Planting Native Herbaceous Species During Land Reclamation: 3-Year Growth Response to Soil Type and Competing Vegetation
Abstract
1. Implications for Practice
2. Introduction
- How does placed topsoil depth affect the growth and survival of these native forbs? We hypothesize that larger quantities of topsoil (=higher nutrition) will lead to higher growth rates, but we concurrently expect to observe a reduction in survival due to increased herbaceous competition.
- Does competing ruderal vegetation have a negative impact on survival and/or growth? We hypothesize that, given similar soil quality, competing vegetation will reduce growth outcomes for each species, but the magnitude of the effect will be proportional to each individual species—we expect Canada goldenrod to show greater resilience as it is known to be an aggressive pioneering species.
3. Methods
3.1. Greenhouse Stock Type Production
3.2. Stock Characterization
3.3. Field Study Site Description and Experimental Design
3.4. Field Measurements
3.5. Statistics
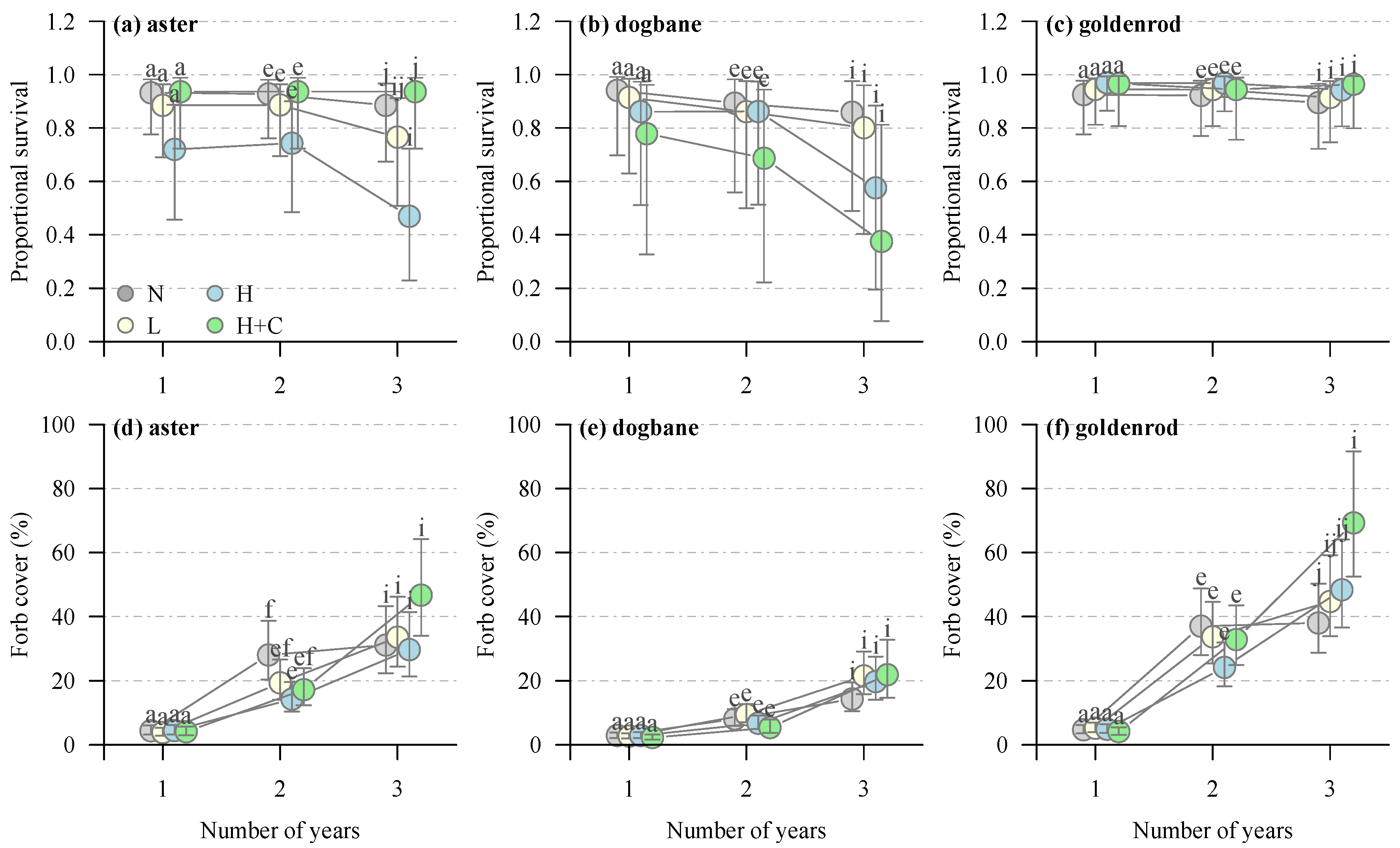
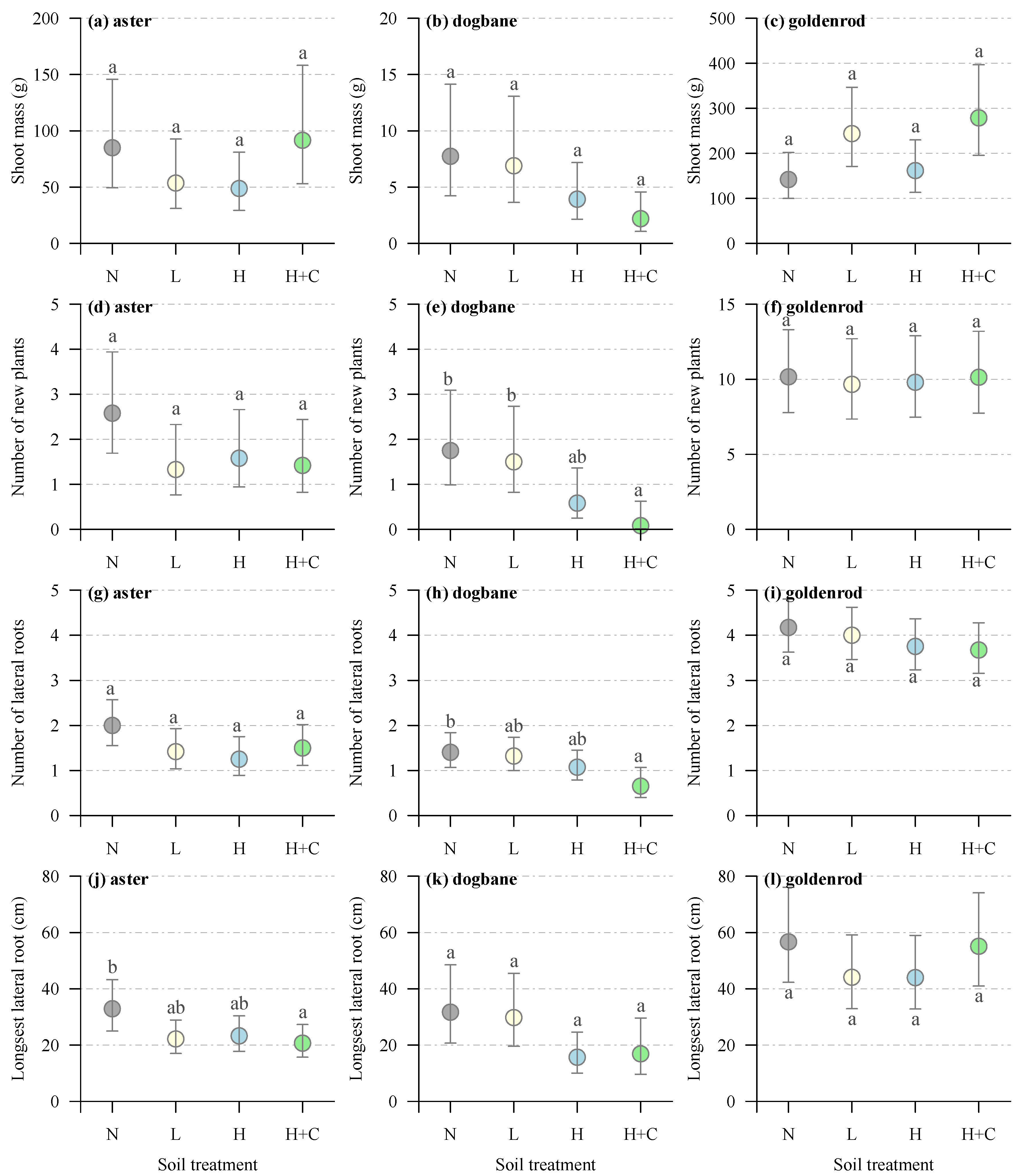
4. Results
4.1. Site Treatment Characteristics
4.2. Canada Goldenrod
4.3. Showy Aster
4.4. Spreading Dogbane
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baah-Acheamfour, M.; Dewey, M.; Fraser, E.C.; Schreiber, S.G.; Schoonmaker, A. Assessing Ecological Recovery of Reclaimed Well Sites: A Case Study from Alberta, Canada. Front. For. Glob. Change 2022, 5, 849246. [Google Scholar] [CrossRef]
- Lupardus, R.C.; McIntosh, A.C.S.; Janz, A.; Farr, D. Succession after reclamation: Identifying and assessing ecological indicators of forest recovery on reclaimed oil and natural gas well pads. Ecol. Indic. 2019, 106, 105515. [Google Scholar] [CrossRef]
- Environment and Sustainable Resource Development (ESRD). 2010 Reclamation Criteria for Wellsites and Associated Facilities for Forested Lands (Updated July 2013); Environment and Sustainable Resource Development: Edmonton, AB, Canada, 2013; p. 81. [Google Scholar]
