Abstract
Tree phenotypes vary because of genotype–climate interactions, and this variation influences host selection by tree-killing bark beetles. As climate-driven bark beetle outbreaks intensify, identifying phenotypic traits that best predict resistance or susceptibility is critical. We examined genetic variation, secondary chemistry, growth rates, and climate sensitivity in interior ponderosa pine (Pinus ponderosa var. scopulorum Engelm.) at two sites in the Black Hills—Devils Tower National Monument (DETO), Wyoming, and Wind Cave National Park (WICA), South Dakota—experiencing low-moderate levels of mountain pine beetle (Dendroctonus ponderosae Hopkins) activity. Genetic structure differed between sites. At DETO, large and small trees formed a single genetic cluster, whereas at WICA, two clusters emerged, one consisting of large trees and another comprising both small and large trees. The concentrations of some terpenes also differed between sites. Compared to beetle-killed trees, surviving trees exhibited distinct lifelong growth patterns and greater sensitivity to climate. Notably, surviving trees showed significant correlations of growth with climate variables, while beetle-killed trees were relatively insensitive. Long-term responsiveness of growth to climate was a stronger predictor of tree susceptibility to beetles than responses in years just before attacks occurred. These findings suggest trees with lower sensitivity to climate may be more vulnerable to beetle attack under changing climatic conditions.
1. Introduction
Tree-killing bark beetles are natural disturbance agents that contribute to the ecological integrity of conifer forests. However, increasingly severe outbreaks driven by climate change [1] have raised concerns that future outbreaks may become more damaging and less regenerative. This has heightened interest in understanding how climate influences tree resistance and susceptibility to bark beetle attack, and how forests may adapt to both a changing climate and escalating bark beetle pressure. Bark beetles are highly selective, targeting specific tree phenotypes that are strongly shaped by climatic conditions [2,3]. Many species preferentially attack weakened trees, and individuals poorly adapted to emerging climate regimes may be more likely to be attacked [2], potentially accelerating adaptive shifts within populations. To inform effective management strategies, we must better understand the extent of genetic variation in forest tree populations and how beetle host selection correlates with a tree’s response to climate. Addressing this challenge requires a multifaceted approach to identify which tree traits are most influenced by climate and most predictive of bark beetle susceptibility.
The mountain pine beetle (Dendroctonus ponderosae Hopkins, MPB) is an aggressive bark beetle that has killed millions of hectares of trees across western North America in recent outbreaks [4]. Unlike most other tree-killing bark beetles that specialize on one or two host tree species, MPB attacks most species of pine. However, the beetle shows preferences for some species, and, within a species, some trees are preferred over others [5,6,7,8]. Most of our understanding of the MPB’s ecology is from studies on lodgepole pine (Pinus contorta Douglas ex. Loudon), the beetle’s most common and co-evolved host, and on whitebark pine (P. albicaulis Engelm.) and jack pine (P. banksiana Lamb.), hosts that are increasingly exposed to the beetle due to climate change [9,10,11,12]. Surprisingly, MPB interactions with ponderosa pine (P. ponderosa Douglas ex. C. Lawson sensu lato), another common host, are little studied. This is particularly true for the eastern portion of the tree’s range that includes the Black Hills, a relatively isolated mountainous region traversing portions of South Dakota and Wyoming, USA.
Ponderosa pine is an iconic western North American tree occupying lower montane elevations with populations extending from southern British Columbia south to the Mexican border, westward to the Pacific coast, and eastward to the western Great Plains [13]. The tree exhibits considerable variability in climatic tolerances, morphology, genetics, and secondary chemistry [14,15,16,17] and consists of a number of distinct species or varieties depending on the traits under consideration [15,18]. A recent study using molecular methods found sufficient differentiation to split P. ponderosa sensu lato into four species that are currently classified as varieties: ponderosa pine (P. ponderosa var. ponderosa Engelm.), Pacific ponderosa pine (P. ponderosa var. benthamiana (Hartw.), Rocky Mountain ponderosa pine (P. ponderosa var. brachyptera Engelm.), and interior ponderosa pine (P. ponderosa var. scopulorum Engelm.) [19]. The current distribution of P. ponderosa sensu lato resulted mostly from a rapid northward migration post-glaciation in the late Holocene [13]. Only one of ten known mitochondrial haplotypes (P. ponderosa var. scopulorum haplotype 6) colonized the Black Hills, arriving as recently as 3900 yr bp [13,15]. Since that time, the tree has come to dominate forested areas of the region.
Management in the Black Hills is among the most intensive within the range of ponderosa pine, and few natural forests remain to use as references of how these ponderosa pine forests might have been structured or responded to natural disturbance in the absence of human influences [20,21]. Black Hills ponderosa pine forests have been subjected to long periods of fire suppression [21,22,23], and most Black Hills forests have been cut two to three times since the arrival of White settlers [20]. Such intense anthropogenic effects, the relatively short time this tree has existed in this region, and the fact that these forests appear to be restricted to one genetic haplotype, make it a challenging, yet interesting, system to investigate genetic diversity and structure, and interactions with insects and climate.
The community of bark beetles in interior ponderosa pine in the Black Hills differs from those in other, more westerly populations of ponderosa pine [24]. Western pine beetle (D. brevicomis LeConte), a major tree-killing bark beetle colonizing other varieties of ponderosa pine, does not occur in interior ponderosa pine and is absent in the Black Hills [25]. Instead, MPB is the primary tree-killing species. MPB found in interior ponderosa pine, including in the Black Hills, are genetically distinct and exhibit full to partial sterility when crossed with those from the west [26]. Indeed, genetic differences between “western” and “eastern” MPB are sufficient to consider them two species [27]. As such, they can be expected to exhibit different behaviors. While MPB can be a serious pest in other varieties of ponderosa pine, its outbreaks are typically short and of limited extent. However, in the Black Hills, MPB is a major agent of tree mortality, and it has developed several extensive outbreaks there [28].
Host selection by MPB is influenced by tree genetics, which affect traits such as secondary chemistry, growth rate, and physiological responses to climate [8,29,30,31,32]. MPB use a combination of cues to locate suitable host trees. Visual orientation to vertical silhouettes allows beetles to identify trees of appropriate size [33]. Although MPB are generally well adapted to the defensive chemistry of their host, these compounds and other secondary metabolites can still influence host choice [33]. Upon closer approach and landing, volatile organic compounds, including terpenes, provide additional cues for assessing host quality [6,34]. Once a beetle begins tunneling, it encounters resin, a constitutive defense that serves as both a physical barrier that can entomb or repel beetles and as a chemical defense via secondary metabolites. The composition and concentration of these secondary metabolites, as well as the quantity of resin produced, influence whether a beetle accepts or rejects a tree. These traits are shaped by both genetic factors and environmental influences on tree physiology [35,36,37].
The growth rate of trees is genetically controlled and responsive to environmental conditions [38]. Annual radial growth reflects individual tree responses to climatic conditions over time and provides insight into their susceptibility to insects and pathogens [32,39,40]. Although growth rate has been correlated with susceptibility to attack, MPB preference for slow- or fast-growing trees has proven inconsistent across pine species and even within populations of the same species. For example, in different populations of whitebark pine and lodgepole pine (P. contorta), MPB has preferentially attacked either faster or slower growing trees [32,40,41] or shown no preference [41]. In contrast, in western ponderosa pine, MPB has been observed to select slower growing individuals [42,43]. Furthermore, genetically distinct groups or age cohorts within populations can exhibit substantially different growth rates [32]. These findings highlight the need to understand better the relationship between genetic diversity, tree growth, and susceptibility to MPB.
