Seven Millennia of Cedrus atlantica Forest Dynamics in the Western Rif Mountains (Morocco)
Abstract
1. Introduction
2. Materials and Methods
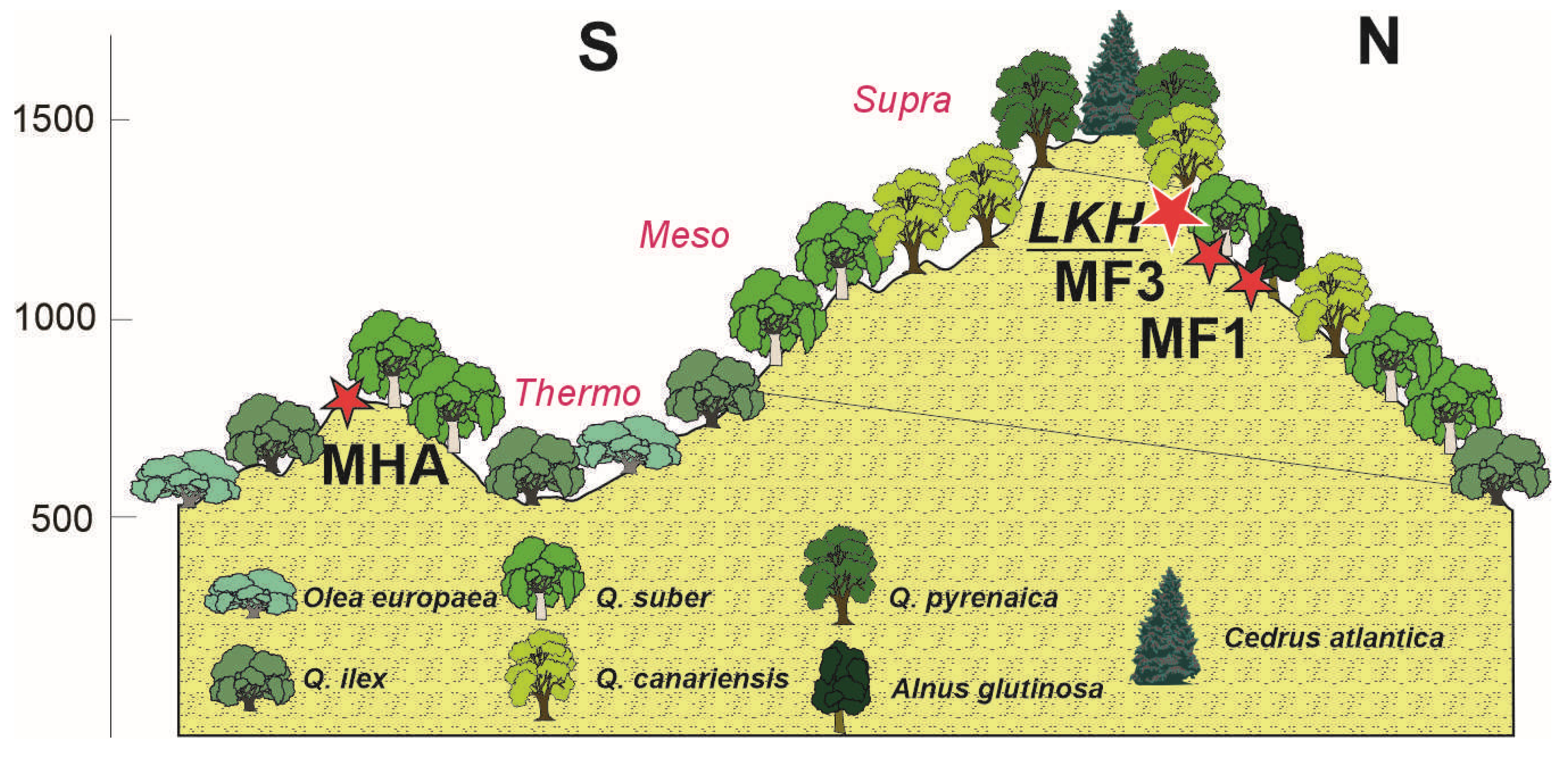
2.1. Study Area

2.2. Coring and Chronology
2.3. Pollen Palynomorphs, NPPs and Charcoal Analyses
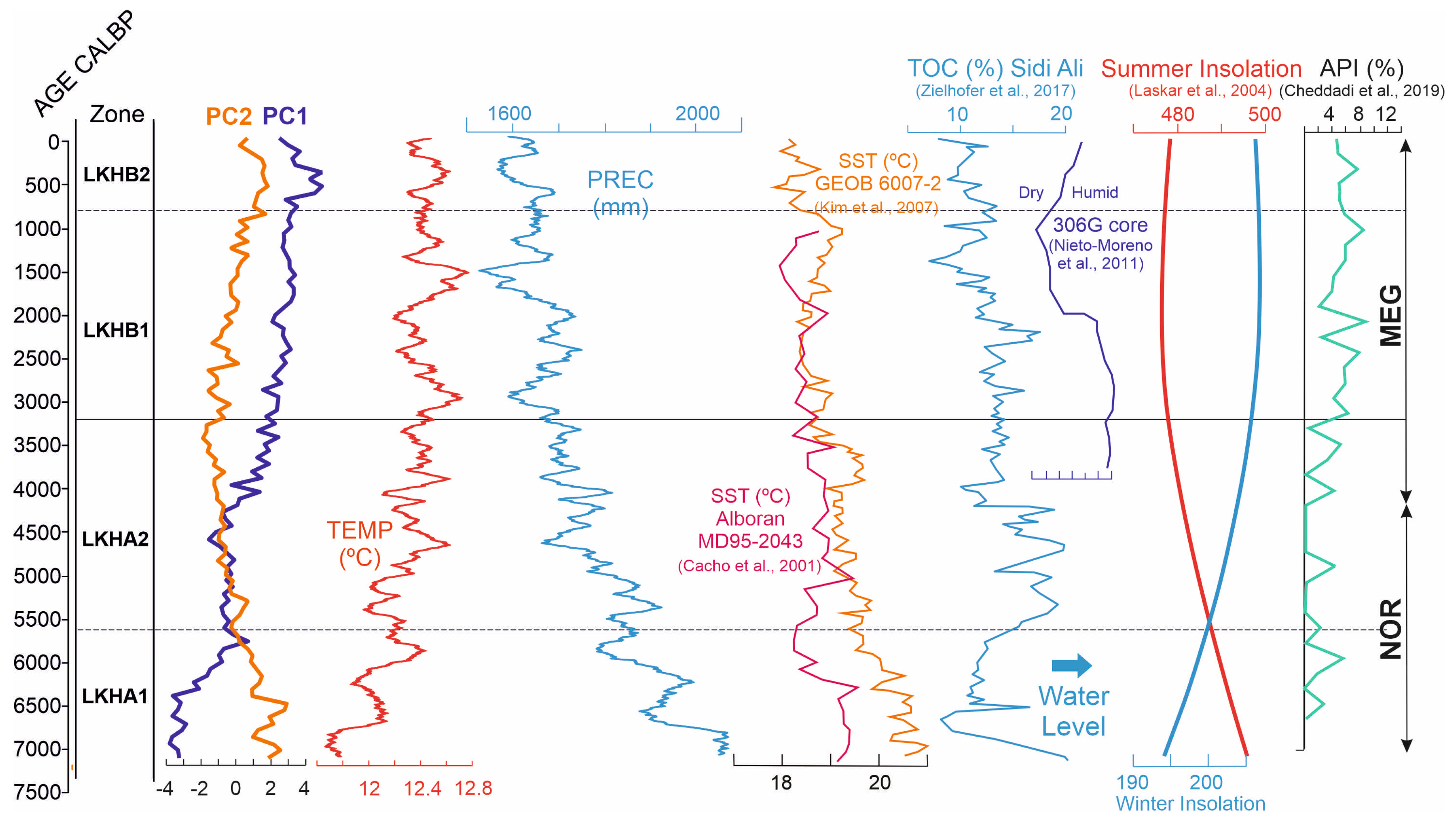
2.4. Paleoclimate Reconstruction
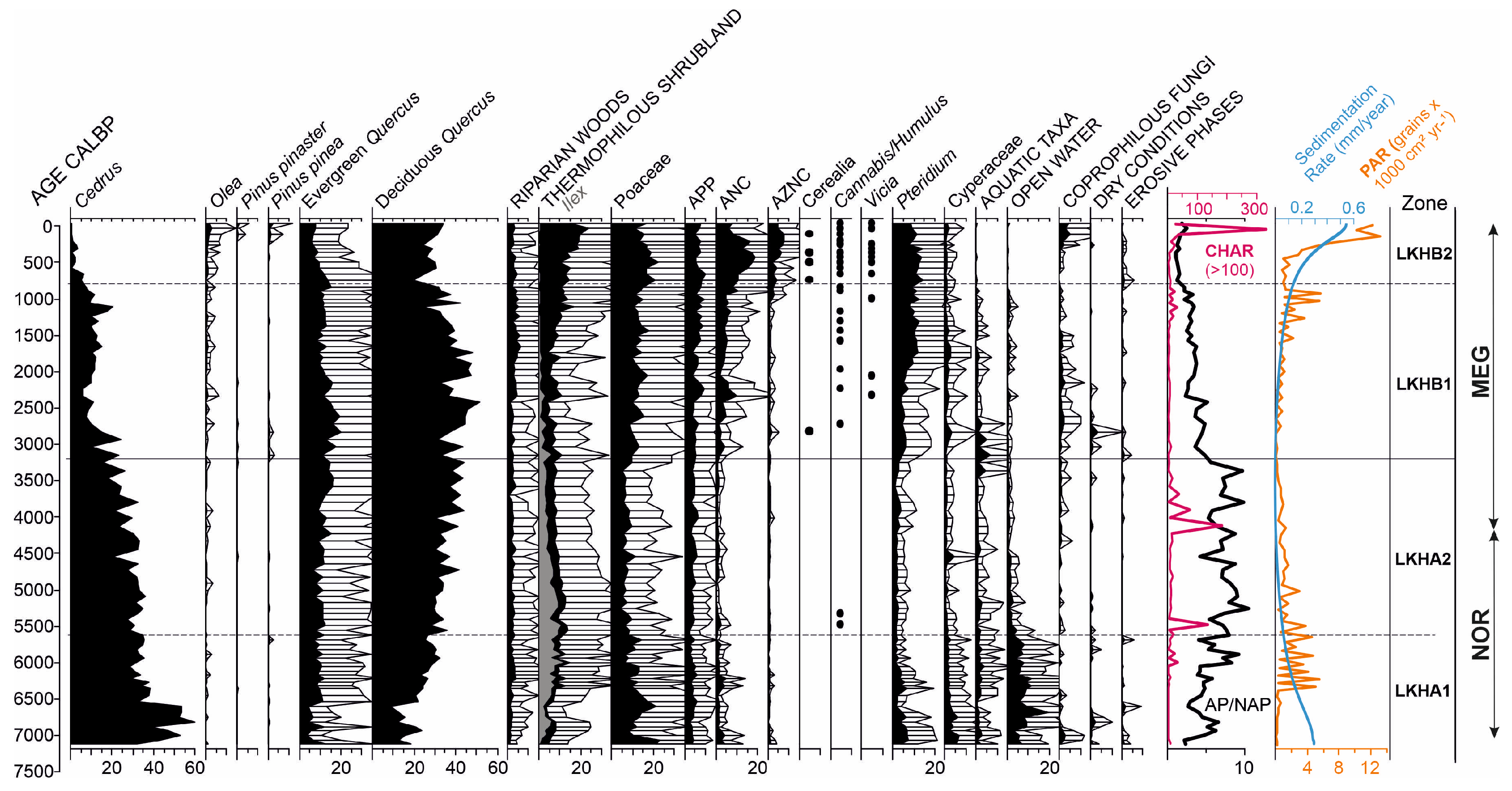
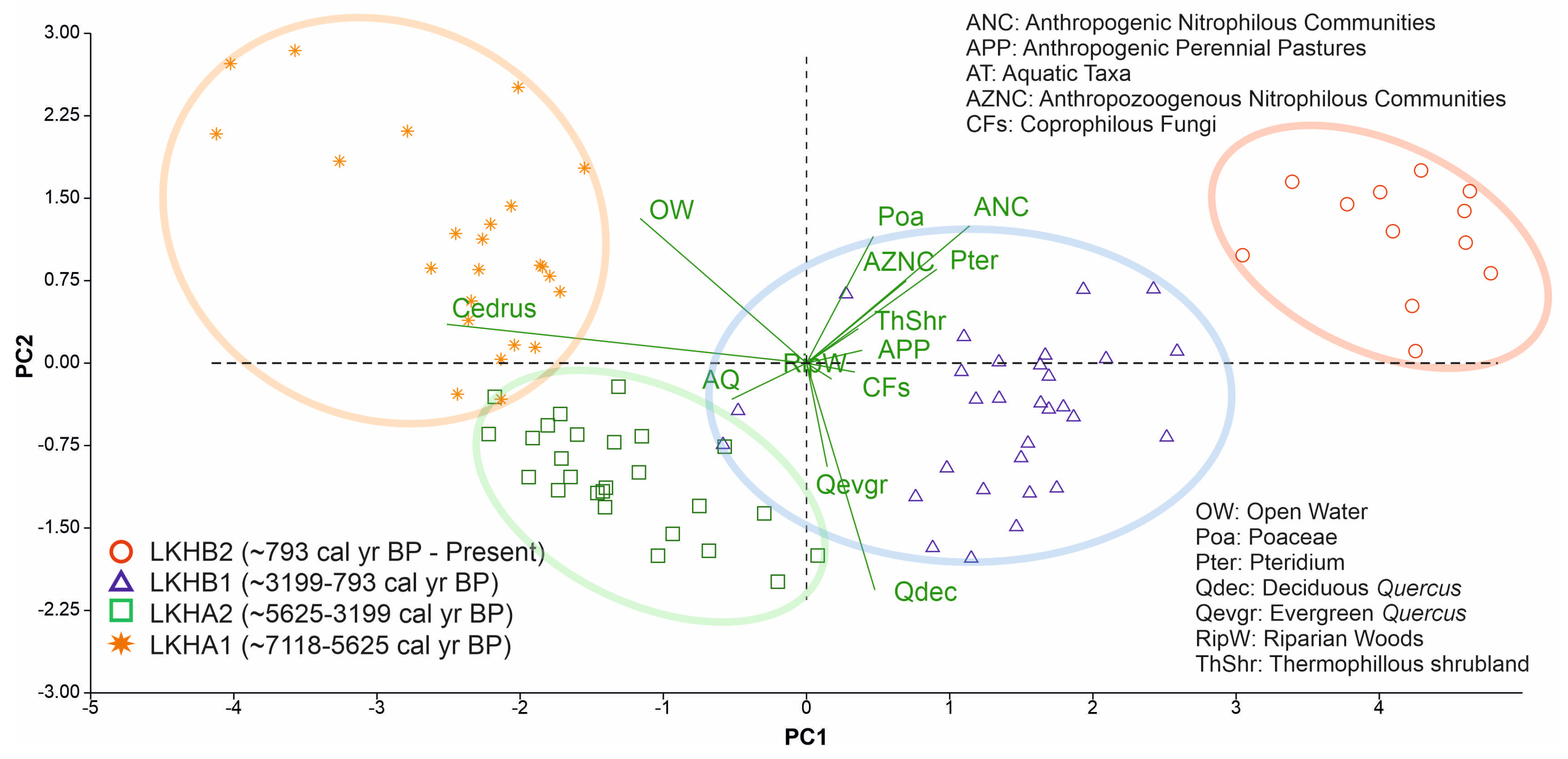
3. Results
4. Discussion
4.1. The Maximum Expansion of Cedar Forests at the Mid-Elevation Mountain Site—Subzone LKHA1 (122–88 cm; ~7118–5625 cal yr BP)
4.2. Cedar-Oak Transition and Early Human Disturbance Under Increasing Climatic Stress—Subzone LKHA2 (88–62 cm; ~5625–3200 ca yrl BP)
4.3. Increasing Human Activity Is Becoming the Main Driver of Tree-Cover Loss—Subzone LKHB1 (62–32 cm; ~3200–800 cal yr BP)
