Silvicultural Practices Shape Fungal Diversity and Community Composition: Metabarcoding Study in a Pinus Forest in Central Mexico
Abstract
1. Introduction
2. Materials and Methods
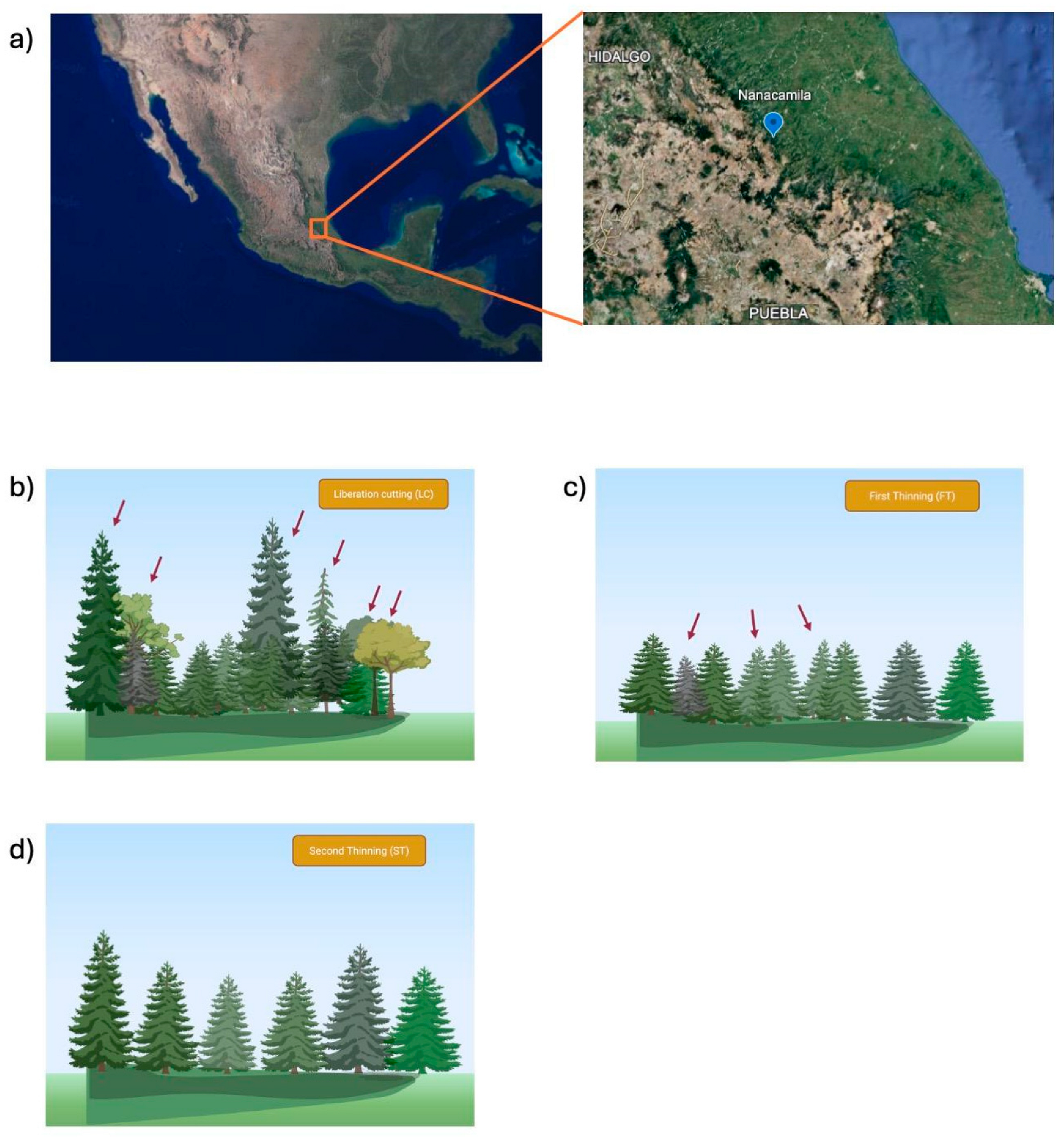
2.1. Study Sites
2.2. Sample Collection
2.3. DNA Extraction, Amplification, and Sequencing
2.4. Statistical and Bioinformatics Analyses
3. Results
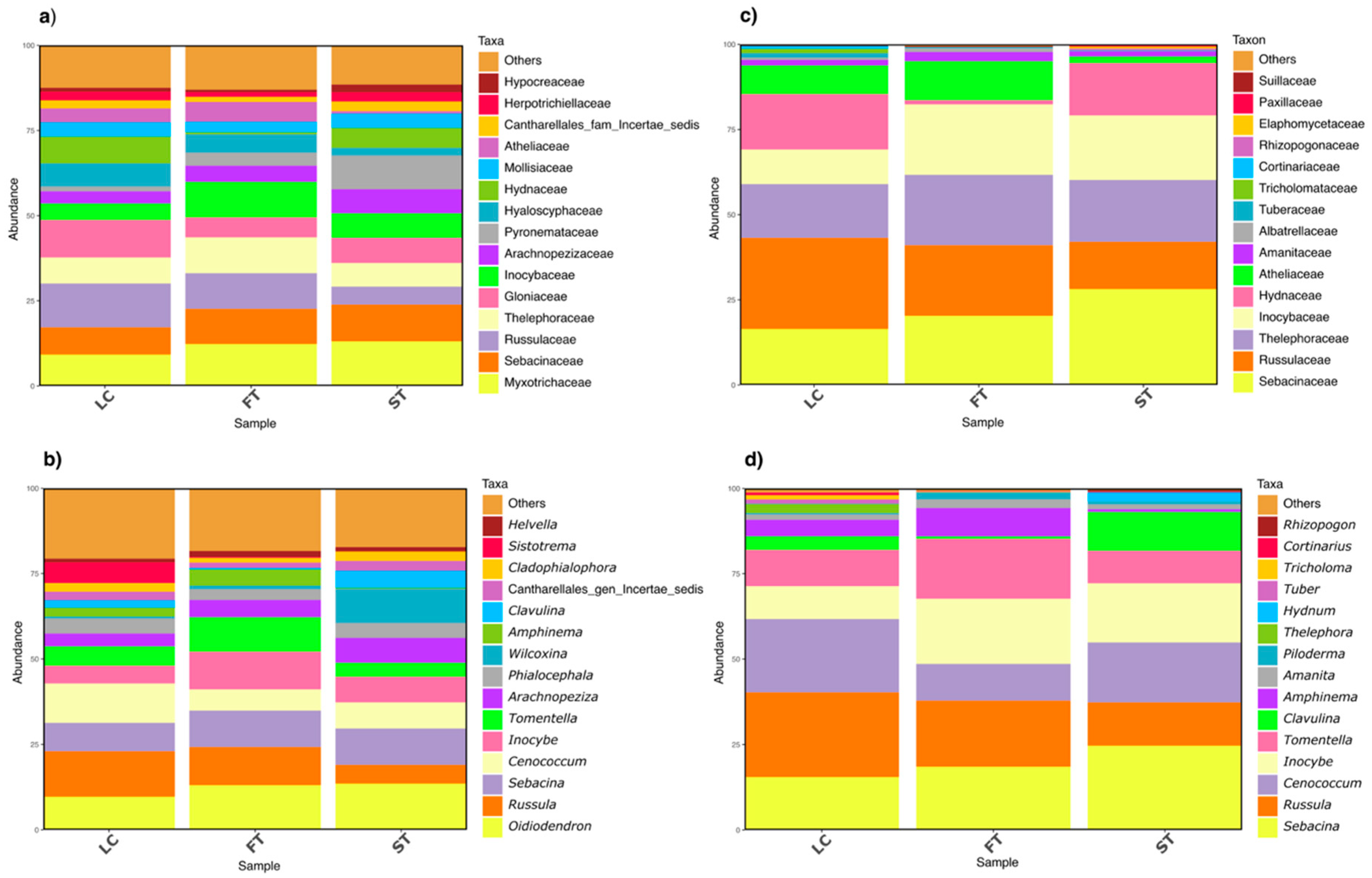
3.1. Fungal ASV Taxonomic Composition
3.2. Effect of Silvicultural Treatments on Fungal Diversity
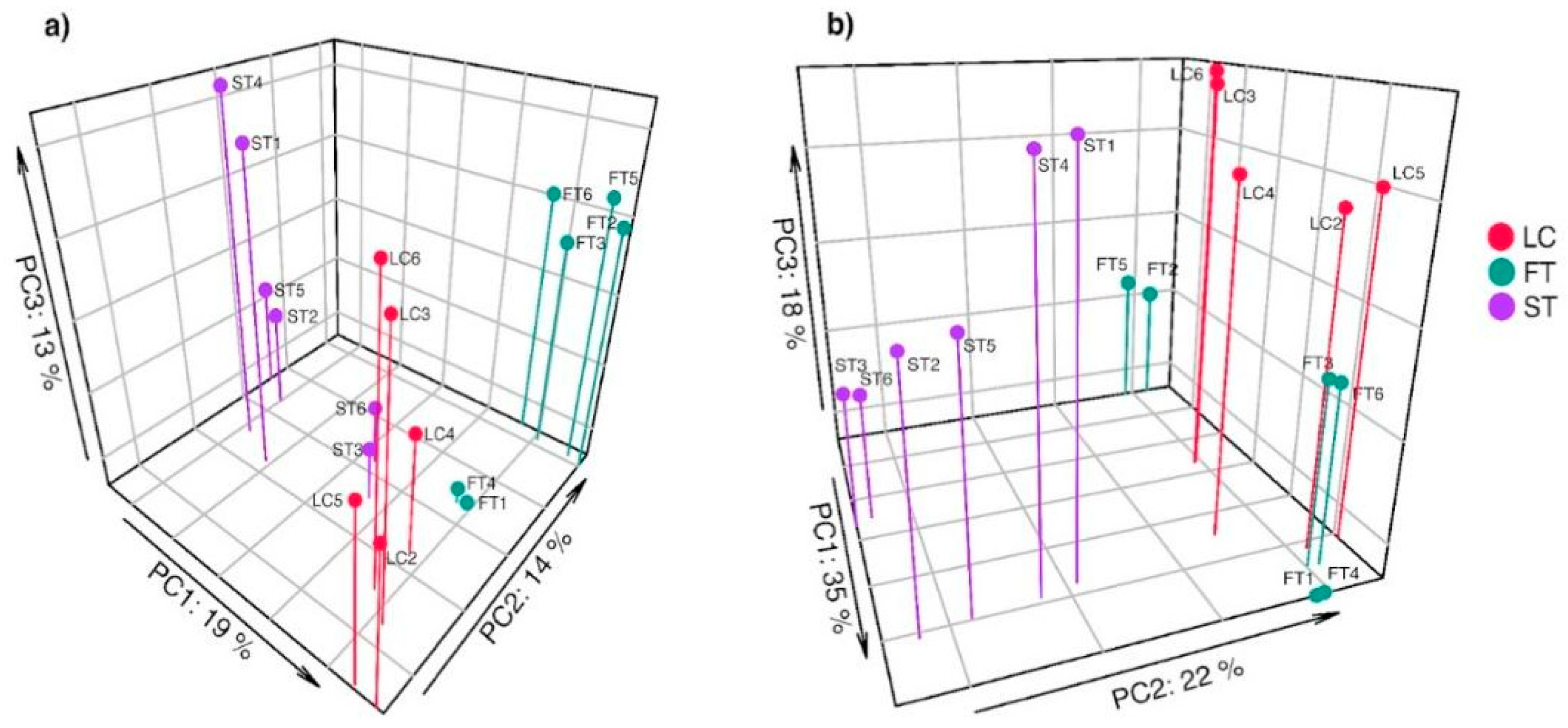
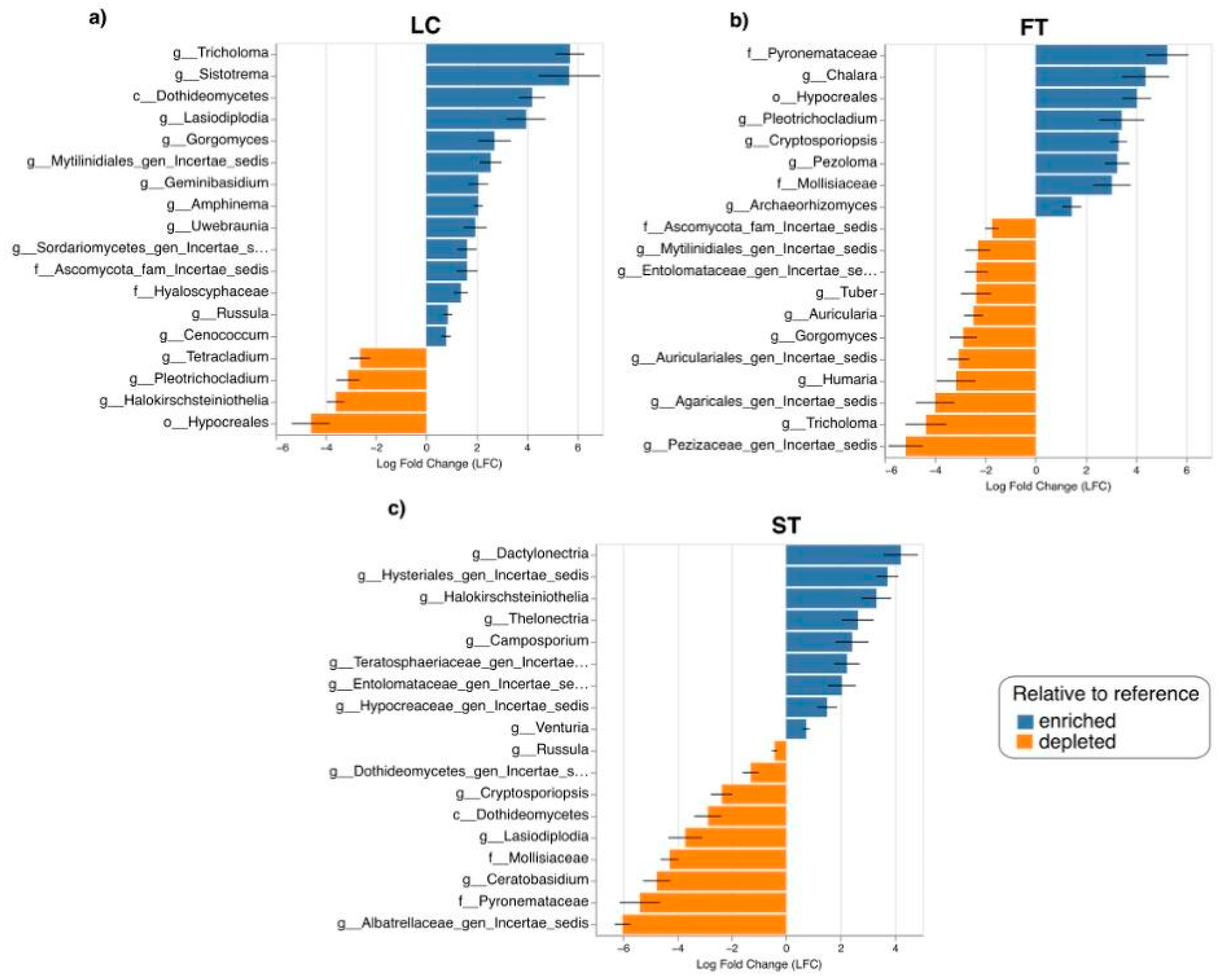
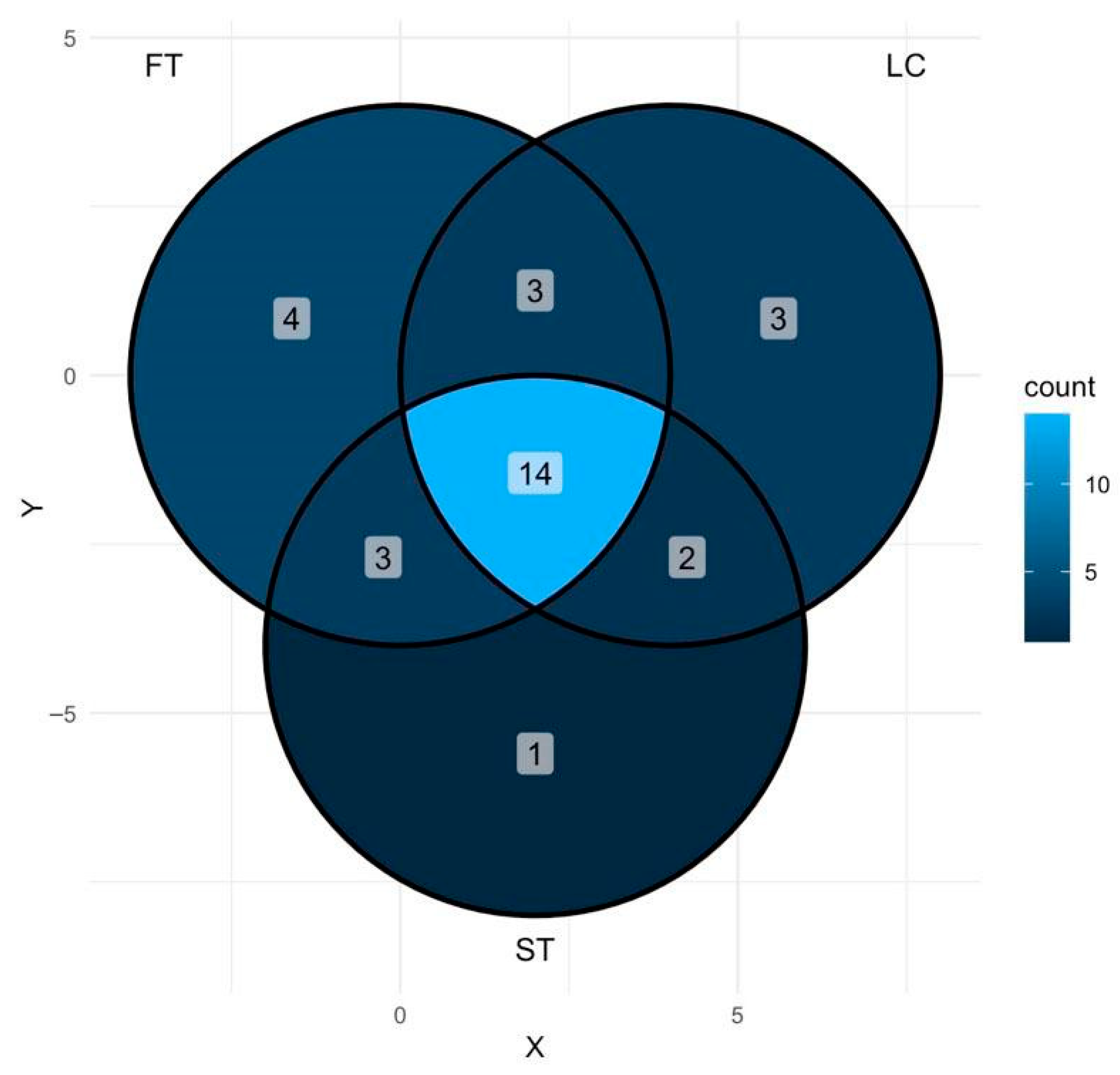
3.3. Effect of Silvicultural Treatments on Fungal Taxa Composition
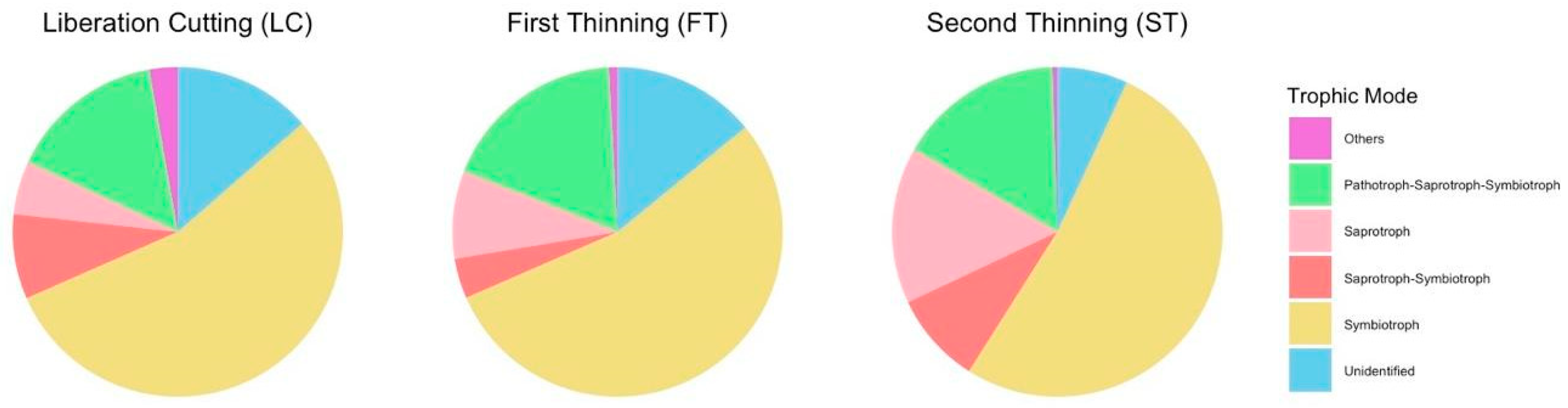
3.4. Changes in Fungal Guild Structure Across Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SDM | Silvicultural development method |
| ECM | Ectomycorrhizal |
| ITS | Internal transcribed spacer |
| LC | Liberation cutting |
| FT | First thinning |
