Abstract
Pine wilt disease (PWD) is a serious forest disease caused by pine wood nematode (PWN). To examine the relationship between coniferous endophytes and PWD resistance, this study investigated endophytic bacterial and fungal communities in five conifer species: two Japanese black pine populations (Pinus thunbergii from Qingdao University, PQ, and Fushan Forest Park, PF), Chinese arborvitae (Platycladus orientalis, PO), cedar (Cedrus deodara, CD), and Masson pine (Pinus massoniana, PM). Results showed a strong correlation between endophytic microbial diversity and PWD resistance. PO with high PWD resistance hosted the most unique bacterial species, while PM with low PWD resistance had the fewest unique bacteria and significantly lower ACE and Shannon indices. At the bacterial genus level, dominant genera in resistant conifers often showed high nematocidal activity, whereas those in susceptible plants boosted nematode reproduction. PQ featured the unique dominant genus Pantoea, and PO’s unique Acinetobacter and the shared genus Bacillus (with CD) both displayed high toxicity to PWNs. In contrast, PF’s Pseudomonas and PM’s Stenotrophomonas significantly promoted nematode reproduction. Fungal community analysis revealed that the unique endophytic fungi in PQ are more abundant than those in PF, and the Shannon index of its endophytic fungi is comparable to that of CD and significantly higher than that of PF. PF’s dominant fungal genus Pestalotiopsis might facilitate nematode invasion, and its fungal Shannon index is significantly lower than PQ’s. Eight bacterial strains were isolated from these five conifer plants, with six highly nematocidal strains originating from PQ, CD, and PO. This study offers evidence that endophytic microbial communities critically influence PWD resistance, offering a microbial basis for developing resistant conifer cultivars through microbiome engineering.
1. Introduction
Pine wilt disease (PWD) is caused by infection with the pine wood nematode (Bursaphelenchus xylophilus, PWN), which is highly devastating to pine trees and ranks among the most severe forest diseases globally [1,2]. PWD has cumulatively killed billions of pine trees in China, resulting in direct and indirect economic losses exceeding hundreds of billions of yuan [3,4,5]. With economic globalization, PWD continues to spread quickly, attracting widespread attention and being listed as a quarantine pest by multiple countries [6,7,8,9,10,11].
Current control strategies for PWD primarily involve physical, chemical, and biological measures targeting both PWN and its vector [12,13,14,15]. Trapping the primary vector (Monochamus alternatus) of PWN and removal of infected pine trees are two major physical control methods, which is labor-intensive, costly, and susceptible to environmental factors [16,17]. Chemical control relies primarily on insecticides, such as thiacloprid [18], imidacloprid [19], abamectin [20], organophosphates [21], and cypermethrin microcapsules (Green Weilai) [22], to kill PWN and its vectors. Nevertheless, long-term heavy use of chemicals risks environmental pollution and may induce nematicide resistance in PWN. Biological control demonstrates unique advantages and significant potential against PWD due to its environmental compatibility and sustainability. The parasitic wasp Sclerodermus guani has been used to kill M. alternatus by parasitizing its body, thereby reducing PWD transmission [23]. Studies also indicated that a Bacillus cereus strain isolated from Pinus elliottii exhibited high nematocidal activity against PWN, while significantly induced defense enzymes in Pinus massoniana [24,25]. Plantazolicin, a metabolite of Bacillus velezensis FZB42 from pepper rhizosphere soil, was reported to possess nematocidal activity [26]. A bacterial strain isolated from rhizosphere soil of P. thunbergii also demonstrated high PWN toxicity and protective effects on pine seedlings [27].
Plant endophytic microbial communities are closely linked to host plant resistance. Certain endophytes enhance their hosts resistance by producing antimicrobial compounds, competing for ecological niches, or inducing systemic resistance in plants [28,29]. Some endophytic bacteria in pines produce nematocidal metabolites that inhibit PWN growth [30]. Researchers also identified proteins with significant toxicity against PWN, including crystal proteins from Bacillus thuringiensis [31,32], alkaline protease Bace16, and neutral protease Bae16 from B. cereus [24,33]. Notably, Botrytis cinerea, which serves as a food source for PWN, produces volatile compounds (e.g., 3-octanol and 1-octen-3-ol) that attract nematodes and promote molting and reproduction [34]. Thus, the stability of endophytic microbiota is crucial for pine health and holds substantial potential for developing eco-efficient PWD control strategies.
With increasing evidence confirming synergistic pathogenicity between Bursaphelenchus xylophilus and its associated bacteria, the pine endophytic microbiome has attracted considerable research attention. P. massoniana and P. thunbergii are well known as susceptible pine species to PWD, Japanese cedar (Cryptomeria japonica) shows susceptibility to PWD in Japan but resistance in China, while there is no reported infection case for Chinese arborvitae (Platycladus orientalis) [35,36]. Preliminary studies in our lab revealed distinct PWN resistance between Japanese black pine Fushan (PF, susceptible) and Japanese black pine Qingdao University (PQ, resistant), accompanied by significant differences in nematode-associated bacterial populations [37]. Therefore, this study tried to analyze the differences in endophytic microbial communities among coniferous plants with different resistances trends and reveal correlation underlying differential PWD resistance among host plants and their endophytic bacteria and fungi.