4.4. Final Collapse of Cedar Forests at Mid-Elevation Under Combined Climatic and Anthropogenic Pressure—Subzone LKHB2 (32–0 cm; ~800 cal yr BP- Present)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Tito, R.; Vasconcelos, H.L.; Feeley, K.J. Mountain Ecosystems as Natural Laboratories for Climate Change Experiments. Front. For. Glob. Change 2020, 3, 38. [Google Scholar] [CrossRef]
- IUCN. Mediterranean Mountains in a Changing World. Guidelines for Developing Action Plans; Regato, P., Salman, R., Eds.; IUCN: Gland, Switzerland; Malaga, Spain, 2008. [Google Scholar]
- Médail, F.; Quézel, P. Biodiversity hotspots in the Mediterranean Basin: Setting global conservation priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Elenga, H.; Peyron, O.; Bonnefille, R.; Prentice, I.C.; Jolly, D.; Cheddadi, R.; Guiot, J.; Andrieu, V.; Bottema, S.; Buchet, G.; et al. Pollen-based reconstruction for southern Europe and Africa 18 000 years ago. J. Biogeogr. 2000, 27, 621–634. [Google Scholar] [CrossRef]
- Kunert, N.; Hajek, P.; Hietz, P.; Morris, H.; Rosner, S.; Tholen, D. Summer temperatures reach the thermal tolerance threshold of photosynthetic decline in temperate conifers. Plant Biol. 2022, 24, 1254–1261. [Google Scholar] [CrossRef]
- Linares, J.C.; Tíscar, P.A.; Camarero, J.J.; Taiqui, L.; Viñegla, B.; Seco, J.; José, M.; Carreira, J.A. Tree growth decline on relict Western-Mediterranean mountain forests: Causes and impacts. In Forest Decline: Causes and Impacts; Jenkins, J.J., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011. [Google Scholar]
- Camarero, J.J.; Sánchez-Salguero, R.; Sangüesa-Barreda, G.; Lechuga, V.; Viñegla, B.; Seco, J.I.; Taiqui, L.; Carreira, J.A.; Linares, J.C. Drought, axe and goats. More variable and synchronized growth forecasts worsening dieback in Moroccan Atlas cedar forests. Sci. Total Environ. 2021, 765, 142752. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.C. The Physical Geography of the Mediterranean; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Kuennecke, B.H. Temperate Forest Biomes; Bloomsbury Publishing: New York, NY, USA, 2008. [Google Scholar]
- Loidi, J.; Navarro-Sánchez, G.; Vynokurov, D. Climatic definitions of the world’s terrestrial biomes. Veg. Classif. Surv. 2022, 3, 231–271. [Google Scholar] [CrossRef]
- Faber-Langendoen, D.; Keeler, T.; Meidinger, D.; Josse, C.; Weakley, A.; Tart, D.; Navarro, G.; Hoagland, B.; Ponomarenko, S.; Fults, G.; et al. Classification and Description of World Formation Types; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2016.
