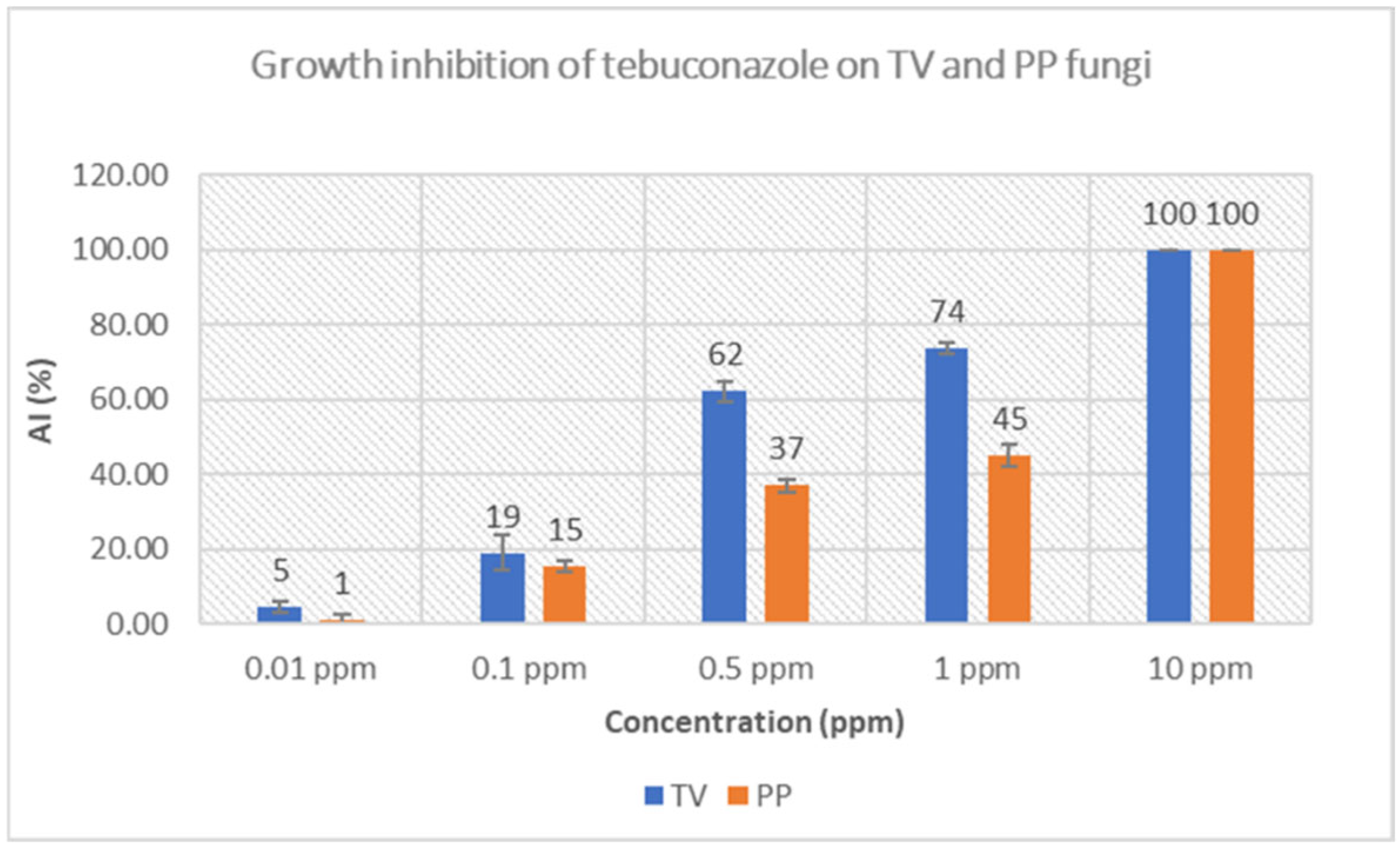
3.3.2. Tebuconazole Growth Inhibition of Fungi
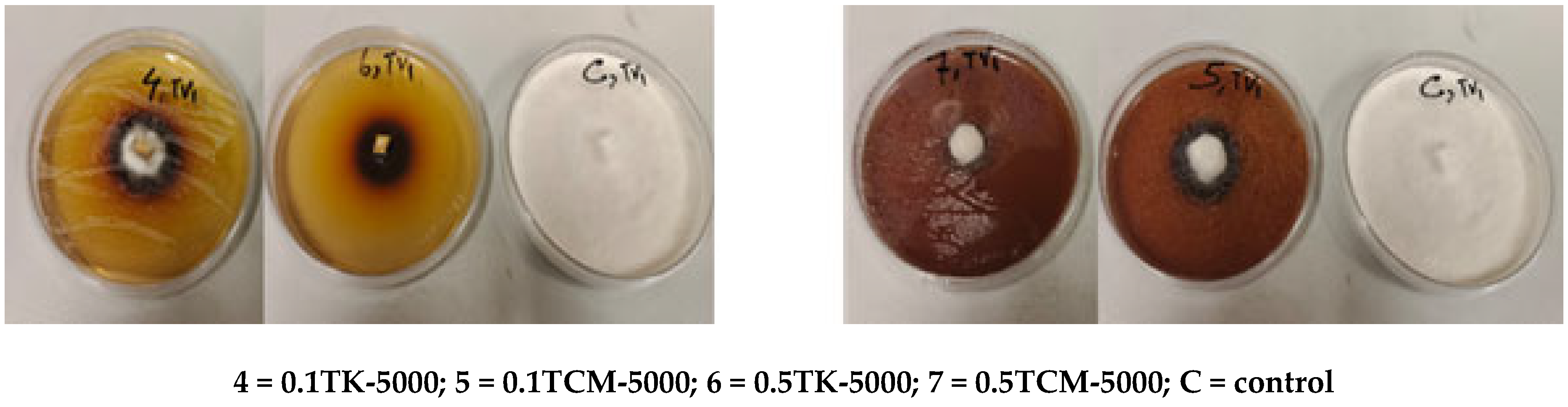
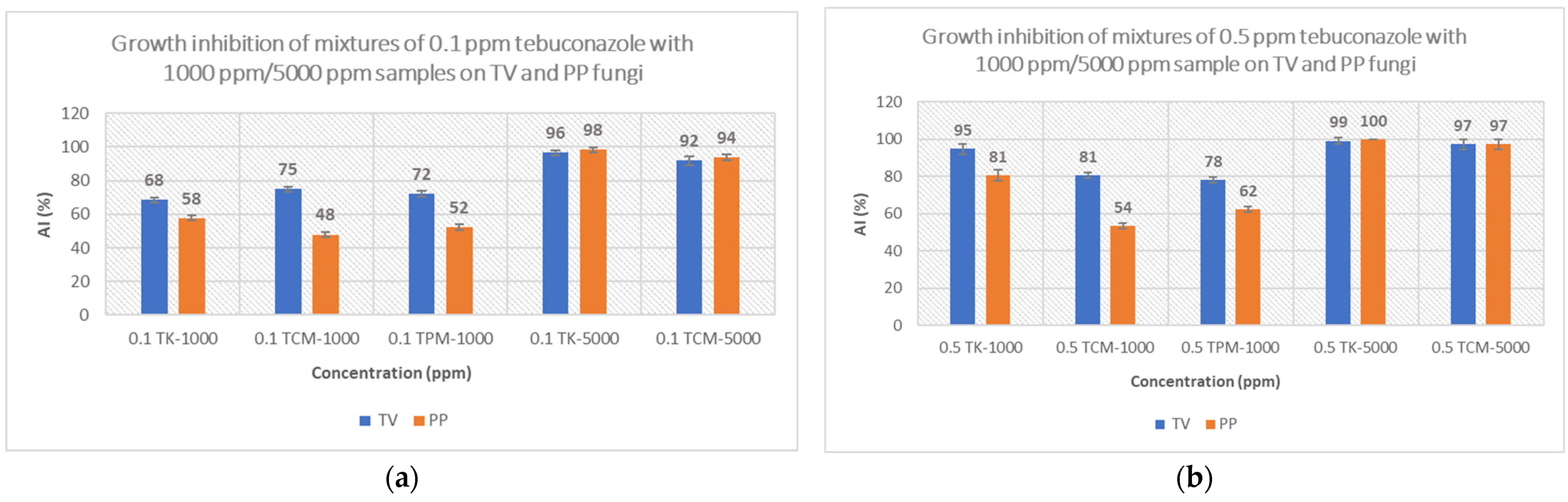
3.3.3. Growth Inhibition of Fungi on 1000 ppm/5000 ppm Sample Mixtures with 0.1 ppm/0.5 ppm Tebuconazole
3.4. Synergistic and Antagonistic Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1H NMR | proton nuclear magnetic resonance |
| 13C NMR | carbon-13 nuclear magnetic resonance |
| FTIR | Fourier transform infrared spectroscopy |
| RPM | revolutions per minute |
| ROS | reactive oxygen species |
| TLC | thin-layer chromatography |
| v/v | volume per volume |
Appendix A

References
- Krügener, K.; Ornik, J.; Jachim, R.; Kietz, B.; Petersen, K.; Mittleman, D.M.; Koch, M.; Viöl, W. Monitoring Fungus Infestation of Common Beech Wood Using Terahertz Radiation. Holzforschung 2020, 74, 635–641. [Google Scholar] [CrossRef]
- Leuschner, C.; Ellenberg, H. Ecology of Central European Forests: Vegetation Ecology of Central Europe; Springer: Cham, Switzerland, 2017; Volume 1. [Google Scholar]
- Leuschner, C.; Ellenberg, H. Ecology of Central European Non-Forest Vegetation: Coastal to Alpine, Natural to Man-Made Habitats: Vegetation Ecology of Central Europe; Springer: Cham, Switzerland, 2017; Volume 2. [Google Scholar]
- Couturier, M.; Berrin, J.-G. The Saccharification Step: The Main Enzymatic Components. In Lignocellulose Conversion: Enzymatic and Microbial Tools for Bioethanol Production; Springer: Berlin/Heidelberg, Germany, 2013; pp. 93–110. [Google Scholar]
- Arantes, V.; Goodell, B. Current Understanding of Brown-Rot Fungal Biodegradation Mechanisms: A Review. In Deterioration and Protection of Sustainable Biomaterials; ACS Publications: Washington, DC, USA, 2014; pp. 3–21. [Google Scholar]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose Degradation: An Overview of Fungi and Fungal Enzymes Involved in Lignocellulose Degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef]
- Fassina Brocco, V.; Gonçalves da Costa, L.; Monteiro de Castro, M.C.; Xavier Barbosa, A.V.; da Costa Lyra, P.H.; Alves Cruz da Conceição, R.C. Antifungal and Antitermitic Potential of Extracts of Industrial Wood Waste from Central Amazon, Brazil. Maderas Cienc. Tecnol. 2025, 27, e0825. [Google Scholar]