In this study, we investigated genetic and chemical profiles, growth rates, and climate sensitivity of interior ponderosa pine in stands experiencing low-moderate MPB activity. Our specific objectives were to: (1) investigate genetic diversity in the tree within and among sites, (2) assess constitutive tree defenses (terpene concentrations and resin flow), and (3) compare tree growth rates and sensitivity to climatic conditions between MPB-killed and neighboring living trees.
2. Methods
2.1. Locations
The study was conducted at Devils Tower National Monument (DETO), WY (44.5902° N, 104.7146° W), and Wind Cave National Park (WICA), SD (43.55647° N, 103.47829° E), USA. Sampling was conducted at DETO in July 2016 and 2017 and at WICA in July 2016. The two sites lie approximately 190 km apart and experienced scattered mortality due to MPB (5%–10% of total mature interior ponderosa pine) over the three years previous to sampling (estimated using National Park Service (NPS) site-specific surveys). Both comprised mixed-age interior ponderosa pine-dominated forests with trees ranging from small saplings to large diameter old growth and were relatively natural compared with other highly managed forests in the region. DETO is located at the northern margin (inclusive of the Bear Lodge Mountains) and WICA at the southern margin of the Black Hills. Historical records of mountain pine beetle outbreaks indicate that, over time, these two locations have experienced lower levels of mortality than the forests occupying the central portions of the Black Hills [44]. Indeed, in the period from 2000 to 2015, when a severe outbreak occurred in the region, trees at DETO and WICA experienced substantially lower mortality [44]. Lower beetle populations in these locations increased the likelihood that beetles chose trees with particular phenotypes rather than more randomly due to high levels of aggregation pheromones (although genetic differences can still override pheromone attraction and result in targeted selection [32]).
At DETO, maps of individual trees killed by MPB in 2015 were available from the NPS. DETO occupies a relatively small area (545 ha), and all MPB-killed trees not located on steep slopes or used ceremonially by Native Americans were sampled. At WICA, general locations of MPB-killed trees were tracked by the NPS and trees were sampled in an area of the park (approx. 120 ha) that contained trees killed by MPB in 2015 (year of death estimated using foliage color and condition).
2.2. Sampling
MPB-killed trees were measured for diameter at breast height (DBH, 1.4 m above soil line) to develop a diameter distribution of trees preferred by the beetle at each site. Beetle-killed trees were then cored to the pith at 1.4 m on two opposing sides. Trees on steep slopes were avoided because of the difficulty of developing accurate growth rates for such trees [45]. For each beetle-killed tree, two nearest-neighbor living trees (hereafter neighbors) that fell within the diameter distribution of trees killed by MPB at the site were cored on opposing sides, avoiding dead areas and coring perpendicular to the slope when present. Needles or phloem (when needles could not be reached) were collected from each neighbor for DNA extraction. All trees killed by the beetle and, consequently, all neighbors sampled, were large/old. Therefore, to provide a better estimate of genetic diversity and to test for within-population genetic sub-structuring among regeneration cohorts, we also collected needles from smaller, mature trees (cone-bearing) (10–15 cm DBH) at each site along variable length transects extending through the same areas where beetle-killed and neighbor trees were sampled. The smaller trees were not aged, but as these were relatively open stands and the trees were not suppressed, they were assumed to be substantially younger than the large neighbor trees. Sample sizes varied by category and can be found in Table 1, Table 2, Table 3, Table 4 and Table 7.

Table 1.
Mean (SD) of resin mass (g), sapwood moisture (SWMC, %), phloem thickness (mm), basal area, DBH (diameter at breast height at 1.4 m, cm), and canopy density (CD, % cover) for neighbor interior ponderosa pine (P. ponderosa var. scopulorum Engelm.) sampled at Devils Tower National Monument, Wyoming (DETO) (2016 and 2017 combined) and Wind Cave National Park, South Dakota (WICA) (2016). Different letters indicate significant differences for a variable between sites (p ≤ 0.05).

Table 2.
Pearson product correlation coefficients for comparisons between canopy density (CD, % cover), basal area, DBH (diameter (cm) at 1.4 m), sapwood moisture content (SWMC, %), resin mass (g), and phloem thickness (mm) for neighbor interior ponderosa pine (P. ponderosa var. scopulorum Engelm.) sampled at Devils Tower National Monument, Wyoming (DETO) (2016 and 2017 combined) and Wind Cave National Park, South Dakota (WICA) (2016).

Table 3.
Mean (SD) for canopy density (% cover), basal area, DBH (diameter at 1.4 m, cm), for beetle-killed and living neighbor interior ponderosa pine (P. ponderosa var. scopulorum Engelm.) sampled at Devils Tower National Monument, Wyoming (DETO) (2016 and 2017 combined) and Wind Cave National Park, South Dakota (WICA) (2016). Different letters indicate significant differences for a variable between beetle-killed and neighbor trees within sites (p ≤ 0.05).

Table 4.
Results of logistic regression of beetle-killed vs. neighbor interior ponderosa pine (P. ponderosa var. scopulorum Engelm.) for canopy density (CD, % cover), basal area, and DBH (diameter at 1.4 m, cm) for Devils Tower National Monument, Wyoming (DETO) (2016 and 2017 combined) and Wind Cave National Park, South Dakota (WICA) (2016). Bold values indicate significance differences (p ≤ 0.05).
A 2.5 cm arch punch was used to collect discs of phloem from two sides of each neighbor for chemical analysis. The thickness of phloem was measured on each disc and then the discs were stored in sealed glass vials and held on dry ice until they could be stored at −80 °C prior to chemical analysis. At the same time, sapwood moisture content (SWMC) was measured for each tree by inserting the probes of a hand-held protimeter (Protimeter Mini2000, BLD, Shanghai, China) into the sapwood where the bark disc had been removed. Then, resin flow was measured from each neighbor tree by attaching plastic funnels under the area where the phloem discs were removed and inserting pre-weighed vials. The vials were retrieved after 24 h and re-weighed to determine resin mass. Canopy density and basal area (BA) were measured at the north side of each tree using a convex densitometer and a 10 BA prism, respectively.
2.3. Genetic Profiling
Three to five needles or a small phloem block from each sample were ground to a fine powder in liquid nitrogen. DNA was extracted from the powder using a Qiagen DNeasy plant extraction kit (Qiagen, Redwood City, CA, USA) following the producer’s protocol. Inter-simple sequence repeats (ISSRs) were used to develop genetic profiles for each tree. ISSRs are variable regions within microsatellite regions [46]. They are highly reproducible and effective for detecting differences among individuals within and among populations. Three ISSR primers were used: 17899A (GTG TGT GTG TGT CA), UBC 818 (CAC ACA CAC ACA CAC AG), and UBC 809 (AGA GAG AGA GAG AGA GG) [46]. A 25 µL reaction mixture was used for PCR combining 12.5 µL Promega Master Mix (Promega, Madison, WI, USA), 2.5 µL RNA-free water, 8 µL 0.5 M primer, and 2 µL DNA template. Reactions were run with one cycle of denaturation at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 1 min, annealing at 47 °C for 2 min, and extension at 72 °C for 1 min [46].
