1. Introduction
Land use changes, biological invasions, and climate change are well established drivers of the global crisis of biodiversity loss [
1,
2]. These factors continue to intensify, resulting in dwindling populations of vulnerable species and accelerating rates of extinction [
3]. Forest ecosystems are particularly affected, facing rapid decline due to deforestation for urban development and agriculture, increasing threats from nonnative pests and diseases, and shifting precipitation regimes [
4,
5]. Many woody plant species are quietly disappearing from the landscape. While in situ conservation efforts are important to prevent continued loss, ex situ approaches are becoming increasingly necessary to ensure the long-term survival of species. To be effective, such efforts must begin well before populations reach critically low levels, ensuring conservation of the full spectrum of genetic diversity [
6].
Ex situ conservation of woody plants presents unique challenges compared to herbaceous species. Many tree and shrub species produce recalcitrant seeds, which do not remain viable in long-term storage, rendering conventional seed banking ineffective [
7,
8]. Instead, “living collections”, such as those maintained by botanic gardens, arboreta, and tree improvement programs, are essential to ensure a stable and consistent seed source of these species for the future [
9]. These efforts often require specialized propagation techniques such as grafting and rooting cuttings and establishment and maintenance of planting sites, along with technical expertise and equipment not widely available to all conservation practitioners [
8]. Moreover, creating plantings that can provide genetically diverse, provenanced seed over time requires significant long-term land commitments [
10]. Such land must not only be biologically suitable for tree growth but also insulated from the risks of development or ownership changes. Long juvenile periods and lower planting densities further limit the speed and scale of seed production compared to herbaceous species. These factors combine to make ex situ woody plant conservation an expensive and logistically complex endeavor [
10].
For many institutions, these challenges prove prohibitive or at least severely limit the breadth of taxa that can be effectively conserved. Such institutions must make difficult choices regarding the tradeoffs between land commitments, number of species conserved, number of genotypes of each species conserved, and the long-term costs of maintaining and managing ex situ collections [
10,
11,
12]. As a result, many at-risk woody species, especially those lacking commercial appeal, are overlooked. Addressing these gaps will require collaboration across federal, state, and private entities, as well as the efficient sharing of knowledge and best practices among practitioners [
13]. Supporting the dissemination of insights gained through practical conservation efforts is a key role for the broader scientific community.
The University of Tennessee’s Tree Improvement Program (UT-TIP) is uniquely equipped to contribute to woody plant germplasm conservation. With strong partnerships across state and federal land management agencies, access to university-managed research centers, appropriate facilities for propagating and housing plant material, and experienced technical staff and equipment, UT-TIP is well positioned to lead conservation projects throughout Tennessee. This report documents the collaborative efforts of UT-TIP and the United States Department of Agriculture’s Natural Resources Conservation Service (NRCS) in the conservation of the Harbison hawthorn (Crataegus harbisonii Beadle), one of the nation’s rarest woody plant species, with the objective of distilling lessons applicable to analogous conservation initiatives in other taxa.
Harbison hawthorn is a small tree or shrub of the Rosaceae family that is endemic to Tennessee (USA) and currently considered “critically imperiled” at the state (state listed S1), national (nationally listed N1), and global (globally listed G1) levels by NatureServe [
14]. Currently, it is known from only two locations, one in Davidson County, Tennessee consisting of a single living individual and another location 220km away in Obion County, Tennessee consisting of an unknown number of individuals spread sparsely over several hectares of private land [
15].
Crataegus harbisonii was first described in 1899 by C.D. Beadle following several collections in the Nashville area, including specimens gathered by the species’ namesake, T.G. Harbison. By 1948, it had been reported in five Tennessee counties. However, no further observations were recorded for nearly 50 years and attempts to relocate historic populations were unsuccessful [
15]. In 1993, the species, by this point long presumed extirpated by state botanists, was rediscovered during a search led by Ron Lance. The discovery consisted of two
C. harbisonii trees growing in two separate wooded areas near Nashville, TN [
16]. Despite intensive surveying of the surrounding area, no additional individuals were located. Since that time, one of the two trees has died [
17]. This Davidson County site has since been the focus of both in situ and ex situ conservation efforts [
17]. Grafted clones of the two originally extant trees are currently preserved in several arboreta including the North Carolina Arboretum (WNCA) and the Arnold Arboretum of Harvard University (AAH).