- Díaz Dellavalle, P.; Cabrera, A.; Alem, D.; Larrañaga, P.; Ferreira, F.; Dalla Rizza, M. Actividad Antifúngica de Extractos de Plantas Medicinales Contra El Hongo Fitopatógeno Alternaria spp. Chil. J. Agric. Res. 2011, 71, 231–239. [Google Scholar]
- Harris, C.A.; Renfrew, M.J.; Woolridge, M.W. Assessing the Risks of Pesticide Residues to Consumers: Recent and Future Developments. Food Addit. Contam. 2001, 18, 1124–1129. [Google Scholar] [CrossRef]
- Kirker, G.T.; Hassan, B.; Mankowski, M.E.; Eller, F.J. Critical Review on the Use of Extractives of Naturally Durable Woods as Natural Wood Protectants. Insects 2024, 15, 69. [Google Scholar] [CrossRef]
- Gérardin, P.; Hentges, D.; Gérardin, P.; Vinchelin, P.; Dumarçay, S.; Audoin, C.; Gérardin-Charbonnier, C. Knotwood and Branchwood Polyphenolic Extractives of Silver Fir, Spruce and Douglas Fir and Their Antioxidant, Antifungal and Antibacterial Properties. Molecules 2023, 28, 6391. [Google Scholar] [CrossRef] [PubMed]
- Joseleau, J.-P.; Ruel, K.; Daouïa, M.; Chopinet, F.; Cardia, J.-D. Plant Polyphenolic Extracts as Natural Pesticides for Wood Protection Treatments. In Proceedings of the American Wood Protection Association: 118th AWPA Meeting, Charleston, SC, USA, 15–17 May 2022. [Google Scholar]
- Gisi, U. Synergistic Interaction of Fungicides in Mixtures. Phytopathology 1996, 86, 1273–1279. [Google Scholar]
- Chaachouay, N. Synergy, Additive Effects, and Antagonism of Drugs with Plant Bioactive Compounds. Drugs Drug Candidates 2025, 4, 4. [Google Scholar] [CrossRef]
- Bittick, S.J. Simultaneous Synergist, Antagonistic, and Additive Interactions between Multiple Local Stressors All Degrade Algal Turf Communities on Coral Reefs. J. Ecol. 2017, 106, 1390–1400. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P. Combining Biocontrol Agents with Chemical Fungicides for Integrated Plant Fungal Disease Control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Ribera, A.E.; Zuñiga, G. Induced Plant Secondary Metabolites for Phytopatogenic Fungi Control: A Review. J. Soil Sci. Plant Nutr. 2012, 12, 893–911. [Google Scholar] [CrossRef]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary Metabolites in Fungus-Plant Interactions. Front. Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef] [PubMed]