- U.S. Department of the Interior. Surface Mining Control Reclamation Act (SMCRA); U.S. Department of the Interior, Office of Surface Mining: Washington, DC, USA, 1977.
- Australia Department of Industry Tourism and Resources. Mine Closure and Completion: Leading Practice Sustainable Development Program for the Mining Industry; Department of Industry, Resources and Tourism: Canberra, Australia, 2006.
- Williamson, T.B.; Edwards, J.E. Adapting Sustainable Forest Management to Climate Change: Criteria and Indicators in a Changing Climate; Canadian Council of Forest Ministers: Ottawa, ON, Canada, 2013. [Google Scholar]
- Hart, S.A.; Chen, H.Y.H. Understory Vegetation Dynamics of North American Boreal Forests. Crit. Rev. Plant Sci. 2006, 25, 381–397. [Google Scholar] [CrossRef]
- Thrippleton, T.; Bugmann, H.; Kramer-Priewasser, K.; Snell, R.S. Herbaceous Understorey: An Overlooked Player in Forest Landscape Dynamics? Ecosystems 2016, 19, 1240–1254. [Google Scholar] [CrossRef]
- Knops, J.M.H.; Nash, I.I.I.T.H.; Schlesinger, W.H. The Influence of Epiphytic Lichens on the Nutrient Cycling of an Oak Woodland. Ecol. Monogr. 1996, 66, 159–179. [Google Scholar] [CrossRef]
- Weber, M.G.; Cleve, K.V. Nitrogen dynamics in the forest floor of interior Alaska black spruce ecosystems. Can. J. For. Res. 1981, 11, 743–751. [Google Scholar] [CrossRef]
- Hanley, T.A.; Deal, R.L.; Orlikowska, E.H. Relations between red alder composition and understory vegetation in young mixed forests of southeast Alaska. Can. J. For. Res. 2006, 36, 738–748. [Google Scholar] [CrossRef]
- Cohen, A.; Naeth, M. Increasing Woody Species Diversity for Sustainable Limestone Quarry Reclamation in Canada. Sustainability 2013, 5, 1340–1355. [Google Scholar] [CrossRef]
- Smreciu, A.; Gould, K. Field emergence of native boreal forest species on reclaimed sites in northeastern Alberta. Nativ. Plants J. 2015, 16, 204–226. [Google Scholar] [CrossRef]