Remarkably, a routine vegetation survey conducted along a rural road in Obion County, TN in 2014, led to the discovery of a group of Harbison hawthorn shrubs approximately 220 km west of the Davidson County locality (Barry Hart, personal communication). This finding not only significantly increased its known census size but also spurred renewed botanical interest in the species. Following this discovery, a comprehensive review of herbarium specimens by Chester et al. [
15] offered valuable insights into the species’ historical and current distribution and addressed questions of taxonomy and species identity.
Despite the enormous potential of the Obion County population to increase the genetic diversity of ex situ Harbison hawthorn collections, no systematic efforts to capture this diversity had been made prior to this project. Using this newly discovered Obion County population as well as existing conservation resources for the Davidson County locality, our work aimed to incorporate as many unique sources as possible of this species into duplicated ex situ conservation orchards designed for large scale seed production.
3. Results
3.1. Characterization of Focal Area
Field surveying of the Obion County site in April 2023 resulted in the documentation of a total of 91 individual hawthorn shrubs ranging in height from 0.6 m to 5.8 m with 24 of these displaying sexual maturity. The average height of all documented individuals was 2.96 m (range: 0.37 m to 5.79 m). The average height of the reproductively mature stems was 4.44 m (range: 3.02 m to 5.79 m). Most plants had two to three codominant stems splitting at or slightly above ground level, with a maximum of 12 stems recorded for a single individual. Around 25% of the individuals were composed of a single dominant stem. Of the total area surveyed, C. harbisonii was found on approximately 30 ha (74 acres).
The site is composed of roughly equal parts mature deciduous forest, various stages of regenerating forest, and open pasture. Harbison hawthorns were found scattered throughout the site in both closed canopy and open-grown conditions. In general, reduced vigor was observed in those stems occurring in closed-canopy settings while greater overall heights, lateral branching, seed production, and occurrence of understory seedlings were observed in the open or partially open canopy locations. We noted the presence of numerous one- to two-year-old seedlings within the drip lines of several larger, reproductively mature trees. When surveying the site during fruit drop, numerous signs of wildlife consumption of hawthorn fruits were evident throughout the site. Competitive release of selected Harbison hawthorns successfully resulted in the observation of more flowers and fruit on these trees in subsequent seasons.
3.2. Propagation Efforts
Seed collection efforts in 2022 yielded 1122 seeds from five sources. Between 19 and 869 seeds were collected from each fruit producing source. Using the warm-cold stratification method, 305 seeds germinated in the first year (27.18%). In the second year, an additional 28 seeds germinated from this seed lot (
Figure 1). Seed collection in 2023 yielded 8013 seeds from 12 sources with between 24 and 3629 seeds collected per source. Using only a single 120 day cold stratification period, 1513 seeds successfully germinated (18.88%). We also note that with this treatment, numerous seeds germinated prior to removing them from cold stratification. A portion of ungerminated seed that was returned to cold stratification was found to have germinated while in cold storage after 90 days, bringing the total germination rate for this seed lot to approximately 22%. Currently, seedlings derived from 12 unique mother trees have been produced.
Seed treated with the warm-cold stratification method had significantly better germination (p < 0.01, binomial logistic regression), although long term survival was greater for germinants from the cold only stratification. Significant differences in germination between sources were also detected for both the 2022 and 2023 seed lots (p < 0.01).