PCR products were visualized in a 1% agarose gel using 1X Tris-borate-EDTA buffer (TBE) (Sigma-Aldrich, Darmstadt, Germany) with the addition of 2 µL ethidium bromide per 100 mL gel mixture. A 100 bp ladder (Promega, Valencia, CA, USA) was added to one lane of each gel for scoring band size. Each gel lane received 5 µL amplified PCR product with bromophenol blue as a running dye and was then run with 1X TBE buffer at 70 mA. Gels were photographed under UV light and then bands were scored manually using only unambiguous clear bands. A subset of 20% of the samples were run a second time to check for consistency in band resolution.
2.4. Terpene Analysis
Phloem samples were analyzed for terpenes at the Montana State University Spectrophotometry Lab (Bozeman, MT, USA) as described in Six et al. [32]. Briefly, phloem was coarsely ground and placed into n-hexane (1 mL/mg) for 1 h. Gas chromatography (GC)-mass spectrometry (MS) was then performed on the extracts using an Agilent 7890A GC (Santa Clara, CA, USA) coupled with Agilent 5975C inert XL EI MSD (Santa Clara, CA, USA) with a triple axis detector. Separation of terpenoids was done using a Phenomenex Zebron ZB-5MS non-polar column (Hyderabad, India). Individual terpenoids were identified by comparing their retention times to those of pure standards and mass spectra from the NIST library (2014) using Agilent Masshunter 8.0 software. Concentrations of each compound in a sample were calculated using six-point calibration curves with known amounts of each standard (Restek Corp., Bellefonte, PA, USA).
2.5. Tree Core Preparation and Measurements
Cores were dried, mounted, and prepped according to standard dendrochronology methods [45]. Ring widths were measured to 0.001 mm on a Velmex measuring stage (Velmex, Bloomfield, NY, USA) using J2X software (v5.0) (Voortech Consulting, Holderness, NH, USA). Cross-dating was conducted visually with plots starting with year of mortality (beetle-killed trees) and year of sampling (neighbor trees). Prior to analysis, BAI was normalized by basal area (BAI/BA) to account for differences in tree size and to provide a more precise estimate of growth in response to climate [47]. Cores that contained rot, numerous false rings, or that could not be cross-dated were excluded.
2.6. Data Analysis
2.6.1. Site and Tree Characteristics
Summary statistics and Pearson product correlation analyses for the variables DBH, canopy density, basal area, SWMC, and thickness of phloem and logistic regression of beetle-killed vs. neighbors for basal area, DBH, and canopy density were performed using Prism GraphPad Software v. 9.1 (San Diego, CA, USA).
2.6.2. Genetic Analysis
A combined binary matrix (band present = 1, band absent = 0) for bands resolved using the three primers was used to conduct analysis of molecular variance (AMOVA) for tree category (site, neighbor, smaller mature) and principal coordinate analysis (PCoA) to explore population (here defined as WICA neighbors, DETO neighbors, WICA smaller mature trees, and DETO smaller mature trees) sub-structuring using GenAIEx 6.5 [48].
2.6.3. Chemistry
T-tests were used to compare terpenoid production between sites. Principle component analysis (PCA) using standardized data and parallel analysis with 1000 simulations was conducted on terpene data by site (WICA and DETO neighbors). Only trees sampled in 2016 were included, as a freezer failure resulted in the loss of 2017 samples. All analyses were conducted using Prism GraphPad v. 9.1.
2.6.4. Tree Growth and Climate Analysis
Climate data from the West Wide Drought Tracker (4 km resolution) [49] located using site-specific centroid coordinates (reported above) were used to investigate the effects of various climate variables on tree growth. To include the most trees, we focused on the time period from 1925 to 2015 (the year of beetle attack on trees sampled). Trees with cores extending to at least 1922 were included. Mean growth of the two cores for each tree were used in all analyses. The climate variables we used were mean annual (MA) and growing season (GS) (April–September) temperature, precipitation, and Palmer Drought Severity Index (PDSI). PDSI is a drought index that uses temperature and precipitation data to estimate the departure from average of soil moisture conditions. Most precipitation falls in this region in late spring and summer, so the April–September period captures most precipitation as well as that which is newly available to trees during growth. Paired t-tests were used to compare the MA growth of beetle-killed and neighbor trees. At each site, Pearson product correlation analyses were used to investigate how the various climate variables influenced growth and whether mean growth of neighbors and beetle-killed trees differed in response to climate over the long time series (1925–2015) and over a shorter series just preceding beetle-caused tree mortality (2000–2015). Then, because mean growth can mask subsets of trees within a category or population that respond differently to climate, we ran correlations for individual neighbors and beetle-killed trees with the same climate variables. Each of these analyses was followed by running the same correlations with a one-year lag to see whether conditions in the previous year affected the next year’s growth and susceptibility to beetle attack.
3. Results
3.1. Tree Characteristics
Summary statistics for neighbor trees at each site are presented in Table 1. Resin production was very low, and resin mass from neighbors did not differ between the two sites (p = 0.54, t = 0.62, df = 49) (Table 1). At DETO, no measurable resin was produced by any of the trees in 2016 and by only four trees out of 14 in 2017, and of those, the amount was very low for three trees. Neighbors at WICA were only measured for resin mass in 2016. At that time, 12 of 14 trees produced some resin, but amounts were very low for all but one tree. SWMC (p = 0.38, t = 0.89, df = 49), phloem thickness (p = 0.54, t = 0.62, df = 49), and basal area (p = 0.11, t = 1.165, df = 49) were similar between the sites (Table 1). Neighbors possessed larger DBH at DETO than at WICA (p = 0.0004, t = 3.81, df = 49) but canopy density was similar between sites (p = 0.07, t = 1.84, df = 49) (Table 1).
Pearson product correlation coefficients for comparisons of neighbor tree characteristics within sites are shown in Table 2. At both DETO and WICA, basal area and canopy density were positively correlated. The strongest correlation was a negative correlation between canopy density and SWMC at DETO; however, there was no correlation between these variables at WICA. At both sites, SWMC and phloem thickness were moderately positively correlated. Phloem thickness was negatively correlated with canopy density at DETO but not at WICA. Resin mass was only correlated with phloem thickness at WICA.
Unpaired t-tests of canopy density of areas surrounding neighbors and beetle-killed trees were significant at WICA (p = 0.01, t = 2.79, df = 30) but not at DETO (p = 0.14, t = 1.52, df = 48) (Table 3). Basal area did not differ between neighbors and beetle-killed trees at either WICA (p = 0.25, t = 1.17, df = 30) or DETO (p = 0.79, t = 0.27, df = 48) (Table 3). Likewise, DBH of neighbors and beetle-killed trees were not different at WICA (p = 0.80, t = 0.26, df = 30) or DETO (p = 0.04, t = 2.16, df = 48) (Table 3). Logistic regressions for canopy density, basal area, and DBH of neighbors versus beetle-killed trees were also not significant except for canopy density at WICA (Table 4).
3.2. Genetic Profiles
AMOVA revealed 76% of genetic variation existed within and 24% among sites (DETO and WICA) supported by a PHiPT value (a measure of how much total genetic variation is due to differences between populations) of 0.238 (Table 5). The PCoA placed neighbors and smaller mature trees at DETO into a single cluster with a slight divergence between trees in these two categories (Figure 1). All DETO trees clustered separately from all WICA trees. Trees at WICA formed two clusters, one composed of only neighbors and another composed of both neighbors and smaller mature trees. There was a considerably greater spread among trees at WICA than at DETO. Overall, 35% of the variation in the data was explained by the first three axes of the PCoA.