2. Materials and Methods
2.1. Materials
Pinus thunbergii (PF) specimens were collected from Fushan Forest Park, Qingdao, China. P. thunbergii (PQ), Pinus massoniana (PM), Cedrus deodara (CD), and Platycladus orientalis (PO) branch samples were obtained from Qingdao University campus. Bursaphelenchus xylophilus was isolated from naturally PWN-infected P. thunbergii in Fushan Forest Park using the Baermann funnel technique [38]. Species identity was confirmed through morphological and molecular characterization [37]. Cultures are maintained at the Microbiology Laboratory, College of Life Sciences, Qingdao University.
2.2. Sample Processing
Healthy vegetative branches were collected from the southern outer side of the lower canopy of target trees. Samples (5–10 cm in length) were excised from disease-free segments located 5–10 cm below the branch apex. For each plant species, three biological replicates were prepared, with branch segments measuring approximately 10 cm in length and 0.5 cm in diameter. Replicate samples of all five species were harvested from the same individual plant to minimize genetic variability.
The samples were processed as follows: the epidermis was removed, samples were surface-sterilized with 75% ethanol, triple-rinsed with sterile water, and surface moisture was removed by blotting with sterile gauze. Samples were segmented (≈2 g), sealed in bags, and stored at 4 °C [39].
2.3. Determination of Endophytic Microbial Diversity and Data Processing and Analysis
DNA extraction, amplification (16S rDNA for bacteria; ITS for fungi) [40], and sequencing were performed by Shanghai Biozeron Biotechnology Co., Ltd., Shanghai, China. Sequences were clustered into Operational Taxonomic Units (OTUs) at a 97% similarity threshold. Taxonomic annotation was performed using reference dat37abases (SILVA for bacteria; UNITE for fungi). Community composition statistics were generated across all taxonomic ranks: Domain, Phylum, Class, Order, Family, Genus, and Species.
Rarefaction analysis was performed using Mothur v.1.21.1 [40] to reveal diversity metrics. Microbial community α-diversity was assessed through ACE (Abundance-based Coverage Estimator) and Shannon indices analysis. Statistical analyses were conducted to examine microbial community structures at different taxonomic levels and stacked bar charts were generated to visualize relative abundances of dominant taxa by R software (R-4.3.2). Three complementary non-parametric multivariate statistical tests (Adonis, ANOSIM, and MRPP) were performed using the vegan package in R to evaluate statistically significant differences in bacterial community diversity indices among samples [41,42,43,44]. Differences were considered significant at p < 0.05. Venn diagrams were constructed to analyze overlapping and unique operational taxonomic units (OTUs) across treatments.
2.4. Isolation of Endophytic Bacteria and Fungi
The five collected experimental materials were rinsed with sterile distilled water, and their barks were removed using horticultural scissors. The materials were surface-sterilized with 75% ethanol followed by washing 3 times with sterile distilled water. Approximately 5 g of each material was fragmented, put into a flask containing 15 mL sterile distilled water, and incubated at 37 °C, 160 rpm for 48 h.
Leachates from the five samples underwent serial 10-fold dilution. Aliquots (100 μL) of each dilution were spread onto Nutrient Agar plates for bacterial isolation or Martin’s agar plates for fungal isolation, and plates were cultured at 37 °C for bacteria and 28 °C for fungi. For culture of bacteria and fungi, single colony of bacteria or single hyphal tip of fungi was transferred into Nutrient Broth (NB) liquid medium or Potato Dextrose Broth (PDB) liquid medium, bacteria were cultured at 37 °C, 160 rpm for 24 h, and fungi were cultured at 28 °C, 180 rpm for 72 h.
2.5. Assay for Nematocidal Activity
The cultures of bacteria and fungi were centrifuged at 4 °C, 10,000× g for 10 min, and the supernatants were collected and filtered with membrane (0.22 μm) for nematocidal assay. A 450 μL aliquot of supernatant per well was dispensed into 48-well plates, followed by addition of 10 μL test PWN suspension (containing 100 PWNs). Each sample group was prepared with a minimum of three biological replicates. Distilled water was added to achieve a final volume of 500 μL/well. Control wells received an equivalent nematode suspension in sterile water. The experiment included four biological replicates. After 24 h incubation at 25 °C in dark, nematode mortality was observed and assessed under a 40× optical microscopy. Death was confirmed when nematodes exhibited straightened bodies with no movement upon probing. Corrected mortality was calculated as [33]:
Corrected mortality (%) = [(Treated mortality − Control mortality)/(1 − Control mortality)] × 100
2.6. Identification of Endophytic Bacteria
Routine bacterial identification referred to the handbook for systematic identification of common bacteria [45]. Molecular identification based on 16S rDNA was performed according to the method described previously [27].
3. Results
3.1. Assessment of Sequencing Depth for Endophytic Microorganisms
Gene library analysis results indicated that the rarefaction curves for bacteria and fungi in the five tested coniferous species asymptotically approached saturation (Figure 1), revealing adequate sequencing depth with high coverage. This depth sufficiently captured the authentic diversity of associated microorganisms in these plants. Bacterial communities clustered into 1648 operational taxonomic units (OTUs), taxonomically classified into 31 phyla, 50 classes, 513 genera and 737 species. Fungal communities formed 1677 OTUs, comprising 8 phyla, 33 classes, 489 genera and 844 species.
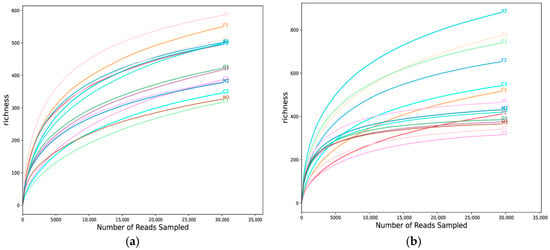
Figure 1.