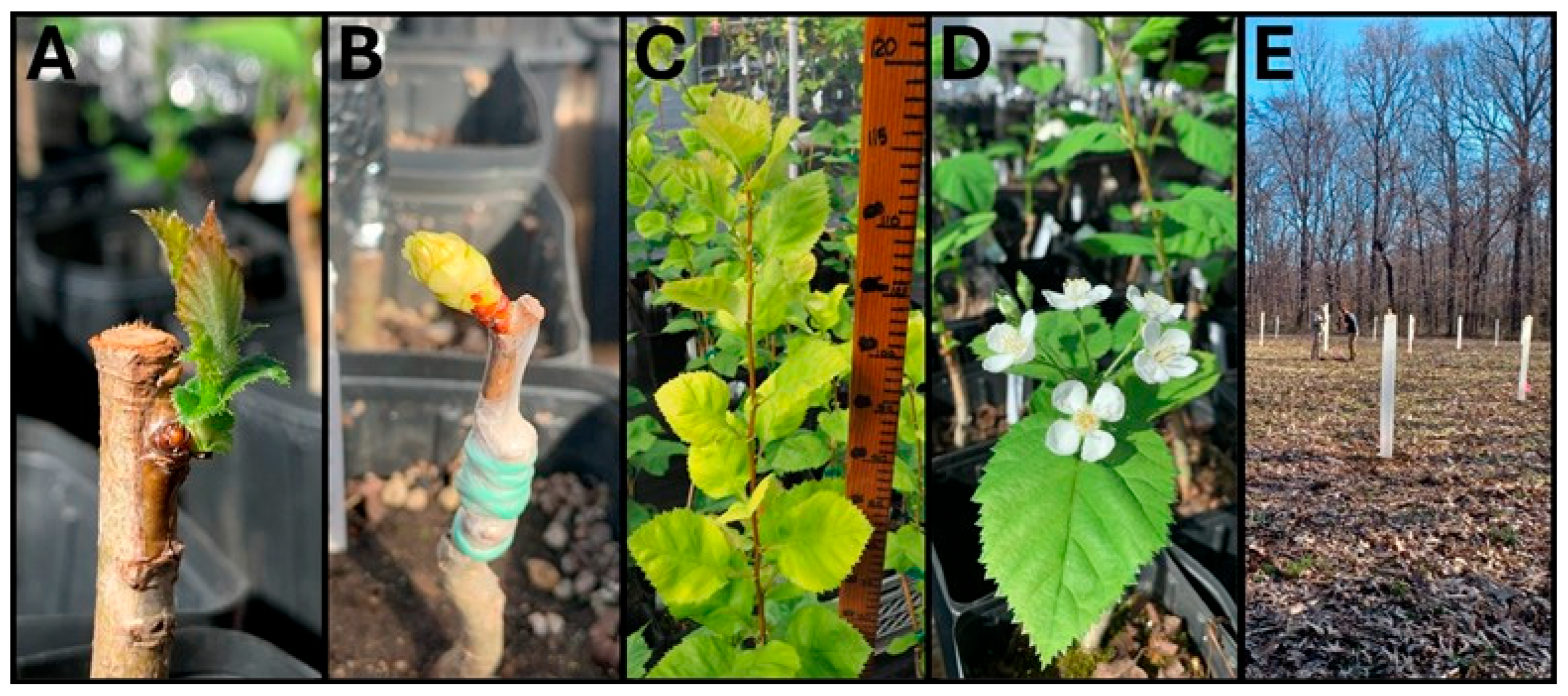
In 2023, 60 chip-bud grafts from 10 sources (six replicates per source) were attempted with 50 successes (83.33%). In 2024, 59 of 72 attempts on 8 additional sources (nine replicates per source) were successful (81.94%) (
Figure 2A). Based on binomial logistic regression, chip-bud success did not differ significantly between 2023 and 2024 (
p = 0.84). Successful grafts grew rapidly with most extending 30–50cm in the first few months after breaking dormancy. Several of those collected from reproductively mature sources also flowered in the first spring after grafting. Eighteen unique sources have been successfully propagated through fall chip-bud grafting.