- Macdonald, S.; Landhäusser, S.; Skousen, J.; Franklin, J.; Frouz, J.; Hall, S.; Jacobs, D.; Quideau, S. Forest restoration following surface mining disturbance: Challenges and solutions. New For. 2015, 46, 703–732. [Google Scholar] [CrossRef]
- Schoonmaker, A.L.; Mathison, A.; Mackenzie, M.D. Hitchhiker planting: Mixed-species container stock planting as a novel tool to increase plant diversity on industrially disturbed sites. Can. J. For. Res. 2023, 53, 905–921. [Google Scholar] [CrossRef]
- Johnson, D.; Kershaw, L.; Mackinnon, A. Plants of the Western Forest: Alberta, Saskatchewan and Manitoba Boreal and Aspen Parkland, 3rd ed.; Partners Publishing: Beaver Dam, NB, Canada, 2020. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Lenth, R.V. Least-squares means The R package lsmeans. J. Stat. Softw. 2018, 69, 1–33. [Google Scholar]
- Dietrich, S.T.; MacKenzie, M.D.; Battigelli, J.P.; Enterina, J.R. Building a Better Soil for Upland Surface Mine Reclamation in Northern Alberta: Admixing Peat, Subsoil and Peat Biochar in a Greenhouse Study with Aspen. Can. J. Soil Sci. 2017, 97, 592–605. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R package version 1.0.7; The R Foundation: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=factoextra (accessed on 5 September 2025).
- Wilson, J.B. A Review of Evidence on the Control of Shoot: Root Ratio, in Relation to Models. Ann. Bot. 1988, 61, 433–449. [Google Scholar] [CrossRef]
- Gedroc, J.J.; McConnaughay, K.D.M.; Coleman, J.S. Plasticity in Root/Shoot Partitioning: Optimal, Ontogenetic, or Both? Funct. Ecol. 1996, 10, 44–50. [Google Scholar] [CrossRef]
- Müller, I.; Schmid, B.; Weiner, J. The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspect. Plant Ecol. Evol. Syst. 2000, 3, 115–127. [Google Scholar] [CrossRef]
- Abhilasha, D.; Quintana, N.; Vivanco, J.; Joshi, J. Do allelopathic compounds in invasive Solidago canadensis s.l. restrain the native European flora? J. Ecol. 2008, 96, 993–1001. [Google Scholar] [CrossRef]