- Benabid, A. Les écosystèmes forestiers, préforestiers et presteppiques du Maroc: Diversité, repartition biogéographique et problèmes posés par leur aménagement. Forêt Méditerranéenne 1985, VII, 53–64. [Google Scholar]
- Cheddadi, R.; Henrot, A.J.; François, L.; Boyer, F.; Bush, M.; Carre, M.; Coissac, E.; De Oliveira, P.E.; Ficetola, F.; Hambuckers, A.; et al. Microrefugia, climate change, and conservation of Cedrus atlantica in the Rif Mountains, Morocco. Front. Ecol. Evol. 2017, 5, 114. [Google Scholar] [CrossRef]
- Cheddadi, R.; Nourelbait, M.; François, L.; Fady, B.; Lefèvre, F.; Khater, C. Come rain or come shine, the species richness will decline in the Moroccan mountains. Glob. Ecol. Conserv. 2024, 52, e02986. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Di Pasquale, G.; Mulligan, M.; Di Martino, P.; Rego, F. (Eds.) Recent Dynamics of the Mediterranean Vegetation and Landscape; John Wiley & Sons: Chichester, UK, 2004. [Google Scholar]
- Thomas, P. Cedrus atlantica. In IUCN Red List of Threatened Species, Version 2013.1; IUCN: Gland, Switzerland, 2013. [Google Scholar] [CrossRef]
- Linstädter, J.; Kehl, M.; Broich, M.; López-Sáez, J.A. Chronostratigraphy, site formation processes and pollen record of Ifri n’Etsedda, NE Morocco. Quatern. Int. 2016, 410, 6–29. [Google Scholar] [CrossRef]
- Ruiz-Alonso, M.; Abel-Schaad, D.; López-Sáez, J.A.; Martínez Sánchez, R.M.; Vera-Rodríguez, J.C.; Pérez-Jordàn, G.; Peña-Chocarro, L.; Alba-Sánchez, F. Late glacial–postglacial North African landscape and forest management: Palynological and anthracological studies in the caves of Kaf Taht el-Ghar and El Khil (Tingitana Peninsula, Morocco). Rev. Palaeobot. Palyno. 2021, 293, 104486. [Google Scholar] [CrossRef]
- Zapata, L.; López-Sáez, J.A.; Ruiz-Alonso, M.; Linstädter, J.; Pérez-Jordà, G.; Morales, J.; Kehl, M.; Peña-Chocarro, L. Holocene environmental change and human impact in NE Morocco: Palaeobotanical evidence from Ifri Oudadane. Holocene 2013, 23, 1286–1296. [Google Scholar] [CrossRef]
- Abel-Schaad, D.; Iriarte, E.; López-Sáez, J.A.; Pérez-Díaz, S.; Ruiz, S.S.; Cheddadi, R.; Alba-Sánchez, F. Are Cedrus atlantica forests in the Rif Mountains of Morocco heading towards local extinction? Holocene 2018, 28, 1023–1037. [Google Scholar] [CrossRef]
- Cheddadi, R.; Bouaissa, O.; Rhoujjati, A.; Dezileau, L. Environmental changes in the Moroccan western Rif mountains over the last 9,000 years. Quaternaire 2016, 27, 15–25. [Google Scholar] [CrossRef]
- Barbero, M.; Bonin, G.; Loisel, R.; Quézel, P. Changes and disturbances of forest ecosystems caused by human activities in the western part of the Mediterranean basin. Vegetatio 1990, 87, 151–173. [Google Scholar] [CrossRef]
- Abel-Schaad, D.; Sabariego-Ruiz, S.; Lopez Saez, J.A.; Alba-Sanchez, F. 69. Targuist mire (Central Rif, Morocco). Grana 2023, 62, 218–220. [Google Scholar] [CrossRef]
- Abel-Schaad, D.; Alba-Sanchez, F.; Lopez Saez, J.A.; Gonzalez-Hernandez, A.; Martin-Girela, M.I.; Cheddadi, R. 78. Fouara (Western Rif, Morocco). Grana 2024, 63, 402–404. [Google Scholar] [CrossRef]
- Birks, H.J.B. Contributions of Quaternary botany to modern ecology and biogeography. Plant Ecol. Divers. 2019, 12, 189–385. [Google Scholar] [CrossRef]
- Cheddadi, R.; Taberlet, P.; Boyer, F.; Coissac, E.; Rhoujjati, A.; Urbach, D.; Remy, C.; Khater, C.; el Antry, S.; Aoujdad, J.; et al. Priority conservation areas for Cedrus atlantica in the Atlas Mountains, Morocco. Conserv. Sci. Pract. 2022, 4, e12680. [Google Scholar] [CrossRef]
- Muller, S.D.; Rhazi, L.; Andrieux, B.; Bottollier-Curtet, M.; Fauquette, S.; Saber, E.-R.; Rifai, N.; Daoud-Bouattour, A. Vegetation history of the western Rif Mountains (NW Morocco): Origin, late-Holocene dynamics and human impact. Veg. Hist. Archaeobot. 2015, 24, 487–501. [Google Scholar] [CrossRef]
- Muller, S.D.; Daoud-Bouattour, A.; Fauquette, S.; Bottollier-Curtet, M.; Rifai, N.; Robles, M.; Saber, E.-R.; El Madihi, M.; Moukrim, S.; Rhazi, L. Holocene history of peatland communities of central Rif (Northern Morocco). Geobios 2022, 70, 35–53. [Google Scholar] [CrossRef]
- Ajbilou, R.; Marañón, T.; Arroyo, J. Ecological and biogeographical analyses of Mediterranean forests of northern Morocco. Acta Oecol. 2006, 29, 104–113. [Google Scholar] [CrossRef]