Whip and tongue grafting in 2024 yielded 46 successes out of 104 attempts for six Obion County sources with 16 to 18 replicates per source (44.23%) (
Figure 2B). Chip-bud grafting was nearly twice as successful as whip & tongue grafting (83% vs. 44%), a difference that was highly significant (
p < 0.01, binomial logistic regression). Growth of these grafts was also slower than chip-bud grafts (see
Figure 2C vs.
Figure 2D). We also successfully grafted 11 clones using scionwood obtained from the North Carolina Arboretum’s Harbison hawthorn accession, derived from the only extant tree occurring at the Davidson County location. Spring whip-and-tongue grafting successfully generated clones of six sources, two of which were not chip-bud grafted.
Additionally, 30 seedlings derived from the Davidson County location were obtained from Ron Lance to include in our collections. Attempts at propagation through rooted cuttings were unsuccessful with all attempts failing to develop roots.
3.3. Outplanting
In mid-March 2025, a total of 60 trees representing 12 sources with five replicates each were planted at the University of Tennessee’s AgResearch and Education Center at Milan site following a randomized complete block design (
Figure 2E). The planting is expected to eventually include 120–130 clones, maximizing representation of the wild population as propagation efforts permit.
In addition to this planting, propagation of clones and seedlings for two additional seed orchards is underway. A seedling seed orchard will be planted at the Ames AgResearch and Recreation Center in southwestern Tennessee, and a duplicate grafted seed orchard will be planted at the Tennessee Department of Agriculture, Division of Forestry’s East Tennessee State Nursery in southeastern Tennessee.
4. Discussion
4.1. Outcomes of Propagation Efforts
One of the key accomplishments of this work is the preservation of critically imperiled Harbison hawthorn wild genotypes in seed orchards with long-term management and stability. To date, 20 sources have been conserved through clonal propagation, and open-pollinated seedlings were produced from 12 unique mother trees. These living collections not only provide a secure repository of germplasm but also serve as a critical seed source for future restoration and research efforts. Given the recalcitrant nature of the seeds of this species, which precludes conventional long-term seed banking, the development of these orchards represents the most sustainable method currently available to maintain genetic diversity and reproductive potential. With 60 ramets derived from 12 ortets already planted at the Milan center and more clones in development, this work represents a significant step forward in ensuring the continued existence of this species in Tennessee.
While the proximity of the Milan site to the natural population is beneficial in that it ensures that the environmental characteristics of the ex situ location are relatively similar to those of the in situ location, it also increases the risk of losing both the wild and domestic populations due to large scale natural disaster, disease outbreak, or another catastrophic event. The planned establishment of two additional seed orchards in disjunct locations in Tennessee will ensure that these germplasm conservation resources are replicated and resilient to disaster and environmental change.
Several outcomes of this work pertaining to hawthorn propagation merit further discussion. First, stratification protocols remain an area of uncertainty. While both warm-cold and cold-only stratification treatments yielded germinants, the rates were variable, and optimal conditions for maximizing germination remain unresolved. Generally, we recommend employing a single cold stratification period for rapid germination. While the initial rate of germination for this method was lower than the warm-cold method (19% vs. 27%), the shorter stratification time provided seedlings with a longer growing season to develop before the first winter and resulted in a higher long time survival rate. Various other combinations of warm-cold treatments could be tested to maximize germination early in the first year while minimizing late season germination.
Our findings underscore the utility of grafting in the rapid establishment of conservation resources. Grafted clones, particularly chip-bud grafts, developed quickly, allowing them to be transplanted into ex situ plantings less than 1.5 years after budwood collection. Many of these clones have retained the reproductive maturity of their ortets and are already producing seed. For a rare species with so few individuals, early seed production can allow the rapid addition of new genotypes and quick initiation of restoration projects [
24]. Grafting success was generally high but varied by source, suggesting genotype-specific differences in compatibility or vigor that should be investigated further. In addition, despite early grafting success (83% success for chip-bud grafting), it remains unclear whether
C. phaenopyrum is the optimal rootstock for Harbison hawthorn propagation in the long term. One particular concern is that the more rapidly growing