OTU-based rarefaction curves of bacteria (a) and fungi (b) in five coniferous plants. X1, X2, X3: CD (Cedrus deodara); Q1, Q2, Q3: PQ (Pinus thunbergii from Qingdao University); C1, C2, C3: PO (Platycladus orientalis); F1, F2, F3: PF (Pinus thunbergii from Fushan Forest Park); M1, M2, M3: PM (Pinus massoniana).
OTU composition was analyzed, and Venn diagram was constructed based on endophytic bacterial and fungal OTU counts. Results showed that for bacterial OTU distribution, P. orientalis (PO) harbored the highest number of unique bacterial OTUs (387), accounting for 24.20% of the total. P. thunbergii Fushan (PF) and Qingdao University (PQ) exhibited 257 and 251 unique bacterial OTUs, representing 16.10% and 15.70%, respectively. P. massoniana (PM) had only 97 unique bacterial OTUs (6.06%) (Figure 2a). For fungal OTU distribution, PF contained 28 unique fungal OTUs, merely 1.83% of its total fungi. PQ showed 120 unique fungal OTUs (7.85%), approximately 4-fold higher than PF counterparts. C. deodara (CD) displayed the most unique fungal OTUs (151, 9.88%). PM and PO had 91 (5.95%) and 65 (4.25%) unique fungal OTUs, respectively (Figure 2b). Notably, only 2 fungal OTUs were shared exclusively between these two species (PM and PO), and the quantity difference is relatively large.
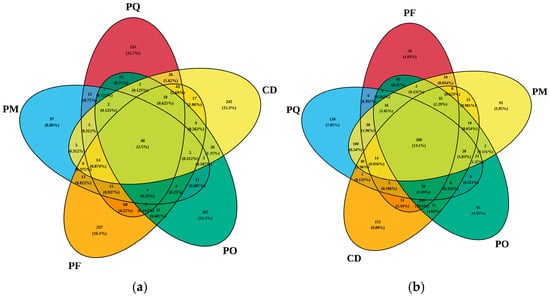
Figure 2.
Venn diagram of OTU number of bacteria (a) and fungi (b) in five coniferous plants.
3.2. Alpha Diversity Analysis of Endophytic Bacterial and Fungal Communities
Diversity analysis was conducted for endophytic bacterial and fungal communities in five coniferous species at the OTU level. Key findings included that for bacterial communities, PM exhibited significantly lower ACE index (richness) and Shannon index (diversity) compared to the other four coniferous species. PQ showed higher ACE and Shannon indices than PF, and PO had balanced ACE and Shannon indices (Figure 3a). For fungal communities, CD possessed a higher ACE index and Shannon indices, PF had a markedly lower Shannon index than PQ. PO displayed relatively consistent ACE and Shannon indices. PM and PQ showed lower ACE indices but a higher Shannon index (Figure 3b).
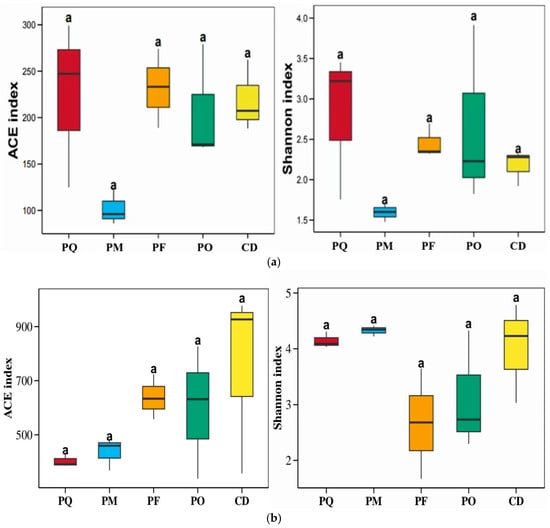
Figure 3.
Microbial community diversity indices of bacteria (a) and fungi (b) in five coniferous plants. Note: Letter “a” indicates significant differences between different plants at the p < 0.05 level.
3.3. Compositional Analysis of Endophytic Microbial Communities
For bacterial community composition, as shown in Figure 4, at the genus level, Burkholderia exhibited the highest relative abundance across all five coniferous species. The ratio was 60.17% (PF), 57.88% (PQ), 67.83% (CD), 57.59% (PO) and 72.47% (PM), respectively, confirming its dominance in these hosts. Other genera displayed distinct relative abundances: Halomonas dominated in PM, accounting for 23.72%. Methylobacterium was the dominant genus in PO (24.62%). Pantoea prevailed in PQ (37.95%). Pelagibacterium was a major genus in PF but showed minimal presence in PQ samples. Saccharopolyspora demonstrated significantly higher abundance in CD than in other species, establishing it as a key genus in this host (Figure 4).