| ST | Second thinning |
| PCoA | Principal coordinate analysis |
References
- Delavaux, C.S.; LaManna, J.A.; Myers, J.A.; Phillips, R.P.; Aguilar, S.; Allen, D.; Alonso, A.; Anderson-Teixeira, K.J.; Baker, M.E.; Baltzer, J.L.; et al. Mycorrhizal Feedbacks Influence Global Forest Structure and Diversity. Commun. Biol. 2023, 6, 1066. [Google Scholar] [CrossRef] [PubMed]
- Dyshko, V.; Hilszczańska, D.; Davydenko, K.; Matić, S.; Moser, W.K.; Borowik, P.; Oszako, T. An Overview of Mycorrhiza in Pines: Research, Species, and Applications. Plants 2024, 13, 506. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; ISBN 1597260401. [Google Scholar]
- Heilmann-Clausen, J.; Barron, E.; Boddy, L.; Dahlberg, A.; Griffith, G.W.; Nordén, J.; Ovaskainen, O.; Perini, C.; Senn-Irlet, B.; Halme, P. A Fungal Perspective on Conservation Biology. Conserv. Biol. 2015, 29, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.M.; Bruns, T.D.; Smith, D.P.; Branco, S.; Glassman, S.I.; Erlandson, S.; Vilgalys, R.; Peay, K.G. Independent