- Benabid, A. Bref aperçu sur la zonation altitudinale de la végétation climacique du Maroc. Ecol. Mediterr. 1982, 8, 301–315. [Google Scholar] [CrossRef]
- Charco, J. El Bosque Mediterráneo en el Norte de África: Biodiversidad y Lucha Contra la Desertización; Agencia Española de Cooperación: Madrid, Spain, 1999. [Google Scholar]
- Taiqui, L.; Cantarino, C.M. Eléments historiques d’analyse écologique des paysages montagneux du Rif Occidental (Maroc). Mediterranea. Ser. De Estud. Biológicos 1997, 16, 23–35. [Google Scholar]
- Carrión Marco, Y.; Vidal-Matutano, P.; Morales, J.; Henríquez Valido, P.; Potì, A.; Kehl, M.; Linstädter, J.; Weniger, G.-C.; Mikdad, A. Late glacial landscape dynamics based on macrobotanical data: Evidence from Ifri El Baroud (NE Morocco). Environ. Archaeol. 2021, 26, 131–145. [Google Scholar] [CrossRef]
- Barton, R.N.E.; Collcutt, S.N.; Carrión Marco, Y.; Clark-Balzan, L.; Debenham, N.D.; Morales-Mateos, J.; Bouzouggar, A. Reconsidering the MSA to LSA transition at Taforalt Cave (Morocco) in the light of new multi-proxy dating evidence. Quat. Int. 2016, 413, 36–49. [Google Scholar] [CrossRef]
- Reimer, P.J.; Austin, W.E.N.; Bard, E.; Babyliss, A.; Blackwell, P.G.; Ramsey, C.B.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Blaauw, M. Methods and code for ‘classical’ age-modelling of radiocarbon sequences. Quat. Geochronol. 2010, 5, 512–518. [Google Scholar] [CrossRef]
- Faegri, K.; Iversen, J. Textbook of Pollen Analysis; John Wiley and Sons: Chichester, UK, 1989. [Google Scholar]
- Goeury, C.; de Beaulieu, J.L. A propos de la concentration du pollen à l’aide de la liqueur de Thoulet dans les sédiments minéraux. Pollen Et Spores 1979, 21, 239–251. [Google Scholar]
- Stockmarr, J. Tablets with spores used in absolute pollen analysis. Pollen Et Spores 1971, 13, 614–621. [Google Scholar]
- Moore, P.D.; Webb, J.A.; Collinson, M.E. Pollen Analysis; Blackwell: Oxford, UK, 1991. [Google Scholar]
- Reille, M. Pollen et Spores d’Europe et d’Afrique du Nord, 2nd ed.; Laboratoire de Botanique Historique et Palynologie: Marseille, France, 1999. [Google Scholar]
- Miola, A. Tools for Non-Pollen Palynomorphs (NPPs) analysis: A list of Quaternary NPP types and reference literature in English language (1972–2011). Rev. Palaeobot. Palynol. 2012, 186, 142–161. [Google Scholar] [CrossRef]
- Mooney, S.D.; Tinner, W. The analysis of charcoal in peat and organic sediments. Mires Peat 2011, 7, 1–18. [Google Scholar]
- Long, C.J.; Whitlock, C. Fire and vegetation history from the coastal rain forest of the western Oregon Coast Range. Quat. Res. 2002, 58, 215–225. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 29 September 2023).
- Cheddadi, R.; Palmisano, A.; López-Sáez, J.A.; Nourelbait, M.; Zielhofer, C.; Tabel, J.; Rhoujjati, A.; Khater, C.; Woodbridge, J.; Lucarini, G.; et al. Human demography changes in Morocco and environmental imprint during the Holocene. Holocene 2019, 29, 816–829. [Google Scholar] [CrossRef]
- Bell, B.A.; Fletcher, W.J. Modern surface pollen assemblages from the Middle and High Atlas, Morocco: Insights into pollen representation and transport. Grana 2016, 55, 286–301. [Google Scholar] [CrossRef]
- Romera-Romera, D.; Alba-Sánchez, F.; Abel-Schaad, D.; Nieto-Lugilde, D. Replicable fine-spatio-temporal climate data for long-term ecology in the Western Mediterranean. Sci. Data 2025, 12, 747. [Google Scholar] [CrossRef] [PubMed]
- Cacho, I.; Grimalt, J.O.; Canals, M.; Sbaffi, L.; Shackleton, N.J.; Schönfeld, J.; Zahn, R. Variability of the western Mediterranean Sea surface temperature during the last 25,000 years and its connection with the Northern Hemisphere climatic changes. Paleoceanography 2001, 16, 40–52. [Google Scholar] [CrossRef]
- Kim, J.H.; Meggers, H.; Rimbu, N.; Lohmann, G.; Freudenthal, T.; Mu, P.J.; Schneider, R.R. Impacts of the North Atlantic gyre circulation on Holocene climate off northwest Africa. Geology 2007, 35, 387–390. [Google Scholar] [CrossRef]
- Zielhofer, C.; Fletcher, W.J.; Mischke, S.; de Batist, M.; Campbell, J.F.E.; Joannin, S.; Tjallingii, R.; El Hamouti, N.; Junginger, A.; Stele, A.; et al. Atlantic forcing of Western Mediterranean winter rain minima during the last 12,000 years. Quat. Sci. Rev. 2017, 157, 29–51. [Google Scholar] [CrossRef]