C. harbisonii will outgrow the relatively slow growing
C. phaenopyrum and result in gradual graft failure over time [
25]. Additional trials with alternative native
Crataegus species, including
C. harbisonii itself, could improve compatibility and resilience. Ultimately, our goal is to graft
C. harbisonii scionwood onto provenanced
C. harbisonii seedling rootstock as suitable hawthorn seedlings become large enough to use as grafting understock.
4.2. Future Directions
Our work thus far has focused on the establishment of ex situ germplasm conservation resources. However, these plantings are also intended to serve as stable seed sources for the generation of plant material to be used statewide in projects related to vegetation restoration, wildlife enhancement, and reforestation. With only two known wild populations of C. harbisonii, one of which is functionally extirpated, thoughtful consideration must be given to future reintroduction or augmentation. This will require coordination with private landowners and careful evaluation of potential outplanting sites for ecological suitability and long-term protection. It will also require further research into the compatibility of this species with large-scale nursery practices and establishing best practices for successful outplanting. The success of our competitive release operations indicates that expanding and refining in situ management practices could both bolster existing populations and improve the likelihood of successful reintroductions.
The mystery of this species’ fluctuating demography over time also warrants focused research. Initial descriptions by C.D. Beadle and T.G. Harbison state that
C. harbisonii was common in the hills surrounding the Nashville area [
16]. Scant reporting for the next century suggests that the species greatly dwindled in numbers. Intensive searches in the 1990s, while successfully rediscovering two Harbison hawthorns, confirmed that the species had likely vanished from the landscape. Barry Hart’s discovery of the robust Obion County population thus represents a stark shift in this narrative of gradual diminution.
Beyond the morphological similarities of the Obion and Davidson County specimens as determined by Ron Lance [
15], the genetic relationship between these two disjunct populations is unknown. Certainly, genetic analysis of all documented individuals of this species is necessary to begin to understand its evolutionary history and ecology. Furthermore, it seems quite possible that more undocumented populations could exist in the wild, particularly on difficult to access private land like that of the Obion County population. Although intensive field searches on private land can be costly and logistically challenging, locating additional populations of this severely endangered species is of great conservation concern and should be prioritized along with ex situ conservation efforts.
Confounding these questions is the complicated biology of
Crataegus, a genus characterized by diverse ploidy levels, apomixis, hybridization, and general taxonomic difficulty. Harbison hawthorn, being both tetraploid [
26] and a putative facultative apomict (based on observation of numerous generations of seedlings; Ron Lance, personal communication), bears some of the hallmarks of a species of hybrid origin [
27]. If, in fact, the species is part of a larger agamic complex, intensive molecular study of this and possible relative species such as
C. ashei Beadle,
C. triflora Chapman, or
C. collina Chapman might illuminate the history of this taxon and provide insights into hawthorn speciation more generally. It is also important to understand these dynamics for practical conservation efforts. The extent of clonality in the wild population, as well the frequency of apomictic seed production in our domesticated plantings, will have profound implications for our assumptions of genetic diversity representation and within orchard interbreeding.
5. Conclusions
The ex situ conservation efforts detailed here represent a critical step toward ensuring the long-term survival of C. harbisonii, a species that remains one of the most imperiled trees in the United States. This study provides a model for woody plant conservation that leverages coordinated field collections, propagation via multiple methods, and duplicated ex situ orchard establishment to conserve a critically endangered species. The lessons learned from C. harbisonii; particularly regarding seed handling, clonal propagation, orchard design, and the infrastructure to support propagation and field establishment can inform broader efforts to safeguard the genetic diversity of other rare and threatened woody plants in the southeastern United States and beyond. Furthermore, with extensive collaboration between university-based researchers, federal land management agencies, conservation professionals, and private landowners, this work exemplifies the value of interagency cooperation in the achievement of conservation goals. As threats to native biodiversity continue to mount, proactive, science-based conservation approaches like those described here will be essential tools to prevent species extinction and maintain ecosystem integrity.