Roles of Ectomycorrhizal and Saprotrophic Communities in Soil Organic Matter Decomposition. Soil. Biol. Biochem. 2013, 57, 282–291. [Google Scholar] [CrossRef]
- Buscot, F.; Weber, G.; Oberwinkler, F. Interactions Between Cylindrocarpon Destructans and Ectomycorrhizas of Picea Abies with Laccaria Laccata and Paxillus Involutus. Trees 1992, 6, 83–90. [Google Scholar] [CrossRef]
- Smith, J.E. Mycorrhizal Symbiosis (Third Edition). Soil Sci. Soc. Am. J. 2009, 73, 694. [Google Scholar] [CrossRef]
- Morin, C.; Samson, J.; Dessureault, M. Protection of Black Spruce Seedlings against Cylindrocladium Root Rot with Ectomycorrhizal Fungi. Can. J. Bot. 1999, 77, 169–174. [Google Scholar]
- Niego, G.A.T.; Lambert, C.; Mortimer, P.; Thongklang, N.; Rapior, S.; Grosse, M.; Schrey, H.; Charria-Girón, E.; Walker, A.; Hyde, K. The Contribution of Fungi to the Global Economy. Fungal Divers. 2023, 121, 95–137. [Google Scholar] [CrossRef]
- Bai, C.; He, X.; Tang, H.; Shan, B.; Zhao, L. Spatial Distribution of Arbuscular Mycorrhizal Fungi, Glomalin and Soil Enzymes under the Canopy of Astragalus Adsurgens Pall. in the Mu Us Sandland, China. Soil. Biol. Biochem. 2009, 41, 941–947. [Google Scholar] [CrossRef]
- McGuire, K.L. Common Ectomycorrhizal Networks May Maintain Monodominance in a Tropical Rain Forest. Ecology 2007, 88, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Nara, K. Ectomycorrhizal Networks and Seedling Establishment during Early Primary Succession. New Phytol. 2006, 169, 169–178. [Google Scholar] [CrossRef]
- Koide, R.T.; Dickie, I.A. Effects of Mycorrhizal Fungi on Plant Populations. In Diversity and Integration in Mycorrhizas; Smith, S.E., Smith, F.A., Eds.; Springer: Dordrecht, The Netherlands, 2002; Volume 244. [Google Scholar] [CrossRef]
- Zamora Morales, B.P.; Zamora-Martínez, M.C.; de Pascual Pola, M.C.d.C.N.; García Campusano, F.T.A. Condiciones Edáficas, Abundancia y Riqueza de Hongos Ectomicorrizógenos Comestibles. Rev. Mex. Cienc. For. 2018, 9, 226–251. [Google Scholar] [CrossRef]
- Collado, E.; Castaño, C.; Bonet, J.A.; Hagenbo, A.; Martínez de Aragón, J.; de-Miguel, S. Divergent Above- and below-Ground Responses of Fungal Functional Groups to Forest Thinning. Soil. Biol. Biochem. 2020, 150, 108010. [Google Scholar] [CrossRef]
- Tomao, A.; Antonio Bonet, J.; Castaño, C.; de-Miguel, S. How Does Forest Management Affect Fungal Diversity and Community Composition? Current Knowledge and Future Perspectives for the Conservation of Forest Fungi. For. Ecol. Manag. 2020, 457, 117678. [Google Scholar] [CrossRef]
- Dove, N.C.; Keeton, W.S. Structural Complexity Enhancement Increases Fungal Species Richness in Northern Hardwood Forests. Fungal Ecol. 2015, 13, 181–192. [Google Scholar] [CrossRef]
- Aguirre-Calderón, O.A. Manejo forestal en el siglo XXI. Madera Y Bosques. 2015, 21, 17–28. [Google Scholar] [CrossRef]
- FAO Soils for Nutrition: State of the Art; FAO: Rome, Italy, 2022.