- Nieto-Moreno, V.; Martínez-Ruiz, F.; Giralt, S.; Jiménez-Espejo, F.; Gallego-Torres, D.; Rodrigo-Gámiz, M.; García-Orellana, J.; Ortega-Huertas, M.; de Lange, G.J. Tracking climate variability in the western Mediterranean during the Late-Holocene: A multiproxy approach. Clim. Past 2011, 7, 1395–1414. [Google Scholar] [CrossRef]
- Laskar, J.; Robutel, P.; Joutel, F.; Gaustineau, M.; Correia, A.C.M.; Levrard, B. A long-term numerical solution for the insolation quantities of the Earth. Astron. Astrophys. 2004, 428, 261–285. [Google Scholar] [CrossRef]
- Walker, M.; Head, M.J.; Lowe, J.; Berkelhammer, M.; Björck, S.; Cheng, H.; Cwynar, L.C.; Fisher, D.; Gkinis, V.; Long, A.; et al. Subdividing the Holocene Series/Epoch: Formalization of stages/ages and subseries/subepochs, and designation of GSSPs and auxiliary stratotypes. J. Quat. Sci. 2019, 34, 173–186. [Google Scholar] [CrossRef]
- Trouet, V.; Esper, J.; Graham, N.E.; Baker, A.; Scourse, J.D.; Frank, D.C. Persistent positive North Atlantic Oscillation mode dominated the Medieval Climate Anomaly. Science 2009, 324, 78–80. [Google Scholar] [CrossRef]
- Linstädter, J.; Broich, M.; Weninger, B. Defining the Early Neolithic of the Eastern Rif, Morocco–Spatial distribution, chronological framework and impact of environmental changes. Quatern. Int. 2018, 472, 272–282. [Google Scholar] [CrossRef]
- Leroy, S.A.; Freitas, M.C.; Andrade, C.; Cearreta, A.; Maanan, M.; Costa, P. A∼ 6,600 year history of vegetation changes and sediment infill of the Moulay Bousselham Lagoon, Atlantic Morocco. J. Afr. Earth Sci. 2025, 223, 105492. [Google Scholar] [CrossRef]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, R.F.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chang. 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Campbell, J.F.; Fletcher, W.J.; Joannin, S.; Hughes, P.; Rhanem, M.; Zielhofer, C. Environmental drivers of Holocene forest development in the Middle Atlas, Morocco. Front. Ecol. Evol. 2017, 5, 113. [Google Scholar] [CrossRef]
- Leunda, M.; González-Sampériz, P.; Gil-Romera, G.; Aranbarri, J.; Moreno, A.; Oliva-Urcia, B.; Sevilla-Callejo, M.; Valero-Garcésa, B. The Late-Glacial and Holocene Marboré Lake sequence (2612 m asl, Central Pyrenees, Spain): Testing high altitude sites sensitivity to millennial scale vegetation and climate variability. Glob. Planet Chang. 2017, 157, 214–231. [Google Scholar] [CrossRef]
- Martín-Puertas, C.; Jiménez-Espejo, F.; Martínez-Ruiz, F.; Nieto-Moreno, V.; Rodrigo, M.; Mata, M.P.; Valero-Garcés, B.L. Late-Holocene climate variability in the southwestern Mediterranean region: An integrated marine and terrestrial geochemical approach. Clim. Past 2010, 6, 807–816. [Google Scholar] [CrossRef]
- Langgut, D.; Cheddadi, R.; Carrión, J.S.; Cavanagh, M.; Colombaroli, D.; Eastwood, W.J.; Greenberg, R.; Litt, T.; Mercuri, A.M.; Miebach, A.; et al. The origin and spread of olive cultivation in the Mediterranean Basin: The fossil pollen evidence. Holocene 2019, 29, 902–922. [Google Scholar] [CrossRef]
- Park, T.K.; Boum, A. Historical Dictionary of Morocco; Scarecrow Press: Lanham, MD, USA, 2005. [Google Scholar]
- Cheddadi, R.; Nourelbait, M.; Bouaissa, O.; Tabel, J.; Rhoujjati, A.; López-Sáez, J.A.; Alba-Sánchez, F.; Khater, C.; Ballouche, A.; Dezileau, L.; et al. A history of human impact on Moroccan mountain landscapes. Afr. Archaeol. Rev. 2015, 32, 233–248. [Google Scholar] [CrossRef]


| Depth (cm) | Lab Code | 14C Age | Age cal yr BP | Probability | Median | |
|---|---|---|---|---|---|---|
| Min | Max | |||||
| 25 | Poz-136570 | 580 ± 30 BP | 505 | 554 | 91.6 | 528.2 |
| 611 | 620 | 3.2 | ||||
| 40 | Poz-136571 | 1385 ± 30 BP | 1276 | 1343 | 95 | 1309.5 |
| 55 | Poz-136497 | 2410 ± 30 BP | 2351 | 2496 | 81.2 | 2458.7 |
| 2596 | 2612 | 2.9 | ||||
| 2637 | 2684 | 10.9 | ||||
| 75 | Poz-136498 | 4175 ± 30 BP | 4588 | 4594 | 1 | 4716 |
| 4614 | 4766 | 73.1 | ||||
| 4783 | 4833 | 20.8 | ||||
| 95 | Poz-136499 | 5350 ± 40 BP | 6001 | 6216 | 85.2 | 6124.8 |
| 6239 | 6271 | 9.7 | ||||
| 121 | Poz-82284 | 6150 ± 50 BP | 6904 | 7169 | 95 | 7036.5 |
| Group | Pollen Types and Non-Pollen Palynomorphs |
|---|---|
| Riparian woods | Alnus, Prunus, Salix |
| Thermophilous shrubland | Cistus, Cytisus, Erica arborea, Helianthemum, Ilex, Phillyrea, Viburnum |