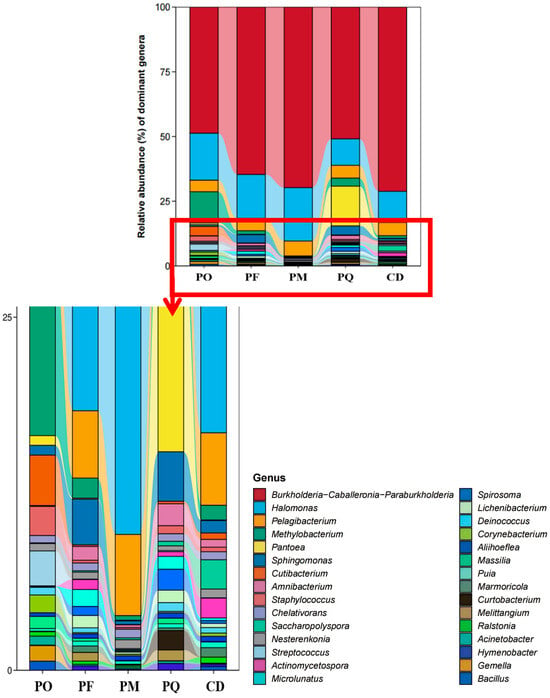
Figure 4.
Bacterial genera from endophytic microorganisms of five species of coniferous plants. Note: abundance threshold > 1%.
Analysis of fungal community composition revealed that Pestalotiopsis was a dominant genus in PF, but not in PQ. Neopestalotiopsis dominated in PO, accounting for ~16.89%. Cladosporium exhibited higher relative abundance in PM and PF compared to other species. Antennariella was a major genus in PQ but showed minimal presence in PF. Fusarium displayed relatively higher abundance in CD, PO, and PQ compared to PM and PF. Diplodia dominated in PF, while its relative abundance was exceptionally low in PQ (Figure 5).
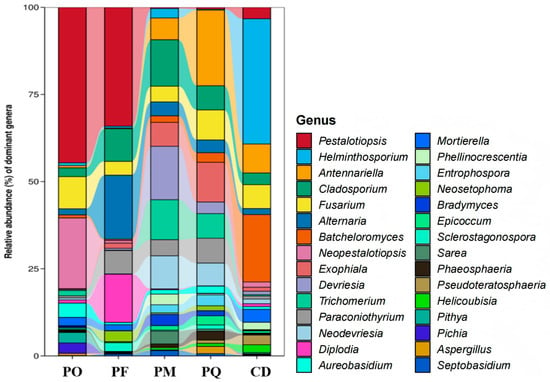
Figure 5.
Fungal genera from endophytic microorganisms of five species of coniferous plants. Note: abundance threshold > 1%.
3.4. Isolation and Nematocidal Analysis of Endophytic Bacteria and Fungi
Eight bacterial strains were isolated from healthy branch samples of five coniferous plant species. The strains distribution was summarized as follows: PF, PM, and CD each yielded one strain (PF1, PM1 and CD1); PQ provided two strains (PQ1 and PQ2); and PO contributed three strains (PO1, PO2 and PO3). The nematocidal activity of the eight bacterial strains was determined, and the results demonstrated that strains PQ1, PQ2, PO1, PO2, PO3, and CD1 all displayed high nematocidal activity at 24 h (p < 0.05). Among these, CD1 and PO3 showed the most prominent activity, achieving up to 100% mortality against B. xylophilus within 24 h. In contrast, strain PF1 exhibited very low nematocidal activity, and the mortality rate of PWN in the PM1 group was even lower than that of the control group, suggesting a potential promotional effect on the nematodes (Figure 6).
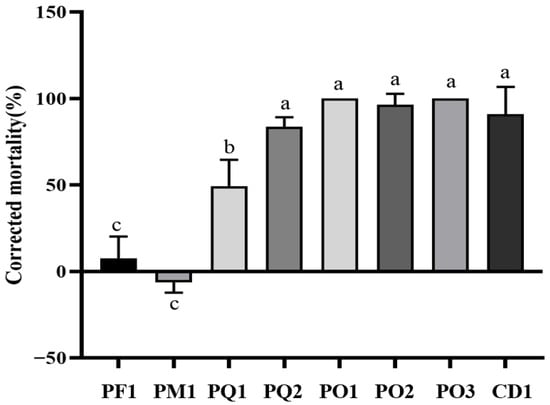
Figure 6.
Corrected mortality of PWNs treated with isolated bacteria. Note: Different lowercase letters indicate significant differences between different strains at the p < 0.05 level.
A total of thirteen fungal strains—F1–F6 from PF, Q1–Q3 from PQ, M1 and M2 from PM, X1 from CD, and C1 from PO—were isolated from healthy branch samples of five coniferous plant species, and their nematocidal activity was assessed using the immersion method. Results indicated that some strains exhibited significant lethality against nematodes (p < 0.05) (Figure 7). Fungi isolated from PM and PO showed approximately 10%–20% nematocidal activity within 24 h. Strain X1 isolated from CD demonstrated relatively higher activity, achieving 41.69% mortality in 24 h. Conversely, strains F1 and F4 displayed no nematocidal activity but instead stimulated nematode proliferation. Given the generally low nematocidal activity observed across all fungal strains, subsequent experiments will exclude fungal investigations.
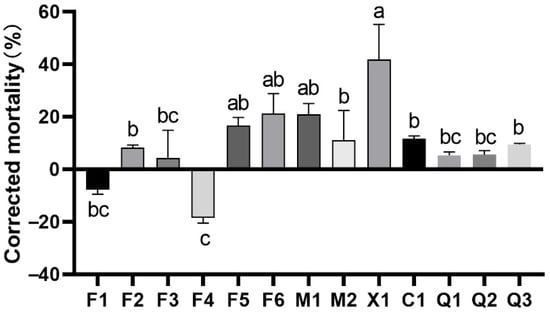
Figure 7.