Table 5.
AMOVA table for comparisons of inter-simple sequence repeats data for neighbor and smaller mature interior ponderosa pine (P. ponderosa var. scopulorum Engelm.) at Devils Tower National Monument, Wyoming (DETO) (2016 and 2017 combined) and Wind Cave National Park, South Dakota (WICA) (2016).
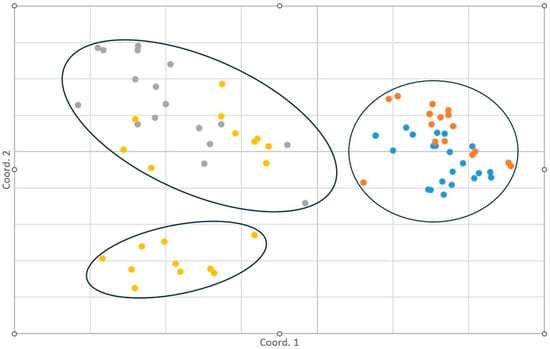
Figure 1.
Principal coordinate analysis (PCoA) developed from binary matrix of inter-simple sequence repeats band data for large neighbor trees and smaller mature interior ponderosa pine (P. ponderosa var. scopulorum Engelm.) at Devils Tower National Monument, Wyoming (DETO) and Wind Cave National Park, South Dakota (WICA). Ellipses encompass trees from each location. Orange = DETO neighbor, blue = DETO small tree, gray = WICA neighbor, yellow = WICA small tree. N = 77 trees. Axes 1–3 accounted for 35% of the variation in the data.
3.3. Chemical Profiles
Concentrations of terpenes were similar in neighbors from the two sites except for the monoterpenes geraniol, terpinolene, and isopolugol, and the sesquiterpene a-humulene, which were significantly higher at DETO and the monoterpene linalool, which was higher at WICA (Table 6). The PCA did not produce any clusters related to site or particular terpene combinations and is not included here.

Table 6.
Comparisons of mean (SD) terpene concentrations (mg/g) of neighbor interior ponderosa pine (P. ponderosa var. scopulorum Engelm.) at Devil’s Tower National Monument, Wyoming (DETO) and Wind Cave National Park, South Dakota (WICA). Significant differences in bold (p ≤ 0.05).
3.4. Climate and Growth Rates
The mean growth of beetle-killed trees was significantly different from that of neighbor trees at both DETO and WICA during the long times series from 1925 to 2015 (t = 2.397, p = 0.018 and t = 10.59, p < 0.0001, respectively) and the short time series from 2000 to 2015 (t = 7.375, <0.0001 and t = 4.368, p = 0.0006, respectively). Growth of neighbor and beetle-killed trees at the two sites differed over time (Figure 2a and Figure 3a). At DETO, both categories of trees grew similarly until the late 1950s when neighbors began to grow more rapidly. However, from 2002 to 2005 growth of neighbors slowed while beetle-killed trees increased growth briefly before dropping back to previous rates. By 2015, when beetle-killed trees at DETO were attacked, the growth of trees in both categories had converged. At WICA, beetle-killed trees grew faster than neighbors until growth converged in the 1980s, after which neighbors grew faster than beetle-killed trees until slowing and converging with beetle-killed trees in 2015.
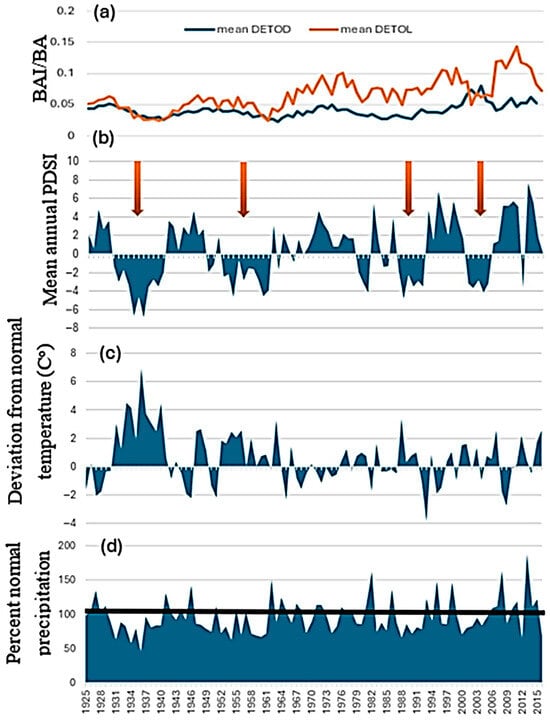
Figure 2.
Tree growth and climate data for Devils Tower National Monument, Wyoming. (a) mean growth curves for neighbor (DETOL) and beetle-killed interior ponderosa pine (P. ponderosa var. scopulorum Engelm.) (DETOD), (b) mean annual PDSI (Palmer Drought Severity Index), (c) deviation from normal temperature during the growing season (April–September), and (d) percent normal precipitation during the growing season (horizontal black line indicates average precipitation for the time period). Arrows denote droughts.
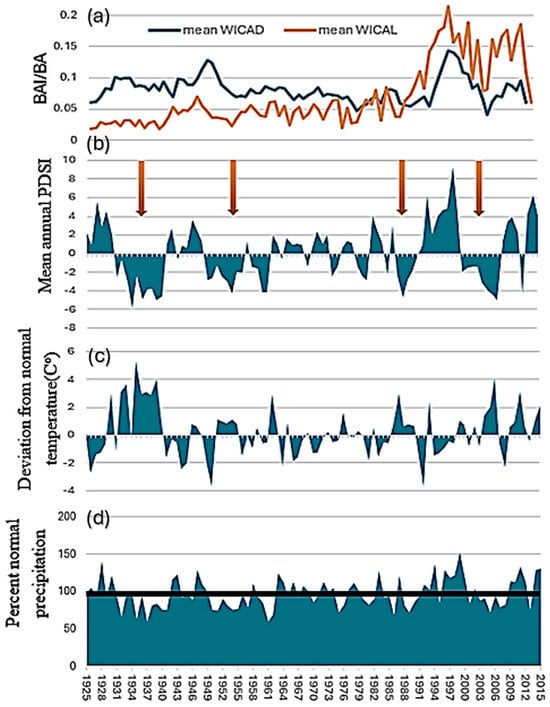
Figure 3.
Tree growth and climate data for Wind Cave National Park, South Dakota. (a) mean growth curves for neighbor (WICAL) and beetle-killed interior ponderosa pine (P. ponderosa var. scopulorum Engelm.) (WICAD), (b) mean annual PDSI (Palmer Drought Severity Index), (c) deviation from normal temperature during the growing season (April–September), and (d) percent normal precipitation during the growing season (horizontal black line indicates average precipitation for the time period). Arrows denote droughts.
The two sites experienced four droughts (periods of negative PDSI for 4–5 or more years) between 1925 and 2015 (Figure 2b and Figure 3b). These occurred approximately 1932 to 1942 (the Dust Bowl period), 1950–1960, the mid-late 1980s, and then the late 1990s–mid-2000s. The first two droughts were hot, while temperatures were more moderate and variable in the latter two droughts (Figure 2c and Figure 3c). From the mid-2010s to the end of the series in 2015, conditions were wetter than normal except for one year (2013) (Figure 2d and Figure 3d).