- Hammel, K.E.; Kapich, A.N.; Jensen, K.A., Jr.; Ryan, Z.C. Reactive Oxygen Species as Agents of Wood Decay by Fungi. Enzyme Microb. Technol. 2002, 30, 445–453. [Google Scholar] [CrossRef]
- Gao, C.; Cui, X.; Matsumura, J. Multidimensional Exploration of Wood Extractives: A Review of Compositional Analysis, Decay Resistance, Light Stability, and Staining Applications. Forests 2024, 15, 1782. [Google Scholar] [CrossRef]
- Castaño, J.D.; Zhang, J.; Anderson, C.E.; Schilling, J.S. Oxidative Damage Control during Decay of Wood by Brown Rot Fungus Using Oxygen Radicals. Appl. Environ. Microbiol. 2018, 84, e01937-18. [Google Scholar] [CrossRef]
- Maraveas, C.; Bayer, I.S.; Bartzanas, T. Recent Advances in Antioxidant Polymers: From Sustainable and Natural Monomers to Synthesis and Applications. Polymers 2021, 13, 2465. [Google Scholar] [CrossRef]
- Kijpornyongpan, T.; Schwartz, A.; Yaguchi, A.; Salvachúa, D. Systems Biology-Guided Understanding of White-Rot Fungi for Biotechnological Applications: A Review. iScience 2022, 25, 104640. [Google Scholar] [CrossRef]
- Sigoillot, J.-C.; Berrin, J.-G.; Bey, M.; Lesage-Meessen, L.; Levasseur, A.; Lomascolo, A.; Record, E.; Uzan-Boukhris, E. Fungal Strategies for Lignin Degradation. Adv. Bot. Res. 2012, 61, 263–308. [Google Scholar]
- Das, A.; Choudhury, S.; Gopinath, V.; Majeed, W.; Chakraborty, S.; Bhairavi, K.S.; Chowdhury, S.; Dubey, V.K.; Akhtar, M.S. Functions of Flavonoids in Plant, Pathogen, and Opportunistic Fungal Interactions. In Opportunistic Fungi, Nematode and Plant Interactions: Interplay and Mechanisms; Springer: Berlin/Heidelberg, Germany, 2024; pp. 91–123. [Google Scholar]
- Koumbi Mounanga, T.; Gérardin, P.; Poaty, B.; Perrin, D.; Gérardin, C. Synthesis and Properties of Antioxidant Amphiphilic Ascorbate Salts. Colloids Surf. Physicochem. Eng. Asp. 2008, 318, 134–140. [Google Scholar] [CrossRef]
- Schultz, T.P.; Nicholas, D.D. Development of Environmentally-Benign Wood Preservatives Based on the Combination of Organic Biocides with Antioxidants and Metal Chelators. Phytochemistry 2002, 61, 555–560. [Google Scholar] [CrossRef]