- Pérez-Rodríguez, F.; Vargas-Larreta, B.; Aguirre-Calderón, O.A.; Corral-Rivas, J.J.; Rojo-Alboreca, A. Proceso analítico jerárquico para seleccionar métodos de manejo forestal en Durango. Rev. Mex. Cienc. For. 2012, 4, 55–72. [Google Scholar]
- Quijada, G.E.M.; Balderas, J.M.M.; Garza, E.J.T.; Calderón, Ó.A.A.; Rodríguez, E.A.; Yamallel, J.I.Y. Diversity, Structure and Floristic Composition of Temperate Forests of Southern Nuevo León State. Rev. Mex. Cienc. For. 2020, 11, 94–123. [Google Scholar] [CrossRef]
- British Columbia. Ministry of Forests. Forest Practices Branch. Silvicultural Systems Handbook for British Columbia. For. Pract. Br., BC. Min. For., Victoria, BC. 2003. Available online: https://www2.gov.bc.ca/assets/gov/farming-natural-resources-and-industry/forestry/stand-tending/silvsystemshdbk-web.pdf (accessed on 19 August 2025).
- Hernández-Salas, J.; Aguirre-Calderón, Ó.A.; Alanís-Rodríguez, E.; Jiménez-Pérez, J.; Treviño-Garza, E.J.; González-Tagle, M.A.; Luján-Álvarez, C.; Olivas-García, J.M.; Domínguez-Pereda, L.A. Efecto Del Manejo Forestal En La Diversidad y Composición Arbórea de Un Bosque Templado Del Noroeste de México. Rev. Chapingo Ser. Cienc. For. Ambiente 2013, 19, 189–199. [Google Scholar] [CrossRef]
- Bautista, L.J.; Damon, A.; Ochoa-Gaona, S.; Tapia, R.C. Impact of Silvicultural Methods on Vascular Epiphytes (Ferns, Bromeliads and Orchids) in a Temperate Forest in Oaxaca, Mexico. For. Ecol. Manag. 2014, 329, 10–20. [Google Scholar] [CrossRef]
- López-Reyes, A.; de la Rosa, J.P.; Ortiz, E.; Gernandt, D.S. Morphological, Molecular, and Ecological Divergence in Pinus Douglasiana and P. maximinoi. Syst. Bot. 2015, 40, 658–670. [Google Scholar] [CrossRef]
- Pérez-López, R.I.; González-Espinosa, M.; Ramírez-Marcial, N.; Pérez-Moreno, J.; Toledo-Aceves, T. Forest Management Effects on the Ectomycorrhizal Macromycete Community in Tropical Montane Forests in Mexico. For. Ecol. Manag. 2021, 501, 119670. [Google Scholar] [CrossRef]
- Abbasi, U.A.; Mattsson, E.; Nissanka, S.P.; Ali, A. Biological, Structural and Functional Responses of Tropical Forests to Environmental Factors. Biol. Conserv. 2022, 276, 109792. [Google Scholar] [CrossRef]
- Rosen, A.; Jörg Fischer, F.; Coomes, D.A.; Jackson, T.D.; Asner, G.P.; Jucker, T. Tracking Shifts in Forest Structural Complexity through Space and Time in Human-Modified Tropical Landscapes. Ecography 2024, 2024, e07377. [Google Scholar] [CrossRef]
- Sabogal, C.C.J.K.W. Silviculture in Natural Forests; FAO: Roma, Italy, 2017. [Google Scholar]
- Tedersoo, L.; Bahram, M.; Zobel, M. How Mycorrhizal Associations Drive Plant Population and Community Biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Howes, C.G.; Vaninsberghe, D.; Yu, H.; Bachar, D.; Christen, R.; Henrik Nilsson, R.; Hallam, S.J.; Mohn, W.W. Significant and Persistent Impact of Timber Harvesting on Soil Microbial Communities in Northern Coniferous Forests. ISME J. 2012, 6, 2199–2218. [Google Scholar] [CrossRef]
- Collado, E.; Bonet, J.A.; Alday, J.G.; Martínez de Aragón, J.; de-Miguel, S. Impact of Forest Thinning on Aboveground Macrofungal Community Composition and Diversity in Mediterranean Pine Stands. Ecol. Indic. 2021, 133, 108340. [Google Scholar] [CrossRef]
- Parladé, J.; Queralt, M.; Pera, J.; Bonet, J.A.; Castaño, C.; Martínez-Peña, F.; Piñol, J.; Senar, M.A.; De Miguel, A.M. Temporal Dynamics of Soil Fungal Communities after Partial and Total Clear-Cutting in a Managed Pinus Sylvestris Stand. For. Ecol. Manag. 2019, 449, 117456. [Google Scholar] [CrossRef]
- Kujawska, M.B.; Rudawska, M.; Wilgan, R.; Leski, T. Similarities and Differences among Soil Fungal Assemblages in Managed Forests and Formerly Managed Forest Reserves. Forests 2021, 12, 353. [Google Scholar] [CrossRef]
- Valdés, M.; Pereda, V.; Ramírez, P.; Valenzuela, R.; Pineda, R.M. The Ectomycorrhizal Community In a Pinus Oaxacana Forest Under Different Silvicultural Treatments. J. Trop. For. Sci. 2009, 21, 88–97. [Google Scholar]