At DETO, both neighbor and beetle-killed trees increased growth in the wet period in the 1990s. Growth of DETO neighbors slowed in the fourth drought, while DETO beetle-killed trees increased growth (Figure 3a). Beetle-killed trees then stabilized while neighbors increased in the wet years but then dropped in 2015 as precipitation returned to normal levels. At WICA, trees in both categories increased growth during the wet period of the 1990s (Figure 2a) but beetle-killed trees to a lesser degree, and both slowed growth substantially just prior to the attack of the beetle-killed trees in 2015, despite this year receiving above normal MA precipitation (Figure 3a). This drop may have been due to a drought in fall truncating growth.
Correlations of mean growth (all trees within a category) with climate: Mean tree growth over the long series (1925–2015) had moderate to strong correlations with all climate variables, including PDSI, temperature, and precipitation for DETO and WICA neighbors for current and lagged years except MA temperature at WICA (Table 7). In contrast, fewer climate variables were correlated with mean growth using the short series (2000–2015). These included positive correlations with MA precipitation, MA PDSI and GS PDSI current and lagged years at DETO, and MA temperature and MA precipitation current year only at WICA. Beetle-killed trees showed far fewer significant correlations with climate variables, especially at WICA. For the long series, there were only negative correlations with GS temperature and positive correlations with MA and GS PDSI in the current year. In the short series for DETO, there were negative correlations with MA precipitation and MA and GS PDSI and none for lagged year at DETO. At WICA, for both the short and long series, beetle-killed trees only showed positive correlations for the long series for lagged year MA precipitation and lagged year GS PDSI (Table 7).

Table 7.
Pearson product correlation coefficients for comparisons of mean growth of neighbor and beetle-killed interior ponderosa pine (P. ponderosa var. scopulorum Engelm.) at Devils Tower National Monument, Wyoming (DETO) and Wind Cave National Park, South Dakota (WICA) for long time series (1925–2015) and short time series (2000–2015) using current year’s climate data and lagged one year. Bold values are significant at ≤0.05. PDSI = Palmer Drought Severity Index, MA = mean annual, GS = growing season (April–September).
Correlations of growth (individual trees within a category) with climate: Because the longer series and current year climate data exhibited more and stronger correlations with climate, they were used for tests investigating individual tree sensitivity. As expected, there was variability among individual trees in their responses to climate. Overall, neighbors responded more similarly to one another than did beetle-killed trees, and correlations were more likely to be all positive or all negative for a particular climate variable. For DETO neighbors, the growth of 70% of trees was positively correlated with MA climate variables, while for DETO beetle-killed, 11% were positively correlated and 17% were negatively correlated (Tables S1 and S2). A total of 85 and 89% of DETO neighbors and 11 and 28% of beetle-killed trees were positively correlated with MA precipitation and MA PDSI, respectively. DETO neighbors were 74% positively correlated with GS precipitation, while beetle-killed trees were 11% positively and 28% negatively correlated. For DETO neighbors, 67% were positively correlated with GS temperature, but no beetle-killed trees showed correlations. A total of 89% of DETO neighbors had positive correlations with GS PDSI, while only 28% of DETO beetle-killed trees did. At DETO, only 4% of neighbors showed no correlations with any climate variables, while 50% of beetle-killed trees had no correlations.
A total of 33% of WICA neighbors and WICA beetle-killed trees were negatively correlated with MA temperature, and 10% of beetle-killed trees were positively correlated (Tables S3 and S4). A total of 92% of WICA neighbors were positively correlated with MA precipitation and MA PDSI whereas 20% of WICA beetle-killed trees were positively and 30% were negatively correlated. A total of 83% of WICA neighbors were negatively correlated with GS temperature, while WICA beetle-killed trees were split with 10% positive and 10% negative correlations. A total of 92% of WICA neighbors were positively correlated with GS precipitation and GS PDSI, while beetle-killed trees had 10% positive and 30% negative correlations for GS precipitation and 30% negative and 30% positive correlations for GS PDSI. Eight percent of WICA neighbors had no correlations with any climate variable, while 30% of WICA beetle-killed trees had no correlations (Tables S3 and S4).
4. Discussion
We used a multipronged approach to investigate how variability within interior ponderosa pine populations in the Black Hills region may influence susceptibility to the eastern form of the mountain pine beetle (MPB) and assess the tree’s adaptive potential to climate change. In general, neighbor and beetle-killed trees within sites did not differ in canopy density, basal area, or DBH, suggesting that the differences observed in terpene composition, growth, and climate sensitivity are likely due to intrinsic differences among trees rather than site-level factors.
We found variation in genetic structure, terpene chemistry, and growth among trees at the two sites. Population sub-structuring by tree size (and presumed age) was evident at WICA, and genetic differentiation between DETO and WICA indicated considerable local adaptation. Genetic diversity at DETO was low, suggesting reduced adaptive capacity in response to environmental change. The most striking results emerged from comparisons of growth patterns between neighbor and beetle-killed trees, revealing that beetle-killed trees were less responsive to climate. This lower responsiveness may impair physiological processes critical for defense, although further investigation is needed. Notably, lifetime growth patterns were more predictive of beetle-induced mortality than growth in the years immediately preceding beetle attack.
ISSR analysis showed that trees from DETO and WICA were genetically distinct. The PCoA of ISSR profiles from neighbors and smaller mature trees revealed three clusters: all DETO trees formed a relatively tight cluster with slight segregation between categories, while WICA smaller mature trees and some WICA neighbors formed a second cluster, and the remaining WICA neighbors formed a third. The greater spread among WICA trees suggests substantially higher genetic diversity in this population, which may confer greater adaptive capacity.
One genetic cluster within WICA included older trees that were distinct from others in the population and did not cluster with any smaller mature trees, suggesting limited genetic contribution to the younger cohort. As neighbor and smaller trees were interspersed, spatial distance cannot explain this pattern. It is possible that an environmental filter at the time of establishment selected against progeny from these older trees. A similar pattern was observed in whitebark pine in Idaho, where a younger cohort was genetically distinct from an interspersed older cohort and exhibited lower diversity [32]. Unfortunately, population genetic studies of pines rarely sample across multiple cohorts, limiting understanding of how genetic variation and adaptive potential shift over time. In species with episodic regeneration, such as conifers, cohorts may establish under different selective pressures, resulting in divergent allele frequencies. Nevertheless, interbreeding among all but the youngest cohorts may preserve overall genetic diversity despite cohort-specific shifts due to selection or drift. Considerations of maintaining multi-aged stands during management may thus be critical, as this structure is often lost through clear-cutting or understory thinning. Sampling across cohorts can improve estimates of genetic diversity, reveal adaptive shifts, and elucidate the role of cohort diversity in maintaining evolutionary potential.
Terpene analysis revealed significant differences in five compounds between the two populations (Table 6), indicating a degree of chemical differentiation. This variation further suggests underlying genetic divergence and potential local adaptation. Although tree secondary metabolites, including terpenes, are used by bark beetles in host selection, the specific compounds involved can vary among and within populations, and their roles remain poorly understood [5,32].