- Mabicka, A.; Dumarçay, S.; Rouhier, N.; Linder, M.; Jacquot, J.P.; Gérardin, P.; Gelhaye, E. Synergistic Wood Preservatives Involving EDTA, Irganox 1076 and 2-Hydroxypyridine-N-Oxide. Int. Biodeterior. Biodegrad. 2005, 55, 203–211. [Google Scholar] [CrossRef]
- Jin, Y.-S. Recent Advances in Natural Antifungal Flavonoids and Their Derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 126589. [Google Scholar] [CrossRef]
- Deresa, E.M.; Diriba, T.F. Phytochemicals as Alternative Fungicides for Controlling Plant Diseases: A Comprehensive Review of Their Efficacy, Commercial Representatives, Advantages, Challenges for Adoption, and Possible Solutions. Heliyon 2023, 9, e13810. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ceja, A.; Loeza-Lara, P.D.; Espinosa-García, F.J.; García-Rodríguez, Y.M.; Medina-Medrano, J.R.; Gutiérrez-Hernández, G.F.; Ceja-Torres, L.F. In Vitro Antifungal Activity of Plant Extracts on Pathogenic Fungi of Blueberry (Vaccinium sp.). Plants 2021, 10, 852. [Google Scholar] [CrossRef]
- Mwangi, E.; Swallow, B. Invasion of Prosopis Juliflora and Local Livelihoods: Case Study from the Lake Baringo Area of Kenya; ICRAF Working Paper No. 3; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2005. [Google Scholar]
- Mbaabu, P.R. Prosopis Juliflora Invasion in Baringo, Kenya: Exploring Its Impacts on LULC Dynamics, Vachellia Tortilis, Selected Ecosystem Services, Livelihoods and Potential Spread Under Climate Change. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 2023. [Google Scholar]
- Schwartzstein, P. An Invasive, Thorny Tree Is Taking over Africa—Can It Be Stopped; National Geographic: Washington, DC, USA, 2019. [Google Scholar]
- Pasiecznik, N. Prosopis Juliflora (Mesquite). Cabi Digital Library. 2017. Available online: https://www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.28113 (accessed on 15 May 2025).
- Maundu, P.; Kibet, S.; Morimoto, Y.; Imbumi, M.; Adeka, R. Impact of Prosopis Juliflora on Kenya’s Semi-Arid and Arid Ecosystems and Local Livelihoods. Biodievrsity 2009, 10, 33–50. [Google Scholar] [CrossRef]
- Sirmah, P.; Dumarçay, S.; Masson, E.; Gerardin, P. Unusual Amount of (−)-Mesquitol from the Heartwood of Prosopis Juliflora. Nat. Prod. Res. 2009, 23, 183–189. [Google Scholar] [CrossRef]