| Anthropogenic Perennial Pastures (APP) | Apiaceae, Artemisia, Caryophyllaceae, Fabaceae, Liliaceae, Lotus, Rosaceae |
| Anthropogenic Nitrophilous Communities (ANC) | Anthemis, Asphodelus albus, Aster, Cardueae, Cichorioideae |
| Anthropozoogenic Nitrophilous Communities (AZNC) | Amaranthaceae, Plantago spp., Rumex spp. |
| Aquatic taxa (AT) | Myriophyllum, Ranunculaceae |
| Open water (OW) | Mougeotia |
| Coprophilous fungi (CFs) | HdV 7A, HdV 55A, HdV 112, HdV 113, HdV 172, HdV 368 |
| Dry conditions | HdV 16 |
| Erosive phases | HdV 207 (Glomus), Pseudoschizaea circula |
| SUBZONE Depth (cm)/Age cal BP | Trees/Thermophilous Shrubland (ThSh) | Herbs | Ferns/Hygrophytes/Aquatic Taxa/Open Water Algae (OW) | NPPs/CHAR/Pollen Concentration (CC) |
|---|---|---|---|---|
| LKHA1 122–88 cm ~7118–5625 cal BP | AP/NAP ~5 Maxima of Cedrus (~60%) ~6800 cal BP and medium levels thereafter (~33%) Increasing levels of both evergreen (~9%) and deciduous (~29%) Quercus, as well as ThSh (4%–10%) Low levels of Riparian woods (~2%) Sporadic occurrences of Pinus and Olea | Decreasing percentages of Poaceae (21%–14%) Low levels of APP (~4%), ANC (~2%) and AZNC (<1%) | Decreasing levels of Pteridium and Cyperaceae Maxima of OW | Sporadic occurrences of Coprophilous fungi Low levels of CHAR Initial minima of Pollen CC |
| LKHA2 88–62 cm ~5625–3199 cal BP | AP/NAP ~7.5 Steady decrease in Cedrus (31%–21%) against evergreen (11%–14%) and deciduous (36%–41%) Quercus Low levels of Riparian woods (~2.5%) Sporadic occurrences of Olea Slight decrease in ThSh (~12%–8%) | Minima of Poaceae (~7%), ANC (~1%) and AZNC (<1%) APP remain (~4%) | Low levels of Pteridium and Cyperaceae Low levels of OW and Aquatic Taxa with a final peak | Sporadic occurrences of Coprophilous fungi Significant peaks of CHAR ~5500 and 4150 cal BP Decreasing pollen CC |
| LKHB1 62–32 cm ~3199–793 cal BP | AP/NAP ~3.5 Steady decline of Cedrus (~18%–9%) Initial maxima of evergreen (20%) and deciduous (51%) Quercus and subsequent slight decrease Increasing levels of Olea, Riparian woods (~3.5%) and ThSh (~8%–12%) | Increasing percentages of Poaceae (~11%–13%), APP (~5%–7%), ANC (~3%–7%) and AZNC (>2%) First occurrences of Cerealia, Cannabis and Vicia | Increasing levels of Pteridium and decline of Cyperaceae Initial significant levels of Aquatic Taxa and subsequent decrease Low levels of OW | Continuous levels of Coprophilous fungi Initial peaks of Dry phases Low levels of CHAR with slight final peaks Increasing Pollen CC |
| LKHB2 32–2 cm ~793 cal BP-present | AP/NAP ~2.6 Demise of Cedrus Decrease in evergreen (~12%–7%) and increase in deciduous (~25%–31%) Quercus after an initial decline Increase in Olea (~2%) and Pinus (>1%) Maxima of ThSh (~19%) | Maxima of Poaceae (~22%), APP (~10%), ANC (~19%) and AZNC (~8%) More continuous occurrences of Cerealia, Cannabis and Vicia | Maxima of Pteridium and low levels of Cyperaceae Virtual demise of both Aquatic Taxa and OW | Maxima of Coprophilous fungi and increase in Erosive phases Final maximum of CHAR Increasing Pollen CC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alba-Sánchez, F.; Abel-Schaad, D.; López-Sáez, J.A.; Romera-Romera, D.; Pérez-Díaz, S.; González-Hernández, A. Seven Millennia of Cedrus atlantica Forest Dynamics in the Western Rif Mountains (Morocco). Forests 2025, 16, 1441. https://doi.org/10.3390/f16091441
Alba-Sánchez F, Abel-Schaad D, López-Sáez JA, Romera-Romera D, Pérez-Díaz S, González-Hernández A. Seven Millennia of Cedrus atlantica Forest Dynamics in the Western Rif Mountains (Morocco). Forests. 2025; 16(9):1441. https://doi.org/10.3390/f16091441
Chicago/Turabian StyleAlba-Sánchez, Francisca, Daniel Abel-Schaad, José Antonio López-Sáez, Daniel Romera-Romera, Sebastián Pérez-Díaz, and Antonio González-Hernández. 2025. "Seven Millennia of Cedrus atlantica Forest Dynamics in the Western Rif Mountains (Morocco)" Forests 16, no. 9: 1441. https://doi.org/10.3390/f16091441
APA StyleAlba-Sánchez, F., Abel-Schaad, D., López-Sáez, J. A., Romera-Romera, D., Pérez-Díaz, S., & González-Hernández, A. (2025). Seven Millennia of Cedrus atlantica Forest Dynamics in the Western Rif Mountains (Morocco). Forests, 16(9), 1441. https://doi.org/10.3390/f16091441