Corrected mortality of PWNs treated with isolated fungi. F1–F6: Isolated from PF; M1, M2: Isolated from PM; X1: Isolated from CD; C1: Isolated from PO; Q1–Q3: Isolated from PQ. Note: Different lowercase letters indicate significant differences between different strains at the p < 0.05 level.
3.5. Identification of Isolated Endophytic Bacteria
The eight bacterial strains isolated from five coniferous species were taxonomically analyzed through morphology and 16S rDNA sequence. Referring handbook for systematic identification of common bacteria, we identified the strains as follows: PQ1, PQ2, and PO1 belong to Pantoea sp.; strain PO3 belongs to Acinetobacter sp.; strains PO2 and CD1 belong to Bacillus sp.; strain PF1 belongs to Pseudomonas sp., while strain PM1 belongs to Stenotrophomonas sp. (Table 1).

Table 1.
Molecular biological identification of the isolated bacteria.
4. Discussion
This study utilized metagenomic sequencing to analyze differences in endophytic microbial communities among five coniferous plants, and results revealed significant correlations between entophytic microbial community diversity and plant resistance to PWD. The ACE index (microbial richness) and Shannon index (diversity), respectively, characterized distinct aspects of plant-associated microbiomes [46]. For entophytic bacterial communities, PO’s relatively balanced ACE and Shannon indices suggest a potential role of unique entophytic microbial groups in its resistance to PWD. In contrast, PM exhibits significantly lower ACE and Shannon indices compared to other species, and the lack of microbial diversity might be one of the key factors behind its high susceptibility to PWN infestation. Compared with PF, PQ’s higher ACE and Shannon indices might contribute to its lower susceptibility to PWD. Genus-level analysis identified Pantoea as a dominant genus uniquely present in PQ but undetected in PF. Pantoea sp. have been documented to modulate the virulence of other phytopathogens [47]. CD’s unique dominant genus was Saccharopolyspora, known for spinosyn production—a potent biopesticide [48]. PO’s characteristic dominant genus was Acinetobacter, with certain species demonstrating high nematocidal activity against PWN [49]. Bacillus served as a shared dominant genus in CD and PO, with established nematocidal efficacy against PWN [50].
Several endophytic bacteria isolated from PQ, PO, and CD exhibited high nematocidal activity. Specifically, Pantoea sp. isolated from PQ and PO showed significant lethality to PWN, potentially attributable to their virulence-modulating properties [47]. Bacillus sp. from resistant species (PO and CD) also demonstrated strong nematocidal effects, aligning with findings by Cao et al. [50]. These bacterial traits might explain the enhanced PWD resistance in these plant species. Pseudomonas sp. from PWD-susceptible PF showed minimal nematocidal activity and might promote nematode reproduction [51]. Stenotrophomonas sp. from vulnerable PM stimulated nematode proliferation, and this genus dominated nematode-associated bacterial communities [49], potentially explaining the high susceptibility of PF and PM.
For the endophytic fungal community, PQ harbors richer unique fungi than PF with higher Shannon index, which may also contribute to its resistance to PWN. CD’s distinctive fungal community might underpin its PWD defense mechanisms. PO and PM contained similar proportions of unique endophytes; PO’s consistent ACE and Shannon indices suggest potential nematode-inhibiting functions, whereas PM’s fungi might facilitate nematode infestation. Pestalotiopsis dominated PF but was scarce in PQ. Fungi in this genus secrete cell-wall-degrading enzymes that convert complex organics into nutrients [52], potentially facilitating nematode colonization and explaining PF’s higher susceptibility. Neopestalotiopsis dominated PO, with certain strains exhibiting potent antioxidant activity against fungal pathogens [53], possibly contributing to low PWN susceptibility. Fusarium, dominant in CD, PO, and PQ, was reported to suppress the PWN vector (M. alternatus) [54,55], potentially impeding disease transmission. Diplodia prevailed in PF and serves as a food source for PWN [56], possibly sustaining nematode development post-infection.
Fungal strains isolated from PF and PM showed negligible PWN suppression or even promoted its growth, whereas CD-derived endophytes exhibited higher nematocidal activity, consistent with their respective resistance/susceptibility patterns. These fungal differences likely contribute to the vulnerability of PF and PM.
Across all five conifers, endophytic bacterial and fungal diversity/richness correlated with infection susceptibility of host plants. Enhanced resistance to PWD in PQ was partially attributed to Pantoea sp., while Acinetobacter sp. and Bacillus sp. contributed to CD and PO’s resistance to PWD. Conversely, nematode-growth-promoting fungi in PF and PM increased their susceptibility to this disease. These findings collectively demonstrate that plant–endophyte interactions modulate defense mechanisms against PWN infection.
5. Conclusions
This study reveals a close relationship between the composition of endophytic microbial communities in five coniferous species and their differential resistance to pine wilt disease (PWD). The diversity and richness of these microbial communities significantly influence plant resistance to PWD. Specifically, conifers with reduced bacterial and fungal diversity, combined with endophytes that promote growth of PWN, show increased susceptibility to PWD. In contrast, those with greater microbial diversity and endophytes that exhibit nematocidal activity against PWN demonstrate enhanced resistance to PWD. This study provides insights into the differential resistance mechanisms of conifers against PWD and establishes a theoretical foundation for biological control strategies and breeding resistant tree varieties.