- Chepkwony, S.C.; Dumarçay, S.; Chapuis, H.; Kiprop, A.; Gerardin, P.; Gerardin-Charbonnier, C. Geographic and Intraspecific Variability of Mesquitol Amounts in Prosopis Juliflora Trees from Kenya. Eur. J. Wood Wood Prod. 2020, 78, 801–809. [Google Scholar] [CrossRef]
- Sirmah, P.; Mburu, F.; Iaych, K.; Dumarcay, S.; Gerardin, P. Potential Antioxidant Compounds from Different Parts of Prosopis Juliflora. J. Trop. For. Sci. 2011, 23, 187–195. [Google Scholar]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Naik, N.M.; Krishnaveni, M.; Mahadevswamy, M.; Bheemanna, M.; Nidoni, U.; Kumar, V.; Tejashri, K. Characterization of Phyto-Components with Antimicrobial Traits in Supercritical Carbon Dioxide and Soxhlet Prosopis Juliflora Leaves Extract Using GC-MS. Sci. Rep. 2023, 13, 4064. [Google Scholar] [CrossRef] [PubMed]
- Owino, J.; Tuimising, J.; Mangin, F.; Gérardin, P.; Kiprop, A.; Gérardin-Charbonnier, C. Synergistic effect of the association of Prosopis juliflora polyphenolic extractives with tebuconazole on the growth inhibition of brown and white rot fungi: A solution to increase the naturality and safety of wood preservation treatment. In Proceedings of the IRG56 Scientific Conference on Wood Protection, Yokohama, Japan, 22–26 June 2025; 10p. [Google Scholar]
- Ivic, D. Curative and Eradicative Effects of Fungicides. In Fungicides; IntechOpen: London, UK, 2010; pp. 3–22. [Google Scholar]
- Hervay, N.T.; Elias, D.; Habova, M.; Jacko, J.; Morvova, M., Jr.; Gbelska, Y. Catechin Potentiates the Antifungal Effect of Miconazole in Candida Glabrata. Folia Microbiol. 2023, 68, 835–842. [Google Scholar] [CrossRef]
- Yamaji, K.; Ichihara, Y. The Role of Catechin and Epicatechin in Chemical Defense against Damping-off Fungi of Current-year Fagus Crenata Seedlings in Natural Forest. For. Pathol. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Bopenga Bopenga, C.S.A.; Dumarçay, S.; Edou Engonga, P.; Gérardin, P. Relationships between Chemical Composition and Decay Durability of Coula Edulis Baill as an Alternative Wood Species in Gabon. Wood Sci. Technol. 2020, 54, 329–348. [Google Scholar] [CrossRef]
- Chang, S.-T.; Wang, S.-Y.; Wu, C.-L.; Su, Y.-C.; Kuo, Y.-H. Antifungal Compounds in the Ethyl Acetate Soluble Fraction of the Extractives of Taiwania (Taiwania cryptomerioides Hayata) Heartwood; De Gruyter Brill: Berlin, Germany, 1999. [Google Scholar]