Constitutive physical defenses, particularly resin production, may be more reliable indicators of individual susceptibility (ref. [7]; but see ref. [50] on the importance of induced defenses). Pines typically produce resin that can repel or kill attacking beetles, preventing successful colonization [51]. Resin exudation upon attack is often interpreted as an indicator of tree vigor, and resin is presumed to be synthesized de novo [37,52]. Because resin production requires substantial carbon investment, drought is expected to reduce resin output via stomatal closure and reduced photosynthesis. However, studies have observed variability in resin flow during drought, indicating that carbon availability may not limit resin exudation, and recent work in P. edulis demonstrated that resin released upon attack originates from stored reserves and is driven by tree water potential rather than current carbon availability [37]. This suggests that water availability, not photosynthate production, governs resin defense at the time of attack.
This mechanism may explain why prior studies have failed to support predictions from the Growth-Differentiation Balance Hypothesis (GDBH), which posits that trees allocate more carbon to defense (e.g., resin) during periods of limited growth [53]. If water pressure, not carbon, controls resin flow, this could account for the observed variability in resin exudation among ponderosa pines exposed to varying drought severity and duration. In our study, most trees showed little to no resin flow after 24 h, suggesting water limitation. However, the several-year period preceding beetle attack and resin sampling in this study was wetter than average, likely allowing the accumulation of resin stores. We sampled 1–2 years post attack in July, a month after MPB typically disperses and attacks new trees in this area. Additionally, resin flow in neighbors at the time of our samples may have differed from that which occurred in 2015 (year of attacks on beetle-killed trees). Climate data suggest that in the year of attack (2015) and in sampling years (2016 for both sites, 2017 DETO only), both sites should have received adequate precipitation (above average for WICA and above average for DETO in 2015 and 2016 and slightly below average for DETO in 2017) to support resin exudation. Therefore, the interaction of water availability and resin defense in this study is unclear. Further, despite low or absent resin flow in neighbor trees during sampling years, we did not observe new mortality due to beetles at either site, suggesting that MPB host selection may involve additional cues beyond resin exudation pressure. Resin duct characteristics may play a role, as greater axial resin duct area has been associated with increased resistance in pines [40,54,55,56,57]. Resin duct area has been shown to influence flow [58,59], and it may also enhance volatile signaling influencing beetle behavior before and during attack [40].
The GDBH also posits that fast-growing trees are less defended due to investment in growth over defense, while slower growing trees are better defended because they prioritize defense over growth. However, this trade-off fails to explain beetle selection patterns. In our study, trees that grew faster prior to attack were more likely to survive, although growth among both categories declined and converged at the time of attacks in 2015, especially at DETO. In whitebark pine, susceptibility in relation to growth rate varied by site, with MPB preferentially attacking either slow- or fast-growing trees. Similarly, in P. contorta, beetle-attacked trees showed a range of growth patterns relative to survivors [41]. These inconsistent patterns suggest that neither slow nor fast growth at the time of attack reliably predicts susceptibility.
In the current study, the strongest predictors of beetle-caused mortality were long-term climate responses. Trees that survived and those that were killed by beetles exhibited distinct growth responses to climate over their lifespans. Surviving neighbors were more responsive to climate fluctuations, growing more during years with greater annual precipitation and cooler temperatures. In contrast, beetle-killed trees exhibited fewer significant correlations and were often unresponsive to any climate variable. Similar patterns have been observed in whitebark pines [32]. Additionally, annual tree growth correlated more strongly with current-year climate than with lagged climate, suggesting that environmental conditions at or just prior to attack are most influential on tree growth [37].
5. Conclusions
Across multiple studies, a common finding is that beetle-killed and surviving trees differ in their long-term climate responses. At the individual level, trees eventually killed by MPB appear less able to adjust physiologically to climatic variation. Understanding how climate sensitivity relates to beetle susceptibility is a key area for future research, especially as climate change intensifies disturbance regimes. The observed relationships between growth and MPB-caused mortality in several pine species underscore the need to explore further the mechanistic and genetic basis of growth–defense trade-offs under variable climate conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16091453/s1, Table S1: Person product correlation coefficients for growth (1925–2015) of individual living neighbor trees vs. climate variables for Devils Tower (DETO); Table S2: Person product correlation coefficients for growth (1925–2015) of individual beetle-killed trees vs. climate variables for Devils Tower (DETO).; Table S3: Person product correlation coefficients for growth (1925–2015) of individual living neighbor trees vs. climate variables for Wind Cave (WICA).; Table S4: Person product correlation coefficients for growth (1925–2015) of individual beetle-killed trees vs. climate variables for Wind Cave (WICA).
Author Contributions
Conceptualization, D.L.S.; methodology, D.L.S.; formal analysis, D.L.S. and H.R.A.; investigation, D.L.S.; resources, DLS; data curation, D.L.S.; writing—original draft preparation, D.L.S. and H.R.A.; writing—review and editing, D.L.S. and H.R.A.; project administration, D.L.S.; funding acquisition, D.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Park Service (NPS) as study #DETO-00054 under the cooperative agreement between the NPS and UM #P14AC00728. Collections were made at both sites under the NPS permit DETO-2017-SCI-0004.
Data Availability Statement
Data are available upon request to the corresponding author.