Author Contributions
Conceptualization, R.L. and G.D.; methodology, S.Z. and G.D.; validation, C.W., Y.P., H.W., J.Y. and M.Z.; formal analysis, S.Z. and G.D.; investigation, S.Z., Q.G. and G.D.; resources, G.D.; data curation, S.Z.; writing—original draft preparation, S.Z.; writing—review and editing, R.L.; visualization, S.Z.; supervision, R.L.; project administration, G.D.; funding acquisition, G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Qingdao Natural Science Foundation (24-4-4-zrjj-183-jch), and Teaching Reform Project for Undergraduate, Shandong Province, China (M2022163).
Data Availability Statement
Research data are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nickle, W.R.; Golden, A.M.; Mamiya, Y.; Wergin, W.P. On the taxonomy and morphology of the pine wood nematode, Bursaphelenchus xylophilus (Steiner &Buhrer 1934) Nickle 1970. J. Nematol. 1981, 13, 385–392. [Google Scholar]
- Wang, X.; Wang, L.F.; Cao, Y.F.; Yuan, Y.Z.; Hu, J.; Chen, Z.H.; Zhu, F.; Wang, X.Z. Bursaphelenchus xylophilus detection and analysis system based on CRISPR-Cas12. Front. Plant Sci. 2022, 13, 1075838. [Google Scholar] [CrossRef]
- Jiang, M.; Huang, B.; Yu, X.; Zheng, W.T.; Jin, Y.L.; Liao, M.N.; Ni, J. Distribution, harm and control measures of pine wood nematode disease. J. Zhejiang For. Sci. Technol. 2018, 38, 83–91. [Google Scholar]
- Ye, J.R.; Huang, L. Several issues in the etiological study of pine wood nematode disease. For. Pest Dis. China 2012, 31, 13–21. [Google Scholar]
- Yang, Z.Q.; Wang, X.Y.; Zhang, Y.N.; Zhang, Y.L. Research progress on integrated control of major forest pests in China with biological control as the main measure. Chin. J. Biol. Control 2018, 34, 163–183. [Google Scholar]
- Zheng, Y.; Khan, M.R. Pine wood nematode in coniferous forests and their management by novel biological and biotechnological interventions. In Novel Biological and Biotechnological Applications in Plant Nematode Management; Springer: Singapore, 2023; pp. 489–514. [Google Scholar]
- Kim, B.N.; Kim, J.H.; Ahn, J.Y.; Kim, S.; Cho, B.K.; Kim, Y.H.; Min, J. A short review of the pinewood nematode, Bursaphelenchus xylophilus. Toxicol. Environ. Health Sci. 2020, 12, 297–304. [Google Scholar] [CrossRef]
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef]
- Ryss, A.Y.; Kulinich, O.A.; Sutherland, J.R. Pine wilt disease: A short review of worldwide research. For. Stud. China 2011, 13, 132–138. [Google Scholar] [CrossRef]
- Ye, J.R.; Wu, X.Q. Research progress of pine wilt disease. For. Pest Dis. China 2022, 41, 1–10. [Google Scholar]
- Choi, W.I.; Nam, Y.; Lee, C.Y.; Choi, B.K.; Shin, Y.J.; Lim, J.H.; Koh, S.H.; Park, Y.S. Changes in major insect pests of pine forests in Korea over the last 50 years. Forests 2019, 10, 692. [Google Scholar] [CrossRef]
- Cheng, H.R.; Lin, M.S.; Qian, R.J. A study on the morphological diagnosis and the pathogenicity of Bursaphelenchus mucronatus. J. Nanjing Agric. Univ. 1986, 2, 55–61. [Google Scholar]
- Zhang, J.J.; Zhang, R.Z.; Chen, J.Y. Species of vector insects of Bursaphelenchus xylophilus and their dispersal ability. J. Zhejiang For. Coll. 2007, 24, 350–356. [Google Scholar]
- Chen, Y.S.; Li, X.Y.; Yu, H.P.; Luo, G.D. Research on biological disinfestation technology for diseased wood of pine wilt disease. China Plant Prot. 2019, 39, 52,82–86. [Google Scholar]
- Espada, M.; Filipiak, A.; Li, H.; Vicente, C.S.L. Editorial: Global occurrence of pine wilt disease: Biological interactions and integrated management. Front. Plant Sci. 2022, 13, 993482. [Google Scholar] [CrossRef]
- Zhu, N.B. Revised and Issued: Technical scheme for prevention and control of pine wood nematode disease and measures for management of pine wood nematode disease epidemic areas and infected woods. For. Pest Dis. China 2019, 38, 47–48. [Google Scholar]
- Meng, Y.J. Monitoring, Identification and prevention of pine wood nematode disease in Dandong City, Liaoning Province. Agric. Technol. 2018, 38, 62–63. [Google Scholar]
- Xiong, H.L.; Wen, X.S. Test report on thiacloprid for controlling Monochamus alternatus, the vector insect of pine wood nematode disease. China Agricultural Industry Economic Development Association. In Proceedings of the 2009 Symposium on New Technologies, New Products of Environmental Protection Pesticides and Biopesticide Technology, Beijing, China, 4–6 November 2009; General Station of Forest Pest Management and Quarantine, State Forestry Administration: Shenyang, China; Jiangxi Provincial Bureau of Forestry Pest Control and Quarantine: Nanchang, China, 2009; pp. 72–76. [Google Scholar]