- Reverchon, F.; del Pilar Ortega-Larrocea, M.; Bonilla-Rosso, G.; Pérez-Moreno, J. Structure and Species Composition of Ectomycorrhizal Fungal Communities Colonizing Seedlings and Adult Trees of Pinus Montezumae in Mexican Neotropical Forests. FEMS Microbiol. Ecol. 2012, 80, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global Diversity and Geography of Soil Fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Muñoz-Castellanos, L.N.; Avila-Quezada, G.D.; Sáenz-De La Riva, G.; Salas, E.; Muñoz-Ramírez, Z.Y.; González-Escobedo, R. Revealing Microbial Patterns in the Rhizosphere of Pecan Trees Asymptomatic and Symptomatic for Texas Root Rot Using a High-Throughput Sequencing Approach. Rhizosphere 2024, 29, 100833. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE Database for Molecular Identification of Fungi: Handling Dark Taxa and Parallel Taxonomic Classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Lin, H.; Peddada, S.D. Analysis of Compositions of Microbiomes with Bias Correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An Open Annotation Tool for Parsing Fungal Community Datasets by Ecological Guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Valdés, M.; Córdova, J.; Gómez, M.; Fierros, A.M. Understory Vegetation and Ectomycorrhizal Sporocarp Diversity Response to Pine Regeneration Methods in Oaxaca, Mexico. West. J. Appl. For. 2003, 18, 101–108. [Google Scholar] [CrossRef]
- Martínez-Peña, F.; de-Miguel, S.; Pukkala, T.; Bonet, J.A.; Ortega-Martínez, P.; Aldea, J.; Martínez de Aragón, J. Yield Models for Ectomycorrhizal Mushrooms in Pinus Sylvestris Forests with Special Focus on Boletus Edulis and Lactarius Group Deliciosus. For. Ecol. Manag. 2012, 282, 63–69. [Google Scholar] [CrossRef]
- Assad, R.; Reshi, Z.A.; Rashid, I.; Wali, D.C.; Bashir, I.; Rafiq, I. Metabarcoding of Root-Associated Ectomycorrhizal Fungi of Himalayan Pindrow Fir through Morphotyping and Next Generation Sequencing. Trees For. People 2021, 6, 100153. [Google Scholar] [CrossRef]
- Katrevičs, J.; Bitenieks, K.; Jansons, Ā.; Jansone, B.; Ruņģis, D.E. Forest Soil Fungal Diversity in Stands of Norway Spruce (Picea abies (L.) Karst.) of Different Ages. Forests 2025, 16, 500. [Google Scholar] [CrossRef]
- Tedersoo, L.; May, T.W.; Smith, M.E. Ectomycorrhizal Lifestyle in Fungi: Global Diversity, Distribution, and Evolution of Phylogenetic Lineages. Mycorrhiza 2010, 20, 217–263. [Google Scholar] [CrossRef] [PubMed]
- Garibay-Orijel, R.; Argüelles-Moyao, A.; Álvarez-Manjarrez, J.; Ángeles-Argáiz, R.E.; García-Guzmán, O.M.; Hernández-Yáñez, H. Diversity and Importance of Edible Mushrooms in Ectomycorrhizal Communities in Mexican Neotropics. In Mushrooms, Humans and Nature in a Changing World: Perspectives from Ecological, Agricultural and Social Sciences; Springer International Publishing: Cham, Switzerland, 2020; pp. 407–424. ISBN 9783030373788. [Google Scholar]
- Boeraeve, M.; Honnay, O.; Jacquemyn, H. Effects of Host Species, Environmental Filtering and Forest Age on Community Assembly of Ectomycorrhizal Fungi in Fragmented Forests. Fungal Ecol. 2018, 36, 89–98. [Google Scholar] [CrossRef]
- Tedersoo, L.; Mikryukov, V.; Zizka, A.; Bahram, M.; Hagh-Doust, N.; Anslan, S.; Prylutskyi, O.; Delgado-Baquerizo, M.; Maestre, F.T.; Pärn, J.; et al. Global Patterns in Endemicity and Vulnerability of Soil Fungi. Glob. Change Biol. 2022, 28, 6696–6710. [Google Scholar] [CrossRef]
- Gómez-Hernández, M.; Ramírez-Antonio, K.G.; Gándara, E. Ectomycorrhizal and Wood-Decay Macromycete Communities along Development Stages of Managed Pinus Patula Stands in Southwest Mexico. Fungal Ecol. 2019, 39, 109–116. [Google Scholar] [CrossRef]
- Toju, H.; Sato, H. Root-Associated Fungi Shared between Arbuscular Mycorrhizal and Ectomycorrhizal Conifers in a Temperate Forest. Front. Microbiol. 2018, 9, 433. [Google Scholar] [CrossRef]
- Bruyant, P.; Moënne-Loccoz, Y.; Almario, J. Root-Associated Helotiales Fungi: Overlooked Players in Plant Nutrition. Soil. Biol. Biochem. 2024, 191, 109363. [Google Scholar] [CrossRef]