Acknowledgments
We thank Rene Ohms of the National Park Service for initiating this study and her support in logistics. We also thank Erika Berglund for her help with tree ring measurements and ISSR analyses, Jordan Lestina for help with cross-dating, and Lorinda Bullington and Shealyn Malone for pre-reviewing the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DETO | Devils Tower National Monument |
| WICA | Wind Cave National Park |
| SWMC | Sapwood moisture content |
| DBH | Diameter breast height |
| MA | Mean annual |
| GS | Growing season |
| MPB | Mountain pine beetle |
| NPS | National Park Service |
| ISSR | Inter-simple sequence repeats |
| PCoA | Principle coordinate analysis |
References
- Bend, B.J.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.; Seybold, S.J. Climate change and bark beetles of the western United States and Canada: Direct and indirect effects. BioScience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Raffa, K.F.; Andersson, M.N.; Schlyter, F. Host Selection by Bark Beetles: Playing the Odds in a High-Stakes Game. In Advances in Insect Physiology; Tittiger, C., Blomquist, G.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 50, pp. 1–74. [Google Scholar]
- Huang, J.; Kautz, M.; Trowbridge, A.M.; Hammerbacher, A.; Raffa, K.F.; Adams, H.D.; Goodsman, D.W.; Xu, C.; Meddens, A.J.H.; Kandasamy, D.; et al. Tree defence and bark beetles in a drying world: Carbon partitioning, functioning and modelling. New Phytol. 2020, 225, 26–36. [Google Scholar] [CrossRef]
- Meddens, A.J.H.; Hicke, J.A.; Ferguson, C.A. Spatiotemporal patterns of observed bark beetle-caused tree mortality in British Columbia and the western United States. Ecol. Appl. 2012, 22, 1876–1891. [Google Scholar] [CrossRef]
- Bend, B.J.; Boone, C.; Raffa, K.F. Tree response and mountain pine beetle attack preference, reproduction and emergence timing in mixed whitebark and lodgepole pine stands. Agric. For. Entomol. 2015, 17, 421–432. [Google Scholar] [CrossRef]
- Gray, C.A.; Runyon, J.B.; Jenkins, M.J.; Giunta, A.D. Mountain pine beetles use volatile cues to locate host limber pine and avoid non-host Great Basin bristlecone pine. PLoS ONE 2015, 10, e0135752. [Google Scholar] [CrossRef] [PubMed]
- West, D.R.; Briggs, J.S.; Jacobi, W.R.; Negrón, J.F. Mountain pine beetle host selection between lodgepole and ponderosa pines in the southern Rocky Mountains. Environ. Entomol. 2015, 45, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Six, D.L.; Vergobbi, C.; Cutter, M. Are survivors different? Genetic and growth analyses of co-occurring whitebark and lodgepole pine after a mountain pine beetle outbreak. Front. Plant Sci. 2018, 9, 993. [Google Scholar] [CrossRef]
- Cullingham, C.I.; Cooke, J.E.K.; Dang, S.; Davis, C.S.; Cooke, B.J.; Coltman, D.W. Mountain pine beetle host-range expansion threatens the boreal forest. Mol. Ecol. 2011, 20, 2157–2171. [Google Scholar] [CrossRef]
- Clark, E.L.; Carroll, A.L.; Huber, D.P.W.; Lindgren, B.S.; Pitt, C. Comparison of lodgepole and jack pine constitutive and induced resin chemistry: Implications for range expansion by the mountain pine beetle, Dendroctonus ponderosae, (Coleoptera: Curculionidae). PeerJ 2014, 2, 240. [Google Scholar] [CrossRef]
- Raffa, K.F.; Mason, C.J.; Bonello, P.; Cook, S.; Erbilgin, N.; Keefover-Ring, K.; Klutsch, J.G.; Villari, C.; Townsend, P.A. Defence syndromes in lodgepole—White bark pine ecosystems relate to degree of historical exposure to mountain pine beetles. Plant Cell Environ. 2017, 40, 1791–1806. [Google Scholar] [CrossRef]
- Burke, J.L.; Bohlmann, J.; Carroll, A.L. Consequences of distributional asymmetry in a warming environment: Invasion of novel forests by the mountain pine beetle. Ecosphere 2017, 8, e0178. [Google Scholar] [CrossRef]
- Norris, J.R.; Betancourt, J.L.; Jackson, S.T. Late Holocene expansion of ponderosa pine (Pinus ponderosa) in the Central Rocky Mountains, USA. J. Biogeogr. 2016, 43, 778–790. [Google Scholar] [CrossRef]
- Smith, R.H.; Peloquin, R.L.; Passof, P.C. Local and Regional Variation in the Monoterpenes of Ponderosa Pine Wood Oleoresin; USDA Forest Service research paper PSW—Volume 56; Pacific Southwest Forest and Range Experiment Station, Forest Service, U.S. Department of Agriculture: Washington, DC, USA, 1969. [Google Scholar]
- Potter, K.M.; Hipkins, V.D.; Mahalovich, M.F.; Means, R.E. Mitiochondrial DNA haplotype diversity patterns in Pinus ponderosa (Pinaceae): Range-wide evolutionary history and implications for conservation. Am. J. Bot. 2013, 100, 1562–1579. [Google Scholar] [CrossRef]
- Potter, K.M.; Hipkins, V.D.; Mahalovich, M.F.; Means, R.E. Nuclear genetic variation across the range of ponderosa pine (Pinus ponderosa): Phylogeographic, taxonomic, and conservation implications. Tree Genet. Genomes 2015, 11, 38. [Google Scholar] [CrossRef]
- Shinneman, D.J.; Means, R.E.; Potter, K.M.; Hipkins, V.D. Exploring climate niches of ponderosa pine (Pinus ponderosa Douglas ex Lawson) haplotypes in the western United States: Implications for evolutionary history and conservation. PLoS ONE 2016, 11, e0151811. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, G.E. Systematics and genetic structure of Ponderosae taxa (Pinaceae) inhabiting the mountain islands of the southwest. Am. J. Bot. 1999, 86, 741–752. [Google Scholar] [CrossRef]
- Willyard, A.; Gernandt, D.S.; Potter, K.; Hipkins, V.; Marquardt, P.; Mahalovich, M.F.; Langer, S.K.; Telewski, F.W.; Cooper, B.; Douglas, E.; et al. Pinus ponderosa: A checkered past obscured by four species. Am. J. Bot. 2017, 104, 161–181. [Google Scholar] [CrossRef]
- Alexander, R.R. Silvicultural Systems, Cutting Methods, and Cultural Practices for Black Hills Ponderosa Pine; Gen. Tech. Rep. RM-139; Rocky Mountain Forest and Range Experiment Station, Forest Service, U.S. Department of Agriculture: Fort Collins, CO, USA, 1987; 32p. [Google Scholar]
- Shinneman, D.J.; Baker, W.L. Nonequilibrium dynamics between catastrophic disturbances and old-growth forests in ponderosa pine landscapes of the Black Hills. Conserv. Biol. 1997, 11, 1276–1288. [Google Scholar] [CrossRef]
- Fisher, R.F.; Jenkins, M.J.; Fisher, W.F. Fire and the Prairie-forest Mosaic of Devils Tower National Monument. Am. Midl. Nat. 1987, 117, 250–257. [Google Scholar] [CrossRef]
- Brown, P.M.; Cook, B. Early settlement forest structure in Black Hills ponderosa pine forests. For. Ecol. Manag. 2006, 223, 284–290. [Google Scholar] [CrossRef]
- Costello, S.L.; Jacobi, W.R.; Negron, J.F. Emergence of Buprestidae, Cerambycidae, and Scolytinae (Coleoptera) from mountain pine beetle-killed and fire-killed ponderosa pines in the Black Hills, South Dakota, USA. Coleopt. Bull. 2013, 67, 149–154. [Google Scholar] [CrossRef]
- Wood, S.L. The Bark and Ambrosia Beetles of North and Central America (Coleoptera: Scolytidae). A Taxonomic Monograph; Brigham Young University Press: Provo, UT, USA, 1982; 1359p. [Google Scholar]
- Dowle, E.J.; Bracewell, R.R.; Prefender, M.E.; Mock, K.E.; Bentz, B.J.; Ragland, G.J. Reproductive isolation and environmental adaptation shape the phylogeography of mountain pine beetle (Dendroctonus ponderosae). Mol. Ecol. 2017, 26, 6071–6084. [Google Scholar] [CrossRef]