- Zhou, H.K. Control Effect of 2% Thiacloprid Microcapsule Powder on Monochamus alternatus. For. Sci. Technol. 2012, 37, 17–18. [Google Scholar]
- Gan, S.Z.; Wang, X.F.; Chen, X.H.; Zhu, F.; Yang, G.Y.; Wang, Y.C.; Wang, S.K.; Liu, S.M.; Xiao, Y.F.; Lin, C.Q. Study on the control of pine wood nematode disease by trunk injection of “Songxianjing” (5% Abamectin emulsifiable concentrate). J. Temp. For. Res. 2023, 6, 42–47. [Google Scholar]
- Min, S.F.; Huang, X.B.; Yao, Q.; Liu, Q.F.; Xu, S.D.; Wang, M.T. Field control effects of chlorpyrifos and abamectin-chlorbenzuron on Monochamus alternatus. Hubei Entomological Society, Hunan Entomological Society, Henan Entomological Society. In Central China Entomological Research (Volume V); Hubei General Station of Forest Pest Control and Quarantine; Yidu Station of Forest Pest Control, Hubei Province; College of Plant Science and Technology, Huazhong Agricultural University: Wuhan, China, 2008; pp. 275–277. [Google Scholar]
- Jing, Z.G.; Zhang, X.H.; Song, J.Q.; Hao, Y.D. Field experiment on controlling anoplophora chinensis with 8% Cypermethrin (Contact-breaking) microcapsule formulation. Shandong For. Sci. Technol. 2021, 51, 58–59. [Google Scholar]
- Kang, W.T.; Tang, C.S.; Liang, N.; Chen, S.Z.; Hang, J.S.; Chen, Q.L. Field Control of Monochamus alternatus with Sclerodermus guani. J. Fujian Agric. For. Univ. Nat. Sci. Ed. 2008, 37, 575–579. [Google Scholar]
- Li, L.L.; Sun, Y.F.; Chen, F.M.; Hao, D.J.; Tian, J.J. An alkaline protease from Bacillus cereus NJSZ-13 can act as a pathogenicity factor in infection of pine wood nematode. BMC Microbiol. 2023, 23, 10. [Google Scholar]
- Li, L.L.; Tan, J.J.; Chen, F.M. Bacillus pumilus strain LYMC-3 shows nematocidal activity against Bursaphelenchus xylophilus via the production of a guanidine compound. Biocontrol Sci. Technol. 2018, 28, 1128–1139. [Google Scholar] [CrossRef]
- Zhang, W.B.; Li, Y.L.; Zhou, L.; Hao, D.J.; Tian, J.J. Nematocidal activity of the representative plant rhizosphere probiotic strain Bacillus velezensis FZB42 against Bursaphelenchus xylophilus. Acta Microbiol. Sin. 2021, 61, 1287–1298. [Google Scholar]
- Sun, Y.X.; Wang, C.; Du, G.C.; Deng, W.J.; Yang, H.; Li, R.G.; Xu, Q. Two nematocidal compounds from lysinimonas m4 against the pine wood nematode, Bursaphelenchus xylophilus. Forests 2022, 13, 1191. [Google Scholar] [CrossRef]
- Batiha, G.; Alqahtani, A.; Ilesanmi, O.; Saati, A.A.; El-Mleeh, A.; Hetta, H.F.; Magdy Beshbishy, A. Avermectin derivatives, pharmacokinetics, therapeutic and toxic dosages, mechanism of action, and their biological effects. Pharmaceuticals 2020, 13, 196. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, J.H.; Liu, M.J.; Jin, C.Z.; Park, D.J.; Kim, J.; Sung, B.H.; Kim, C.J. New discovery on the nematode activity of aureothin and alloaureothin isolated from endophytic bacteria Streptomyces sp. AE170020. Sci. Rep. 2022, 12, 3947. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Haas, D.; Heeb, S. Extracellular protease of Pseudomonas fuorescens CHA0, a biocontrol factor with activity against the root-knot nematode Meloidogyne incognita. Appl. Environ. Microbiol. 2005, 71, 5646–5649. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Wang, Y.T.; Zhu, J.C.; Zhou, G.; Liu, J. Screening and regulatory mechanisms of inter-root soil nematocidal bacteria of Pinus massoniana. Forests 2023, 14, 2230. [Google Scholar] [CrossRef]
- Niu, Q.H.; Huang, X.W.; Tian, B.Y.; Yang, J.K.; Liu, J.; Zhang, L.; Zhang, K.Q. Bacillus sp. B16 kills nematodes with a serine protease identified as a pathogenic factor. Appl. Microbiol. Biotechnol. 2006, 69, 722–730. [Google Scholar] [CrossRef]
- Liu, M.J.; Hwang, B.S.; Jin, C.Z.; Li, W.J.; Park, D.J.; Seo, S.T.; Kim, C.J. Screening, isolation and evaluation of a nematocidal compound from actinomycetes against the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 2019, 75, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, K.; Izumi, S.; Watanabe, H. Hormone-like Action of 3-Octanol and 1-Octen-3-ol from Botrytis cinerea on the Pine Wood Nematode, Bursaphelenchus xylophilus. Agric. Biol. Chem. 1989, 53, 1777–1781. [Google Scholar] [CrossRef]
- Ye, J.R. Epidemic status, control technologies and countermeasures of pine wilt disease in China. Sci. Silvae Sin. 2019, 55, 1–10. [Google Scholar]