- Colby, S.R. Calculating Synergistic and Antagonistic Responses of Herbicide Combinations. Weeds 1967, 15, 20–22. [Google Scholar] [CrossRef]
- Aguiar, N.F.B. Tebuconazole and Azoxystrobin: Understanding the Fungicide Potential of the Combination Used in a Commercial Formulation. Master’s Thesis, Universidade de Aveiro, Aveiro, Portugal, 2021. [Google Scholar]
- Joaquín-Ramos, A.d.J.; López-Palestina, C.U.; Pinedo-Espinoza, J.M.; Altamirano-Romo, S.E.; Santiago-Saenz, Y.O.; Aguirre-Mancilla, C.L.; Gutiérrez-Tlahque, J. Phenolic Compounds, Antioxidant Properties and Antifungal Activity of Jarilla (Barkleyanthus Salicifolius ENT# 91; KunthENT# 93; H. Rob & Brettell). Chil. J. Agric. Res. 2020, 80, 352–360. [Google Scholar]
- Simonetti, G.; Brasili, E.; Pasqua, G. Antifungal Activity of Phenolic and Polyphenolic Compounds from Different Matrices of Vitis Vinifera L. against Human Pathogens. Molecules 2020, 25, 3748. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, J.; Huang, Y.; Liu, Y.; Hu, G.; Yan, W.; Yan, G.; Guo, Q.; Shi, J.; Han, R. Sustainable Pest Management Using Plant Secondary Metabolites Regulated Azadirachtin Nano-Assemblies. Nat. Commun. 2025, 16, 1721. [Google Scholar] [CrossRef]

| Solvent | Yield [%] |
|---|---|
| Cyclohexane | 0.27 ± 0.05 |
| Acetone | 5.86 ± 0.26 |
| X Sample | Y Sample | Combination | Expected Response (E) | Observed Response (O) | Comparison | Synergistic/Additive/Antagonistic Effect |
|---|---|---|---|---|---|---|
| 0.1 ppm T | 1000 ppm K | 0.1 TK-1000 | 44 | 68 | O > E | Synergistic |
| 0.1 ppm T | 1000 ppm CM | 0.1 TCM-1000 | 38 | 75 | O > E | Synergistic |
| 0.1 ppm T | 1000 ppm PM | 0.1 TPM-1000 | 64 | 72 | O > E | Synergistic |
| 0.5 ppm T | 1000 ppm K | 0.5 TK-1000 | 74 | 95 | O > E | Synergistic |
| 0.5 ppm T | 1000 ppm CM | 0.5 TCM-1000 | 71 | 81 | O > E | Synergistic |
| 0.5 ppm T | 1000 ppm PM | 0.5 TPM-1000 | 83 | 78 | E ≈ O | Additive |
| 0.1 ppm T | 5000 ppm K | 0.1 TK-5000 | 74 | 96 | O > E | Synergistic |
| 0.1 ppm T | 5000 ppm CM | 0.1 TCM-5000 | 61 | 92 | O > E | Synergistic |
| 0.5 ppm T | 5000 ppm K | 0.5 TK-5000 | 88 | 99 | O > E | Synergistic |
| 0.5 ppm T | 5000 ppm CM | 0.5 TCM-5000 | 82 | 97 | O > E | Synergistic |