- Pinno, B.; Li, E.; Khadka, B.; Schoonmaker, A. Germination and early growth of boreal understory plants on 3 reclamation soil types under simulated drought conditions. Nativ. Plants J. 2017, 18, 92–105. [Google Scholar] [CrossRef]
- Craig, A.; Macdonald, S.E. Threshold effects of variable retention harvesting on understory plant communities in the boreal mixedwood forest. For. Ecol. Manag. 2009, 258, 2619–2627. [Google Scholar] [CrossRef]
- Van der Sloot, M.; Kleijn, D.; De Deyn, G.B.; Limpens, J. Carbon to nitrogen ratio and quantity of organic amendment interactively affect crop growth and soil mineral N retention. Crop Environ. 2022, 1, 161–167. [Google Scholar] [CrossRef]

| Soil Treatment | Year 1 | Year 2 | Year 3 |
|---|---|---|---|
| N | 0.05 ab (0.03–0.08) | 0.19 a (0.14–0.25) | 0.25 b (0.19–0.32) |
| L | 0.09 b (0.06–0.13) | 0.22 a (0.16–0.28) | 0.40 a (0.33–0.49) |
| H | 0.07 ab (0.04–0.10) | 0.21 a (0.16–0.28) | 0.49 a (0.40–0.57) |
| HC | 0.03 a (0.01–0.05) | 0.16 a (0.11–0.21) | 0.35 ab (0.28–0.43) |
| Response | Forb | Fixed Effects | Chi-Square Value | DF | p-Value |
|---|---|---|---|---|---|
| Site total vegetation cover | Soil treatment | 10.5560 | 3 | 0.0144 | |
| Year | 407.4110 | 2 | <0.0001 | ||
| Soil treatment × year | 28.1310 | 6 | <0.0001 | ||
| Survival | Aster | Soil treatment | 11.7969 | 3 | 0.0081 |
| Year | 10.3931 | 2 | 0.0055 | ||
| Soil treatment × year | 1.8467 | 6 | 0.9332 | ||
| Dogbane | Soil treatment | 3.0627 | 3 | 0.3820 | |
| Year | 15.5265 | 2 | 0.0004 | ||
| Soil treatment × year | 3.158 | 6 | 0.7887 | ||
| Goldenrod | Soil treatment | 1.5083 | 3 | 0.6803 | |
| Year | 1.4814 | 2 | 0.4768 | ||
| Soil treatment × year | 0.7845 | 6 | 0.9925 | ||
| Forb cover | Aster | Soil treatment | 2.4401 | 3 | 0.4862 |
| Year | 475.7452 | 2 | <0.0001 | ||
| Soil treatment × year | 14.1875 | 6 | 0.0276 | ||
| Dogbane | Soil treatment | 1.842 | 3 | 0.6058 | |