- Teste, F.P.; Lieffers, V.J.; Strelkov, S.E. Ectomycorrhizal Community Responses to Intensive Forest Management: Thinning Alters Impacts of Fertilization. Plant Soil 2012, 360, 333–347. [Google Scholar] [CrossRef]
- Goldmann, K.; Schöning, I.; Buscot, F.; Wubet, T. Forest Management Type Influences Diversity and Community Composition of Soil Fungi across Temperate Forest Ecosystems. Front. Microbiol. 2015, 6, 1300. [Google Scholar] [CrossRef]
- Alem, D.; Dejene, T.; Oria-de-Rueda, J.A.; Geml, J.; Martín-Pinto, P. Soil Fungal Communities under Pinus Patula Schiede Ex Schltdl. & Cham. Plantation Forests of Different Ages in Ethiopia. Forests 2020, 11, 1109. [Google Scholar] [CrossRef]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Põlme, S.; Riit, T.; Liiv, I.; Kõljalg, U.; Kisand, V.; Nilsson, R.H.; Hildebrand, F.; et al. Shotgun Metagenomes and Multiple Primer Pair-Barcode Combinations of Amplicons Reveal Biases in Metabarcoding Analyses of Fungi. MycoKeys 2015, 10, 1–43. [Google Scholar] [CrossRef]
- Wittebolle, L.; Marzorati, M.; Clement, L.; Balloi, A.; Daffonchio, D.; Heylen, K.; De Vos, P.; Verstraete, W.; Boon, N. Initial Community Evenness Favours Functionality under Selective Stress. Nature 2009, 458, 623–626. [Google Scholar] [CrossRef]
- Põlme, S.; Abarenkov, K.; Henrik Nilsson, R.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A User-Friendly Traits Database of Fungi and Fungus-like Stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Baldrian, P. Forest Microbiome: Diversity, Complexity and Dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef]
- Purahong, W.; Kahl, T.; Schloter, M.; Bauhus, J.; Buscot, F.; Krüger, D. Comparing Fungal Richness and Community Composition in Coarse Woody Debris in Central European Beech Forests under Three Types of Management. Mycol. Prog. 2014, 13, 959–964. [Google Scholar] [CrossRef]
- Tanney, J.B.; Seifert, K.A. Mollisiaceae: An Overlooked Lineage of Diverse Endophytes. Stud. Mycol. 2020, 95, 293–380. [Google Scholar] [CrossRef]
- Koukol, O. New Species of Chalara Occupying Coniferous Needles. Fungal Divers. 2011, 49, 75–91. [Google Scholar] [CrossRef]
- Flores-Rentería, D.; Barradas, V.L.; Álvarez-Sánchez, J. Ectomycorrhizal Pre-Inoculation of Pinus Hartwegii and Abies Religiosa Is Replaced by Native Fungi in a Temperate Forest of Central Mexico. Symbiosis 2018, 74, 131–144. [Google Scholar] [CrossRef]
| Treatment | Raw Sequences | High-Quality Sequences | Chao1 | Shannon | Simpson |
|---|---|---|---|---|---|
| Liberation Cutting (LC) | 1,936,447 | 577,612 | 432.8 ± 39.52 | 7.2 ± 0.09 | 0.99 ± 0.002 |
| First Thinning (FT) | 2,367,577 | 703,262 | 459.17 ± 27.9 | 7.22 ± 0.06 | 0.99 ± 0.002 |
| Second Thinning (ST) | 2,198,592 | 692,752 | 382 ± 46.5 | 6.91 ± 0.17 | 0.98 ± 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Valencia, L.E.; González-Escobedo, R.; Zamora-Martínez, M.C.; Pérez-García, J.; Garibay-Orijel, R.; García-Campusano, F. Silvicultural Practices Shape Fungal Diversity and Community Composition: Metabarcoding Study in a Pinus Forest in Central Mexico. Forests 2025, 16, 1397. https://doi.org/10.3390/f16091397
García-Valencia LE, González-Escobedo R, Zamora-Martínez MC, Pérez-García J, Garibay-Orijel R, García-Campusano F. Silvicultural Practices Shape Fungal Diversity and Community Composition: Metabarcoding Study in a Pinus Forest in Central Mexico. Forests. 2025; 16(9):1397. https://doi.org/10.3390/f16091397
Chicago/Turabian StyleGarcía-Valencia, Liliana E., Román González-Escobedo, Marisela Cristina Zamora-Martínez, Jocelyn Pérez-García, Roberto Garibay-Orijel, and Florencia García-Campusano. 2025. "Silvicultural Practices Shape Fungal Diversity and Community Composition: Metabarcoding Study in a Pinus Forest in Central Mexico" Forests 16, no. 9: 1397. https://doi.org/10.3390/f16091397
APA StyleGarcía-Valencia, L. E., González-Escobedo, R., Zamora-Martínez, M. C., Pérez-García, J., Garibay-Orijel, R., & García-Campusano, F. (2025). Silvicultural Practices Shape Fungal Diversity and Community Composition: Metabarcoding Study in a Pinus Forest in Central Mexico. Forests, 16(9), 1397. https://doi.org/10.3390/f16091397