| X Sample | Y Sample | Combination | Expected Response (E) | Observed Response (O) | Comparison | Synergistic/Additive/Antagonistic Effect |
|---|---|---|---|---|---|---|
| 0.1 ppm T | 1000 ppm K | 0.1 TK-1000 | 28 | 58 | O > E | Synergistic |
| 0.1 ppm T | 1000 ppm CM | 0.1 TCM-1000 | 45 | 48 | O > E | Synergistic |
| 0.1 ppm T | 1000 ppm PM | 0.1 TPM-1000 | 52 | 52 | E = O | Additive |
| 0.5 ppm T | 1000 ppm K | 0.5 TK-1000 | 46 | 81 | O > E | Synergistic |
| 0.5 ppm T | 1000 ppm CM | 0.5 TCM-1000 | 59 | 54 | E ≈ O | Additive |
| 0.5 ppm T | 1000 ppm PM | 0.5 TPM-1000 | 65 | 62 | E ≈ O | Additive |
| 0.1 ppm T | 5000 ppm K | 0.1 TK-5000 | 49 | 98 | O > E | Synergistic |
| 0.1 ppm T | 5000 ppm CM | 0.1 TCM-5000 | 79 | 94 | O > E | Synergistic |
| 0.5 ppm T | 5000 ppm K | 0.5 TK-5000 | 62 | 100 | O > E | Synergistic |
| 0.5 ppm T | 5000 ppm CM | 0.5 TCM-5000 | 84 | 97 | O > E | Synergistic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owino, J.I.; Tuimising, J.; Mangin, F.; Gerardin, P.; Kiprop, A.; Gerardin-Charbonnier, C. Activity of Flavanols Extracted from Prosopis juliflora Mesquite on Growth Inhibition of Wood-Decaying Fungi and Their Synergistic Effect with Tebuconazole. Forests 2025, 16, 1462. https://doi.org/10.3390/f16091462
Owino JI, Tuimising J, Mangin F, Gerardin P, Kiprop A, Gerardin-Charbonnier C. Activity of Flavanols Extracted from Prosopis juliflora Mesquite on Growth Inhibition of Wood-Decaying Fungi and Their Synergistic Effect with Tebuconazole. Forests. 2025; 16(9):1462. https://doi.org/10.3390/f16091462
Chicago/Turabian StyleOwino, John Isemeki, Judith Tuimising, Floriane Mangin, Philippe Gerardin, Ambrose Kiprop, and Christine Gerardin-Charbonnier. 2025. "Activity of Flavanols Extracted from Prosopis juliflora Mesquite on Growth Inhibition of Wood-Decaying Fungi and Their Synergistic Effect with Tebuconazole" Forests 16, no. 9: 1462. https://doi.org/10.3390/f16091462
APA StyleOwino, J. I., Tuimising, J., Mangin, F., Gerardin, P., Kiprop, A., & Gerardin-Charbonnier, C. (2025). Activity of Flavanols Extracted from Prosopis juliflora Mesquite on Growth Inhibition of Wood-Decaying Fungi and Their Synergistic Effect with Tebuconazole. Forests, 16(9), 1462. https://doi.org/10.3390/f16091462