| Year | 481.161 | 2 | <0.0001 | ||
| Soil treatment × year | 14.028 | 6 | 0.0293 | ||
| Goldenrod | Soil treatment | 1.2905 | 3 | 0.7313 | |
| Year | 1115.0579 | 2 | <0.0001 | ||
| Soil treatment × year | 24.5578 | 6 | 0.0004 | ||
| Shoot biomass | Aster | Soil treatment | 8.4853 | 3 | 0.0369 |
| Dogbane | Soil treatment | 5.5917 | 3 | 0.1333 | |
| Goldenrod | Soil treatment | 9.0481 | 3 | 0.0286 | |
| Number of new plants | Aster | Soil treatment | 4.8667 | 3 | 0.1818 |
| Dogbane | Soil treatment | 11.753 | 3 | 0.0082 | |
| Goldenrod | Soil treatment | 0.0955 | 3 | 0.9924 | |
| Number of lateral roots | Aster | Soil treatment | 5.7915 | 3 | 0.1222 |
| Dogbane | Soil treatment | 9.4796 | 3 | 0.0235 | |
| Goldenrod | Soil treatment | 1.8619 | 3 | 0.6016 | |
| Longest lateral root | Aster | Soil treatment | 8.5626 | 3 | 0.0357 |
| Dogbane | Soil treatment | 9.014 | 3 | 0.0291 | |
| Goldenrod | Soil treatment | 2.5434 | 3 | 0.4675 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chartrand-Pleau, C.; Degenhardt, D.; Schoonmaker, A. Planting Native Herbaceous Species During Land Reclamation: 3-Year Growth Response to Soil Type and Competing Vegetation. Forests 2025, 16, 1442. https://doi.org/10.3390/f16091442
Chartrand-Pleau C, Degenhardt D, Schoonmaker A. Planting Native Herbaceous Species During Land Reclamation: 3-Year Growth Response to Soil Type and Competing Vegetation. Forests. 2025; 16(9):1442. https://doi.org/10.3390/f16091442
Chicago/Turabian StyleChartrand-Pleau, Camille, Dani Degenhardt, and Amanda Schoonmaker. 2025. "Planting Native Herbaceous Species During Land Reclamation: 3-Year Growth Response to Soil Type and Competing Vegetation" Forests 16, no. 9: 1442. https://doi.org/10.3390/f16091442
APA StyleChartrand-Pleau, C., Degenhardt, D., & Schoonmaker, A. (2025). Planting Native Herbaceous Species During Land Reclamation: 3-Year Growth Response to Soil Type and Competing Vegetation. Forests, 16(9), 1442. https://doi.org/10.3390/f16091442