- Bracewell, R.R.; Vanderpool, D.; Good, J.M.; Six, D.L. Cascading speciation among mutualists and antagonists in a tree-beetle-fungal interaction. R. Soc. B Biol. Sci. 2018, 285, 20180694. [Google Scholar] [CrossRef]
- McMillan, J.; Allen, K.K. Evaluation of Mountain Pine Beetle Activity in the Black Hills National Forest; USFS Rocky Mountain Region: Lakewood, CO, USA, 2001. [Google Scholar]
- Thoss, V.; Byers, J.A. Monoterpene chemo diversity of ponderosa pine in relation to herbivory and bark beetle colonization. Chemoecology 2006, 16, 51–58. [Google Scholar] [CrossRef]
- Keefover-Ring, K.; Trowbridge, A.; Mason, C.J.; Raffa, K.F. Rapid induction of multiple terpenoid groups by ponderosa pine in response to bark beetle-associated fungi. J. Chem. Ecol. 2016, 42, 1–12. [Google Scholar] [CrossRef]
- Chiu, C.C.; Keeling, C.I.; Bohlmann, J. Toxicity of pine monoterpenes to mountain pine beetle. Nat. Sci. Rep. 2017, 7, 8858. [Google Scholar] [CrossRef]
- Six, D.L.; Trowbridge, A.; Howe, M.; Perkins, D.; Berglund, E.; Brown, P.; Hicke, J.A.; Balasubramanian, G. Growth, chemistry, and genetic profiles of whitebark pine forests affected by climate-driven mountain pine beetle outbreaks. Front. For. Glob. Change 2021, 4, 671510. [Google Scholar] [CrossRef]
- Campbell, S.A.; Borden, J.H. Integration of visual and olfactory cues of hosts and non-hosts by three bark beetles (Coleoptera: Scolytidae). Ecol. Entomol. 2006, 31, 437–449. [Google Scholar] [CrossRef]
- Moeck, H.A.; Simmons, C.S. Primary attraction of mountain pine beetle, Dendroctonus ponderosae Hopk., (Coleoptera: Scolytidae) to bolts of lodgepole pine. Can. Entomol. 1991, 123, 299–304. [Google Scholar] [CrossRef]
- Trowbridge, A.M.; Stoy, P.C.; Adams, H.D.; Law, D.J.; Breshears, D.D.; Helmig, D.; Monson, R.K. Drought supersedes warming in determining volatile and tissue defenses of pinon pine (Pinus edulis). Environ. Res. Lett. 2019, 14, 065006. [Google Scholar] [CrossRef]
- Trowbridge, A.M.; Adams, H.D.; Collins, A.; Dickman, L.T.; Grossiord, C.; Hofland, M.; Malone, S.; Wever, D.K.; Sevanto, S.; Stoy, P.C.; et al. Hotter droughts alter resource allocation to chemical defenses. Oecologia 2021, 197, 921–938. [Google Scholar] [CrossRef] [PubMed]
- Malone, S.C.; Thompson, R.A.; Chow, P.S.; de Oliveira, C.R., Jr.; Landhäusser, S.M.; Six, D.L.; McCulloh, K.A.; Adams, H.D.; Trowbridge, A.M. Water, not carbon, drives drought-constraints on stem terpene defense against simulated bark beetle attack in Pinus edulis. New Phytol. 2025, 245, 318–331. [Google Scholar] [CrossRef]
- Kremer, A.; Ronce, O.; Robledo-Arnuncio, J.J.; Guillaume, F.; Bohrer, G.; Nathan, R.; Bridle, J.R.; Gomulkiewicz, R.; Klein, E.K.; Ritland, K.; et al. Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecol. Lett. 2012, 15, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Stine, A.R.; Huybers, P. Implications of Liebig’s Law of the Minimum for tree-ring reconstructions of climate. Environ. Res. Lett. 2017, 12, 114018. [Google Scholar] [CrossRef]
- Kichas, N.E.; Trowbridge, A.M.; Raffa, K.F.; Malone, S.C.; Hood, S.M.; Everett, R.G.; McWethy, D.B.; Pederson, G.T. Growth and defense characteristics of white bark pine (Pinus albicaulis) and lodgepole pine (Pinus contorta var latifolia) in a high elevation, disturbance prone mixed forest in northwestern Montana, USA. For. Ecol. Manag. 2021, 493, 119286. [Google Scholar] [CrossRef]
- Cooper, L.A.; Reed, C.C.; Ballantyne, A.P. Mountain pine beetle attack faster growing lodgepole pine at low elevations in western Montana, USA. For. Ecol. Manag. 2019, 427, 200–207. [Google Scholar] [CrossRef]
- Keen, R.M.; Voelker, S.L.; Bentz, B.J.; Wang, S.-Y.S.; Farrell, R. Stronger influence of growth rate than severity of drought stress on mortality of large ponderosa pines during the 2012–2015 CA drought. Oecologia 2020, 194, 359–370. [Google Scholar] [CrossRef]
- Reed, C.C.; Hood, S.M. Few generalizable patterns of tree-level mortality during extreme drought and concurrent bark beetle outbreaks. Sci. Total Environ. 2019, 750, 141306. [Google Scholar] [CrossRef]
- Graham, R.T.; Asherin, L.A.; Battaglia, M.A.; Jain, T.B.; Mata, S.A. Mountain Pine Beetle: A Century of Knowledge, Control Attempts, and Impacts to the Black Hills; USDA RMRS Gen. Tech. Rep. RMS-GTR-353; USDA RMRS: Washington, DC, USA, 2016; p. 2016. [Google Scholar]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010. [Google Scholar]
- Parasharami, V.A.; Thengane, S.R. Inter population genetic diversity analysis using ISSR markers in Pinus roxburghii (Sarg.) from Indian provenances. Int. J. Biodivers. Conserv. 2012, 4, 219–227. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D.; Marshall, L. Growth, carbon-isotope discrimination, and drought-associated mortality across a Pinus ponderosa elevational transect. Glob. Change Biol. 2010, 16, 399–415. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Abatzoglou, J.T.; McEvoy, D.J.; Redmond, K.T. The west-wide drought tracker: Drought monitoring at fine spatial scales. Bull. Am. Meteorol. Soc. 2017, 98, 1815–1820. [Google Scholar] [CrossRef]
- Howe, M.; Yanchuk, A.; Wallin, K.F.; Raffa, K.F. Quantification of heritable variation in multiple lodgepole pine chemical and physical traits that contribute to defense against mountain pine beetle (Dendroctonus ponderosae). For. Ecol. Manag. 2024, 553, 121660. [Google Scholar] [CrossRef]
- Gaylord, M.L.; Kolb, T.E.; Wallin, K.F.; Wagner, M.R. Seasonal dynamics of tree growth, physiology, and resin defenses in a northern Arizona ponderosa pine forest. Can. J. For. Res. 2007, 37, 1173–1183. [Google Scholar] [CrossRef]
- Krokene, P. Conifer defense and resistance to bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Academic Press: Cambridge, MA, USA, 2015; pp. 177–207. [Google Scholar]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Kane, J.M.; Kolb, T.E. Importance of resin ducts in reducing ponderosa pine mortality from bark beetle attack. Oecologia 2010, 164, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Ferrenberg, S.; Kane, J.M.; Mitton, J.B. Resin duct characteristics associated with tree resistance to bark beetles across lodgepole and limber pines. Oecologia 2014, 174, 1283–1292. [Google Scholar] [CrossRef]
- Zhao, S.; Erbilgin, N. Larger resin ducts are linked to the survival of lodgepole pine trees during mountain pine beetle outbreak. Front. Plant Sci. 2019, 10, 1459. [Google Scholar] [CrossRef]
- Kichas, N.E.; Pederson, G.T.; Hood, S.M.; Everett, R.G.; McWethy, D.B. Increased whitebark pine (Pinus albicaulis) growth and defense under a warmer and regionally drier climate. Front. For. Glob. Chang. 2023, 6, 1089138. [Google Scholar] [CrossRef]
- Hood, S.; Sala, A. Ponderosa pine resin defenses and growth: Metrics matter. Tree Physiol. 2015, 35, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Valor, T.; Hood, S.M.; Pique, M.; Larranaga, A.; Casals, P. Resin ducts and bark thickness influence pine resistance to bark beetles after prescribed fire. For. Ecol. Manag. 2021, 494, 119322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).