- Ju, Y.W.; Zhao, B.G.; Pu, C.H. Differences in pathogenicity to Cedrus deodara between pine wood nematodes from China and Japan. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2007, 31, 139–140. [Google Scholar]
- Dong, W.Y.; Du, G.C.; Zhang, T.T.; Yang, H.; Guo, Q.Q.; Li, R.G. Bacterial diversity and pathogenicity of pine wood nematode (Bursaphelenchus xylophilus) from two different sources. Chiang Mai J. Sci. 2022, 49, 284–298. [Google Scholar] [CrossRef]
- Han, Z.M.; Hong, Y.D.; Zhao, B.G. A study on pathogenicity of bacteria carried by pine wood nematodes. J. Phytopathol. 2010, 151, 683–689. [Google Scholar] [CrossRef]
- Liu, Y.J.; Han, S.C.; Gao, J.L.; Yu, X.F.; Qing, G.E.; Hu, S.P.; Guo, J.A.; Zhao, X.Y. Effects of different tillage methods combined with straw returning on the diversity of endophytic bacteria in maize. Acta Microbiol. Sin. 2024, 64, 2522–2538. [Google Scholar]
- Bullington, L.S.; Larkin, B.G. Using direct amplification and next-generation sequencing technology to explore foliar endophyte communities in experimentally inoculated western white pines. Fungal Ecol. 2015, 17, 170–178. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, Y.; Friman, V.P.; Kowalchuk, G.A.; Xu, Y.C.; Shen, Q.R.; Jousset, A. Initial soil microbiome composition and functioning predetermine future plant health. Sci. Adv. 2019, 5, eaaw0759. [Google Scholar] [CrossRef]
- Xiong, W.; Song, Y.; Yang, K.; Gu, Y.; Wei, Z.; Kowalchuk, G.A.; Xu, Y.C.; Jousset, A.; Shen, Q.R.; Stefan, G. Rhizosphere protists are key determinants of plant health. Microbiome 2020, 8, 27–35. [Google Scholar] [CrossRef]
- O’Reilly, F.J.; Mielke, P.W. Asymptotic normality of MRPP statistics from invariance principles of u-statistics. Commun. Stat.-Theory Methods 1980, 9, 629–637. [Google Scholar] [CrossRef]
- Dong, X.Z.; Cai, M.Y. Handbook for Systematic Identification of Common Bacteria; Science Press: Beijing, China, 2001. [Google Scholar]
- Zheng, D.W.; Zhou, Y.; Xie, F.L.; Xie, Z.B.; Liu, F.; Zhu, H.J. Screening, Identification and biocontrol potential of antagonistic bacteria against Phytophthora capsici. J. Hunan Agric. Univ. (Nat. Sci. Ed.) 2023, 49, 442–447. [Google Scholar]
- Abo-Elyousr, K.A.M.; Hassan, S.A. Biological control of Ralstonia solanacearum (Smith), the causal pathogen of bacterial wilt disease by using Pantoea spp. Egypt. J. Biol. Pest Control 2021, 31, 113. [Google Scholar]
- Sheng, Z.; Chen, K.; Li, X. Research progress on the biosynthesis of spinosad. Acta Microbiol. Sin. 2016, 56, 397–405. [Google Scholar]
- Northeast Forestry University. A Strain of Acinetobacter Pittii T-150 for Controlling Pine Wood Nematode Disease and Its Application; CN117210374A; Northeast Forestry University: Harbin, China, 2024. [Google Scholar]
- Tian, X.L.; Zhang, Q.L.; Chen, G.H.; Mao, Z.C.; Yang, J.R.; Xie, B.Y. Metagenomic Analysis of the Diversity of Bacteria Associated with Bursaphelenchus xylophilus. Acta Microbiol. Sin. 2010, 50, 909–916. [Google Scholar]
- Lin, F.; Zhao, B.G. Effect of bacteria on the reproduction of Bursaphelenchus xylophilus. J. Beijing For. Univ. 2006, 4, 135–138. [Google Scholar]
- Tang, X.B.; Ni, Y.J.; Hu, Y.C.; Bai, J.H.; Wang, L.; Chen, Q.X.; Wen, Z.F. Isolation, identification of pathogen causing leaf spot disease of Vitis davidii by Pestalotiopsis clavispora and screening of its control agents. Plant Prot. 2020, 46, 110–115+154. [Google Scholar]
- Zheng, Z.; Chai, S.; Chen, J.; Yang, H.; Chang, J.; Yang, G. Isolation and identification of flavonoid-producing endophytic fungi from Loranthus tanakae Franch. & Sav that exhibit antioxidant and antibacterial activities. J. Appl. Microbiol. 2022, 133, 1892–1904. [Google Scholar] [CrossRef]
- Ma, L.J.; Zhang, L.Q.; Lin, H.P.; Mao, S.F. Study on pathogens of Monochamus alternatus and their pathogenicity. Chin. J. Biol. Control 2009, 25, 220–225. [Google Scholar]
- Petersen-Silva, R.; Inácio, L.; Henriques, J.; Naves, P.; Sousa, E.; Pujade-Villar, J. Susceptibility of larvae and adults of Monochamus galloprovincialis to entomopathogenic fungi under controlled conditions. Int. J. Pest Manag. 2015, 61, 106–112. [Google Scholar] [CrossRef]
- Fukushige, H. Propagation of Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) on Fungi Growing in Pine-Shoot Segments. Appl. Entomol. Zool. 1991, 26, 371–376. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).